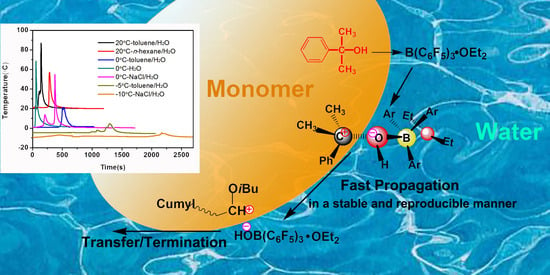
Characteristics and Mechanism of Vinyl Ether Cationic Polymerization in Aqueous Media Initiated by Alcohol/B(C6F5)3/Et2O
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. States of Suspension and Emulsion
2.3. Polymerizations
2.3.1. Vinyl Ether Cationic Polymerization in Suspension
2.3.2. Vinyl Ether Cationic Polymerization in Emulsion
2.4. Measurements
2.5. Computational Method
3. Results and Discussion
3.1. Testing of Different Initiators
3.2. Polymerization in Aqueous Suspension
3.2.1. Effect of Initiator Concentration
3.2.2. Effect of Diluents
3.2.3. Effect of Temperature
3.2.4. Other Vinyl Ethers
3.3. Polymerization in Aqueous Emulsions
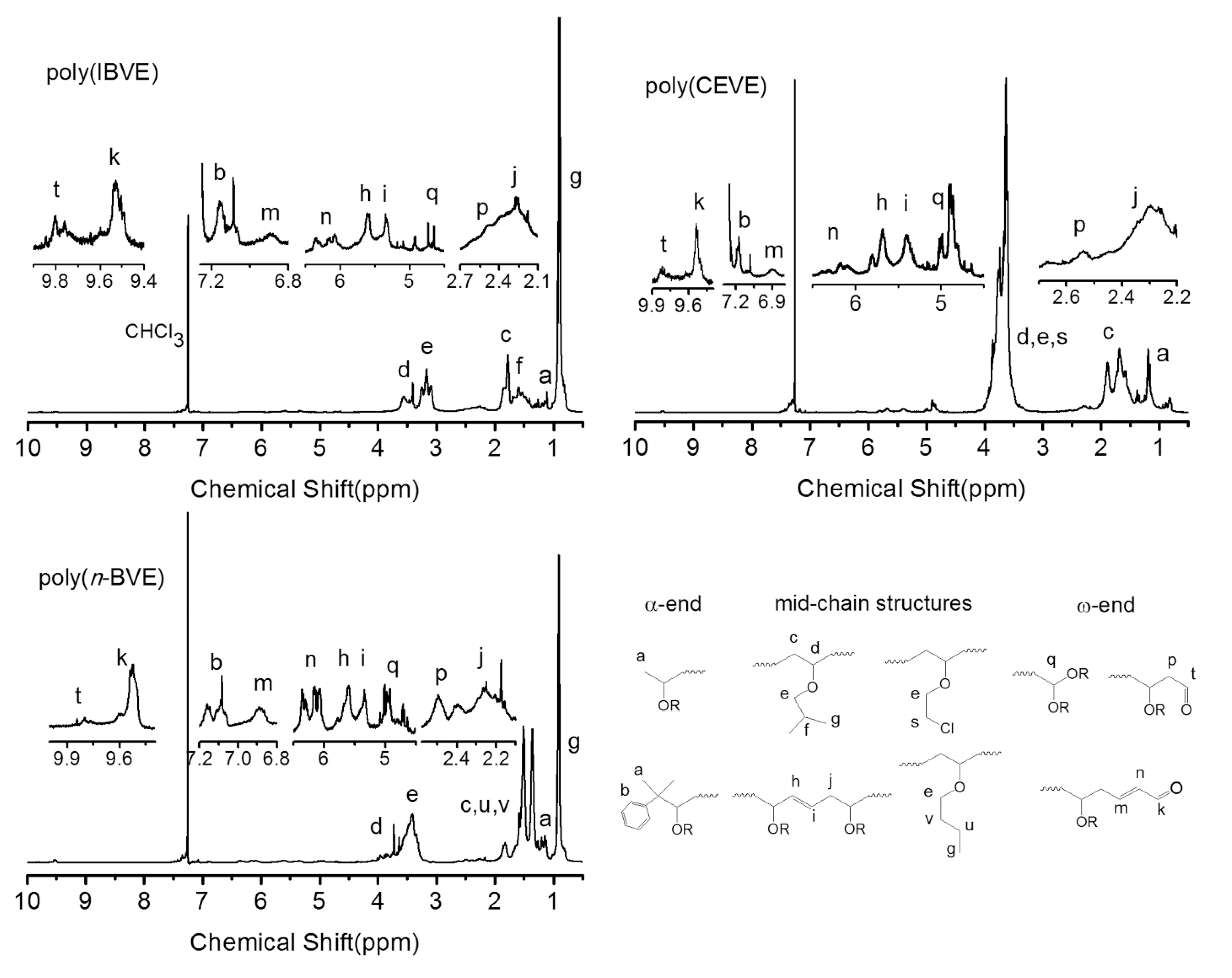
3.4. Polymer Characterization
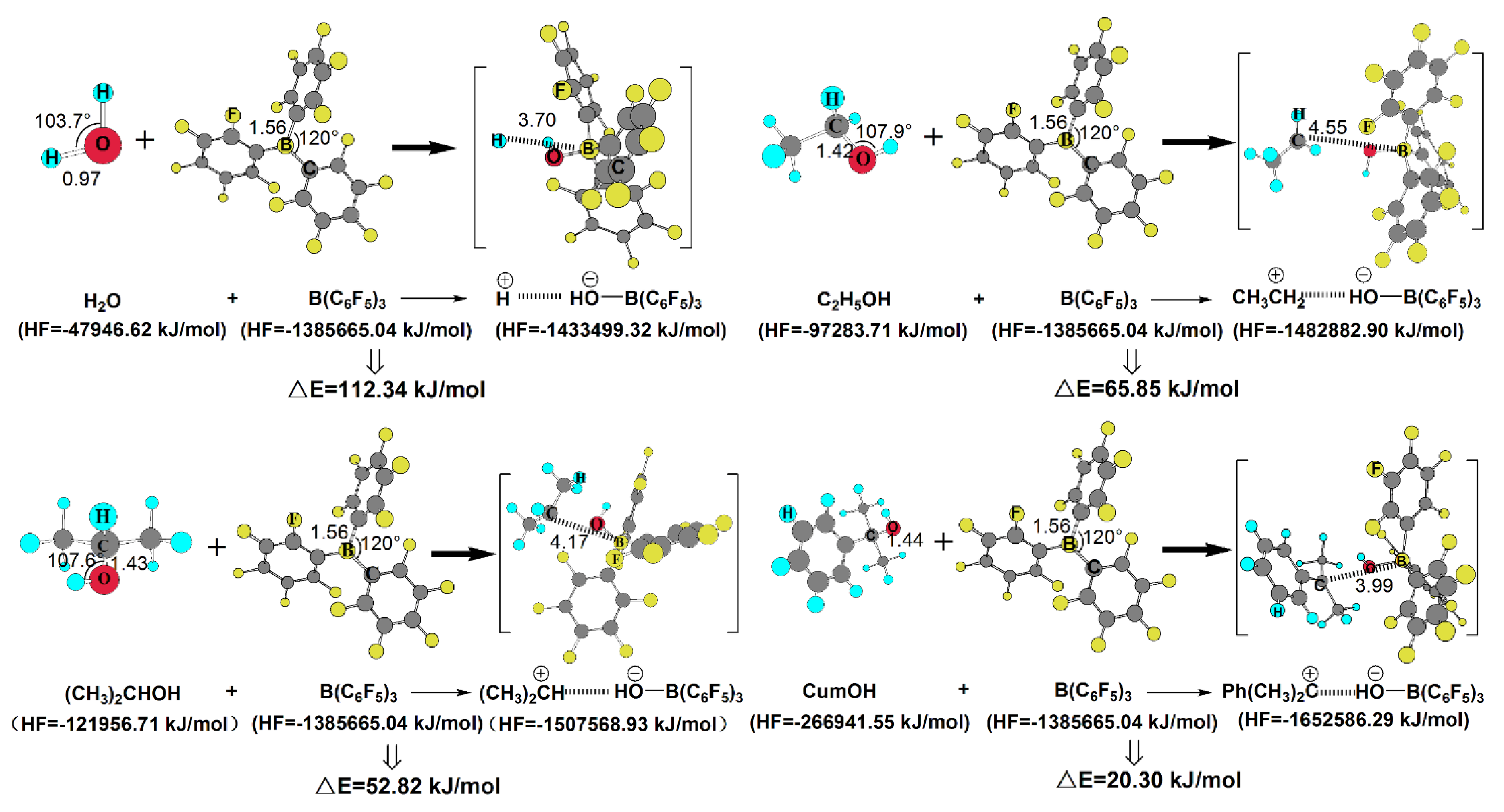
3.5. Possible Interactions Between the Initiator and Co-initiator
3.6. Proposed Mechanism for Polymerization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rajasekhar, T.; Emert, J.; Wolf, L.M.; Faust, R. Controlled catalytic chain transfer polymerization of isobutylene in the presence of tert-butanol as exp-enhancer. Macromolecules 2018, 51, 3041–3049. [Google Scholar] [CrossRef]
- Hadjikyriacou, S.; Faust, R. Amphiphilic block copolymers by sequential living cationic polymerization: Synthesis and characterization of poly(isobutylene-b-methyl vinyl ether). Macromolecules 1996, 29, 5261–5267. [Google Scholar] [CrossRef]
- Kanazawa, A.; Kanaoka, S.; Aoshima, S. Living cationic polymerization of vinyl ether with methanol/metal chloride initiating system: Relationship between polymerization behavior and the nature of Lewis acids. Macromolecules 2010, 43, 2739–2747. [Google Scholar] [CrossRef]
- Kanazawa, A.; Kanaoka, S.; Aoshima, S. Heterogeneously catalyzed living cationic polymerization of isobutyl vinyl ether using iron(III) oxode. J. Am. Chem. Soc. 2007, 129, 2420–2421. [Google Scholar] [CrossRef] [PubMed]
- Aoshima, S.; Kanaoka, S. A renaissance in living cationic polymerization. Chem. Rev. 2009, 109, 5245–5287. [Google Scholar] [CrossRef]
- De, P.; Faust, R. Living carbocationic polymerization of p-methoxystyrene using p- methoxystyrene hydrochloride/SnBr4 initiating system: Determination of the absolute rate constant of propagation for ion pairs. Macromolecules 2004, 37, 7930–7937. [Google Scholar] [CrossRef]
- Wu, Y.; Ren, P.; Guo, W.; Li, S.; Yang, X.; Shang, Y. Living cationic sequential block copolymerization: Synthesis and characterization of poly(4-(2-hydroxyethyl)styrene-b-isobutylene-b-4-(2-hydroxyethyl)styrene)triblock copolymers. Polym. J. 2010, 42, 268–272. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Y.; Li, S.; Mao, J.; Guo, W.; Chang, J.; Zhang, T.; Wu, S. Synthesis of branched butyl rubber with bimodal distribution by cationic polymerization and its structures and properties. Acta Polym. Sin. 2015, 9, 1028–1035. [Google Scholar]
- Anastas, P.T.; Kirchhoff, M.M. Origins, current status, and future challenges of green chemistry. Acc. Chem. Res. 2002, 35, 686–694. [Google Scholar] [CrossRef]
- Horváth, I.T.; Anastas, P.T. Innovations and green chemistry. Chem. Rev. 2007, 107, 2169–2173. [Google Scholar] [CrossRef]
- Kostjuk, S.V.; Vasilenko, I.V.; Shiman, D.I.; Frolov, A.N.; Gaponik, L.V. Highly reactive polyisobutylenes via cationic polymerization of isobutylene by AlCl3/Ether complexes in non-polar media: Scope and limitations. Macromol. Symp. 2015, 349, 94–103. [Google Scholar] [CrossRef]
- Vasilenko, I.V.; Shiman, D.I.; Kostjuk, S.V. Highly reactive polyisobutylenes via AlCl3OBu2-coinitiated cationic polymerization of isobutylene: Effect of solvent polarity, temperature, and initiator. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 750–758. [Google Scholar] [CrossRef]
- Xie, Y.; Chang, J.; Wu, Y.; Yang, D.; Wang, H.; Zhang, T.; Li, S.; Guo, W. Synthesis and properties of bromide-functionalized poly(isobutylene-co-p-methylstyrene) random copolymer. Polym. Int. 2017, 66, 468–476. [Google Scholar] [CrossRef]
- Mingotaud, A.F.; Cansell, F.; Gilbert, N.; Soum, A. Cationic and anionic ring-opening polymerization in supercritical CO2 preliminary results. Polym. J. 1999, 153, 77–86. [Google Scholar] [CrossRef]
- Clark, M.R.; Desimone, J.M. Cationic polymerization of vinyl and cyclic ethers in supercritical and liquid carbon dioxide. Macromolecules 1995, 28, 3002–3004. [Google Scholar] [CrossRef]
- Wu, Y.; Han, L.; Zhang, X.; Mao, J.; Gong, L.; Guo, W.; Gi, K.; Li, S. Cationic polymerization of isobutyl vinyl ether in an imidazole-based ionic liquid: Characteristics and mechanism. Polym. Chem. 2015, 6, 2560–2568. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, W.; Wu, Y.; Gong, L.; Li, W.; Li, X.; Li, S.; Shang, Y.; Yang, D.; Wang, H. Cationic polymerization of p-methylstyrene in selected ionic liquids and polymerization mechanism. Polym. Chem. 2016, 7, 5099–5112. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, W.; Wu, Y.; Li, W.; Li, S.; Shang, Y.; Zhang, J. Solubility of monomers for chain polymerization in ionic liquids predicted by the conductor-like screening model for real solvents. Ind. Eng. Chem. Res. 2017, 56, 14694–14703. [Google Scholar] [CrossRef]
- Han, L.; Wu, Y.; Dan, Y.; Wang, H.; Zhang, X.; Wei, X.; Guo, W.; Li, S. Characteristics and mechanism of styrene cationic polymerization in 1-butyl-3-methylimidazolium hexafluorophosphate ionic liquid. RSC Adv. 2016, 6, 105322–105330. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, W.; Wu, Y.; Shang, Y.; Li, S.; Xiong, W. Synthesis of random copolymer of isobutylene with p-methylstyrene by cationic polymerization in ionic liquids. E-polymers 2018, 18, 423–431. [Google Scholar] [CrossRef]
- Kostjuk, S.V.; Ganachaud, F. Cationic polymerization of vinyl monomers in aqueous media: From monofunctional oligomers to long-lived polymer chains. Acc. Chem. Res. 2010, 43, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wu, Y.; Dan, J. Cationic polymerization of isobutyl vinyl ether coinitiated with heteropolyacid or its salts in aqueous medium. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 546–556. [Google Scholar] [CrossRef]
- Satoh, K.; Kamigaito, A.M.; Sawamoto, M. Controlled cationic polymerization of p-methoxystyrene in aqueous media with Yb(OTf)3. Macromolecules 1999, 32, 3827–3832. [Google Scholar] [CrossRef]
- Satoh, K.; Kamigaito, A.M.; Sawamoto, M. Direct living cationic polymerization of p-hydroxystyrene with boron trifluoride etherate in the presence of water. Macromolecules 2000, 33, 5405–5410. [Google Scholar] [CrossRef]
- Satoh, K.; Kamigaito, A.M.; Sawamoto, M. Metal triflates and tetrafluoroborates as water-tolerant Lewis Acids for cationic polymerization in aqueous media. Macromolecules 2000, 33, 5836–5840. [Google Scholar] [CrossRef]
- Touchard, V.; Graillat, C.; Boisson, C.; D’Agosto, A.F.; Spitz, R. Use of a Lewis Acid surfactant combined catalyst in cationic polymerization in miniemulsion apparent and hidden initiators. Macromolecules 2004, 37, 3136–3142. [Google Scholar] [CrossRef]
- Vasilenko, I.V.; Yeong, H.Y.; Delgado, M.; Ouardad, S.; Peruch, F.; Voit, B.; Ganachaud, F.; Kostjuk, S.V. A catalyst platform for unique cationic (co)polymerizationin aqueous emulsion. Angew. Chem. Int. Ed. 2015, 54, 12728–12732. [Google Scholar] [CrossRef]
- Vasilenko, I.V.; Ganachaud, F.; Kostjuk, S.V. New insights into the cationic polymerization in emulsion catalyzed by water-dispersible Lewis Acid surfactant complexes: A case study with p-methoxystyrene. Macromolecules 2016, 49, 3264–3273. [Google Scholar] [CrossRef]
- Cauvin, S.; Ganachaud, F.; Moreau, M.; Hémery, P. High molar mass polymers by cationic polymerisation in emulsion and miniemulsion. Chem. Commun. 2005, 21, 2713–2715. [Google Scholar] [CrossRef]
- Satoh, K.; Nakashima, J.; Kamigaito, M.; Sawamoto, M. Novel BF3OEt2/R-OH initiating system for controlled cationic polymerization of styrene in the presence of water. Macromolecules 2001, 34, 396–401. [Google Scholar] [CrossRef]
- Radchenko, A.V.; Kostjuk, S.V.; Vasilenko, I.V.; Ganachaud, F.; Kaputsky, F.N. Controlled/living cationic polymerization of styrene with BF3OEt2 as a coinitiator in the presence of water: Improvements and limitations. Eur. Polym. J. 2007, 43, 2576–2583. [Google Scholar] [CrossRef]
- Kostjuk, S.V.; Ganachaud, F. Cationic polymerization of styrene in solution and aqueous suspension using B(C6F5)3 as a water-tolerant Lewis Acid. Macromolecules 2006, 39, 3110–3113. [Google Scholar] [CrossRef]
- Kostjuk, S.V.; Ganachaud, F.; Radchenko, A.V.; Vasilenko, I.V. Cationic polymerization of styrene derivatives and cyclopentadiene catalyzed by B(C6F5)3 in aqueous media: Comparison of suspension, emulsion and dispersion processes. Macromol. Symp. 2011, 308, 1–7. [Google Scholar] [CrossRef]
- Kostjuk, S.V.; Radchenko, A.V.; Ganachaud, F. Controlled cationic polymerization of cyclopentadiene with B(C6F5)3 as a coinitiator in the presence of water. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 4734–4747. [Google Scholar] [CrossRef]
- Kostjuk, S.V.; Ouardad, S.; Peruch, F.; Deffieux, A.; Absalon, C.; Puskas, J.E.; Ganachaud, F. Carbocationic polymerization of isoprene co-initiated by B(C6F5)3: An alternative route toward natural rubber polymer analogues? Macromolecules 2011, 44, 1372–1384. [Google Scholar] [CrossRef]
- Mathers, R.T.; Lewis, S.P. Aqueous cationic olefin polymerization using tris(pentafluorophenyl)gallium and aluminum. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1325–1332. [Google Scholar] [CrossRef]
- Radchenko, A.V.; Kostjuk, S.V.; Ganachaud, F. Cationic polymerization of isobutyl vinyl ether in aqueous media: Physical chemistry tricks to fight against thermal runaway. Polym. Chem. 2013, 4, 1883–1892. [Google Scholar] [CrossRef]
- Lewis, S.P.; Henderson, L.D.; Chandler, B.D.; Parvez, M.; Piers, W.E.; Collins, S. Aqueous suspension polymerization of isobutene initiated by 1,2-C6F4[B(C6F5)2]2. J. Am. Chem. Soc. 2005, 127, 46–47. [Google Scholar] [CrossRef]
- Lewis, S.P.; Chai, J.; Collins, S. Isobutene polymerization using chelating diboranes: Polymerization in aqueous suspension and hydrocarbon solution. Organometallics 2009, 28, 249–263. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A At. Mol. Opt. Phys. 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B Condens. Matter 1986, 33, 8822–8824. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Dimitrov, P.; Bartelson, K.J.; Emert, J.; Faust, R. Polymerization of isobutylene by GaCl3 or FeCl3/ether complexes in nonpolar solvents. Macromolecules 2012, 45, 8598–8603. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, Y.; Yan, P.; Zhang, Y.; Xu, R. Polyisobutylene with high exo-olefin content via β-H elimination in the cationic polymerization of isobutylene with H2O/FeCl3/dialkyl ether initiating system. Macromolecules 2011, 44, 1866–1875. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, K.; Lu, Y. Effects of ether on the cationic polymerization of isobutylene catalyzed by AlCl3. ACS Omega 2018, 3, 2033–2039. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Y.; Li, X.; Yang, D.; Zhang, M.; Wang, H.; Shang, Y.; Ren, P.; Mu, X.; Li, S.; et al. Characteristics and mechanism of styrene cationic polymerization in aqueous media initiated by cumyl alcohol/B(C6F5)3. Macromol. Chem. Phys. 2019, 220. [Google Scholar] [CrossRef]
- Sipos, L.; De, P.; Faust, R. Effect of temperature, solvent polarity, and nature of Lewis Acid on the rate constants in the carbocationic polymerization of isobutylene. Macromolecules 2003, 36, 8282–8290. [Google Scholar] [CrossRef]
- Sawamoto, M. Modern cationic vinyl polymerization. Prog. Polym. Sci. 1991, 16, 111–172. [Google Scholar] [CrossRef]
- Kostjuk, S.V.; Radchenko, A.V.; Ganachaud, F. Controlled living cationic polymerization of p-methoxystyrene in solution and aqueous dispersion using tris(pentafluorophenyl)borane as a Lewis Acid acetonitrile does the Job. Macromolecules 2007, 40, 482–490. [Google Scholar] [CrossRef]
- Fuji, Y.; Ando, T.; Kamigaito, M.; Sawamoto, M. Iron-catalyzed suspension living radical polymerizations of acrylates and styrene in water. Macromolecules 2002, 35, 2949–2954. [Google Scholar] [CrossRef]
- Vasilenko, I.V.; Frolov, A.N.; Kostjul, S.V. Cationic polymerization of isobutylene using AlCl3OBu2 as a coinitiator: Synthesis of highly reactive polyisobutylene. Macromolecules 2010, 43, 5503–5507. [Google Scholar] [CrossRef]
- Ouardad, S.; Deffieux, A.; Peruch, F. Polyisoprene synthesized via cationic polymerization: State of the art. Pure Appl. Chem. 2012, 84, 2065–2080. [Google Scholar] [CrossRef]








| run | Initiator (content) (mmol) | Conv.b (±S) (%) | Mnc (GPC) (g mol−1) | Mw/Mnc |
|---|---|---|---|---|
| 1 | H2O (∞) | 30.1 (±15.4) | 4690 | 1.88 |
| 2 | EtOH (0.25) | 45.2 (±15.6) | 4310 | 2.01 |
| 3 | IPA (0.25) | 43.3 (±7.7) | 5940 | 1.57 |
| 4 | CumOH (0.25) | 48.3 (±1.6) | 6270 | 1.39 |
| Run | Medium | T (°C) | Time (min) | Conv. (%) | Mn (GPC) (g mol−1) | Mw/Mn |
|---|---|---|---|---|---|---|
| 5 | H2O | 20 | 5 | 48.9 | 6500 | 1.39 |
| 6 | H2O | 0 | 10 | 27.4 | 5020 | 1.41 |
| 7 | n-hexane/H2O | 20 | 17 | 44.2 | 4810 | 1.32 |
| 8 | n-hexane/H2O | 0 | 21 | 40.7 | 4690 | 1.28 |
| 9 | n-hexane/H2O | −5 | 27 | 24.7 | 4410 | 1.36 |
| 10 | Toluene/H2O | 20 | 17 | 42.6 | 4610 | 1.37 |
| 11 | Toluene/H2O | 0 | 21 | 35.1 | 4550 | 1.40 |
| 12 | Toluene/H2O | −5 | 27 | 22.7 | 4090 | 1.32 |
| 13 | NaCl solution b | 20 | 15 | 37.3 | 4800 | 1.36 |
| 14 | NaCl solution b | 0 | 27 | 28.7 | 3040 | 1.42 |
| 15 | NaCl solution b | −10 | 47 | 23.0 | 2120 | 1.31 |
| run | Media | Acetal b (%) | Aldehyde b (%) | Alkenal b (%) | Internal olefin c (per chain) |
|---|---|---|---|---|---|
| 16 | H2O | 31.8 | 31.5 | 26.7 | 0.4 |
| 17 | n-hexane/H2O | 28.2 | 35.1 | 28.7 | 0.3 |
| 18 | Toluene/H2O | 27.0 | 36.0 | 27.0 | 0.4 |
| 19 | NaCl solution | 17.0 | 46.1 | 30.0 | 0.5 |
| 20 | CTAB | 27.9 | 39.6 | 23.5 | 0.1 |
| 21 | NP-40 | 28.8 | 33.4 | 27.9 | 0.2 |
| 22 | SDBS | 39.8 | 34.8 | 19.5 | 0.1 |
| 23 d | H2O | 33.8 | 20.6 | 3.2 | 0.4 |
| 24 e | H2O | 11.7 | 41.6 | 38.6 | 0.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wu, Y.; Chen, K.; Zhang, M.; Gong, L.; Yang, D.; Li, S.; Guo, W. Characteristics and Mechanism of Vinyl Ether Cationic Polymerization in Aqueous Media Initiated by Alcohol/B(C6F5)3/Et2O. Polymers 2019, 11, 500. https://doi.org/10.3390/polym11030500
Zhang J, Wu Y, Chen K, Zhang M, Gong L, Yang D, Li S, Guo W. Characteristics and Mechanism of Vinyl Ether Cationic Polymerization in Aqueous Media Initiated by Alcohol/B(C6F5)3/Et2O. Polymers. 2019; 11(3):500. https://doi.org/10.3390/polym11030500
Chicago/Turabian StyleZhang, Jinghan, Yibo Wu, Kaixuan Chen, Min Zhang, Liangfa Gong, Dan Yang, Shuxin Li, and Wenli Guo. 2019. "Characteristics and Mechanism of Vinyl Ether Cationic Polymerization in Aqueous Media Initiated by Alcohol/B(C6F5)3/Et2O" Polymers 11, no. 3: 500. https://doi.org/10.3390/polym11030500
APA StyleZhang, J., Wu, Y., Chen, K., Zhang, M., Gong, L., Yang, D., Li, S., & Guo, W. (2019). Characteristics and Mechanism of Vinyl Ether Cationic Polymerization in Aqueous Media Initiated by Alcohol/B(C6F5)3/Et2O. Polymers, 11(3), 500. https://doi.org/10.3390/polym11030500