Preparation of Layered Polyethylene Oxide/rGO Composite: Flexible Lateral Heat Spreaders
Abstract
1. Introduction
2. Experiments
2.1. Materials
2.2. Synthesis of Graphene Oxide (GO)
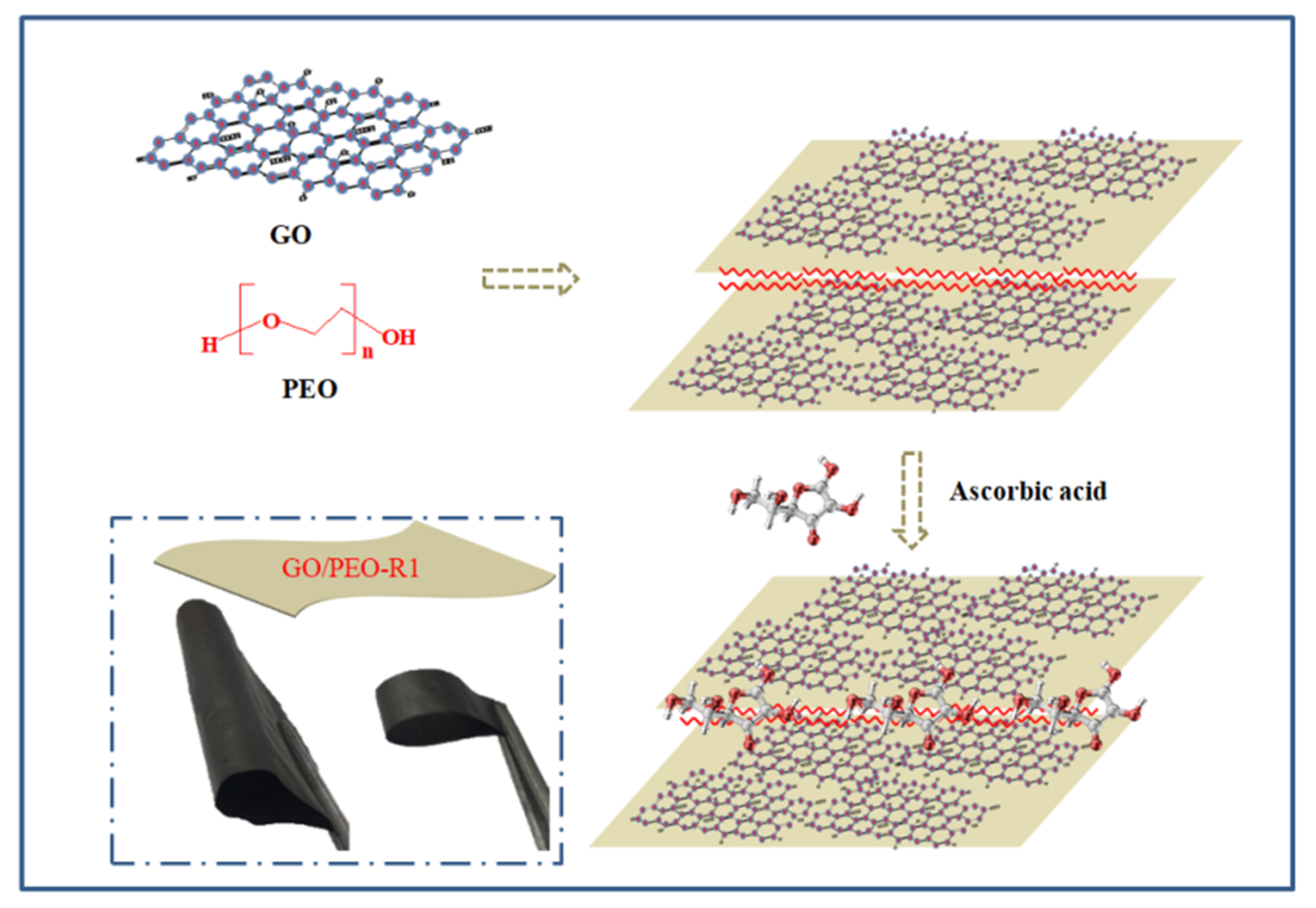
2.3. Synthesis of the Composites
2.4. Material Characterization
3. Results and Discussion
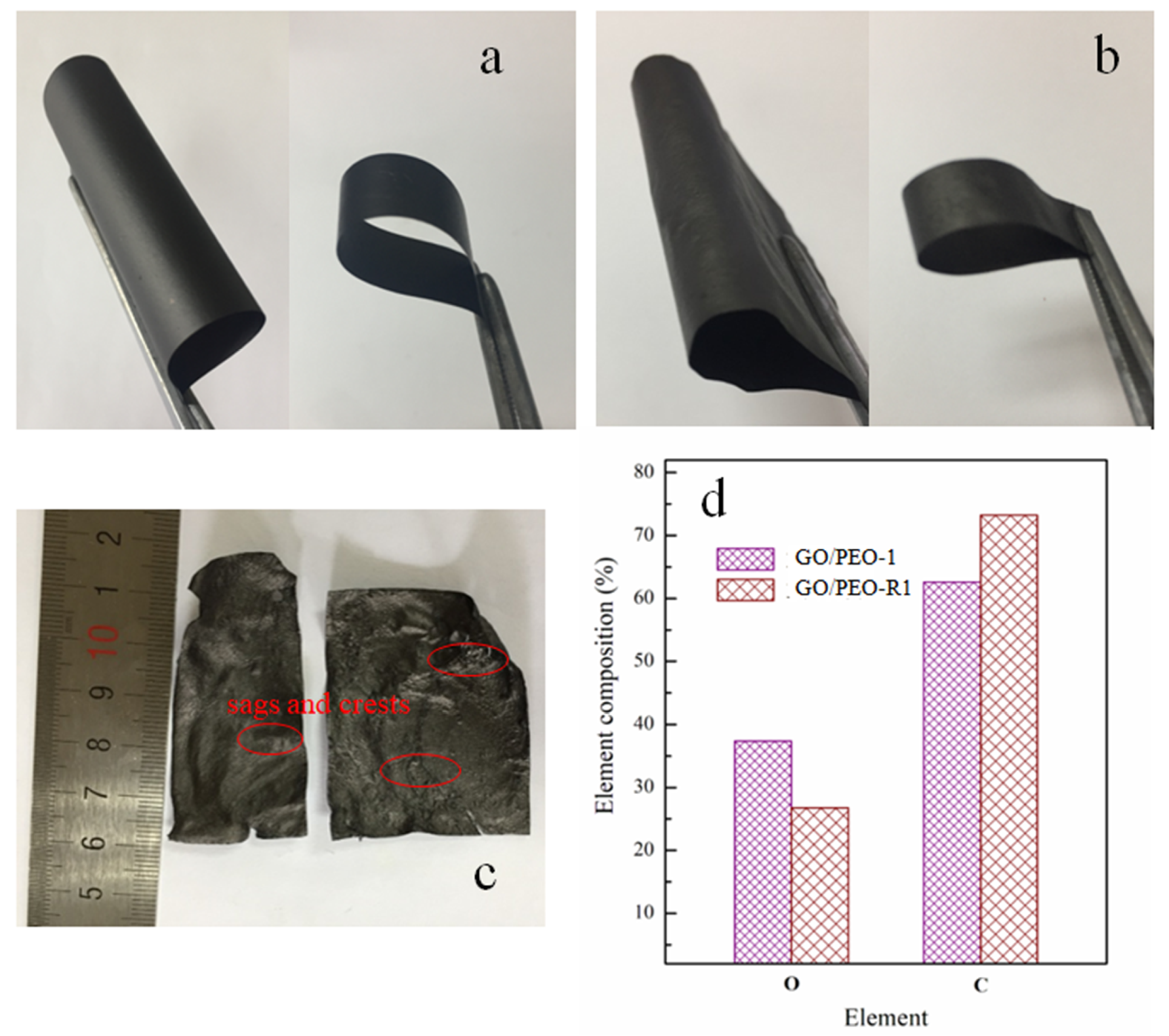
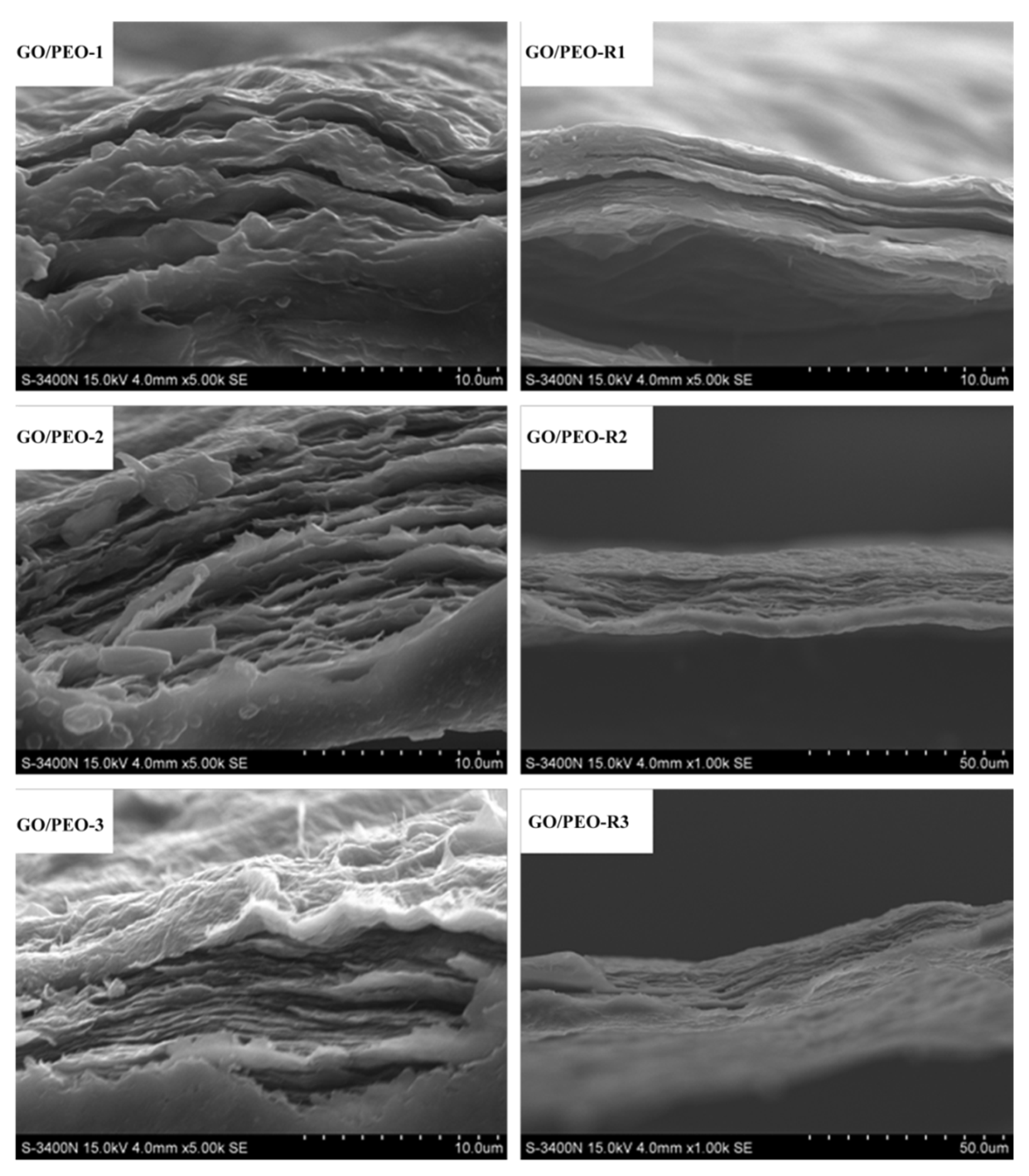
3.1. Morphology Characterization of the Films
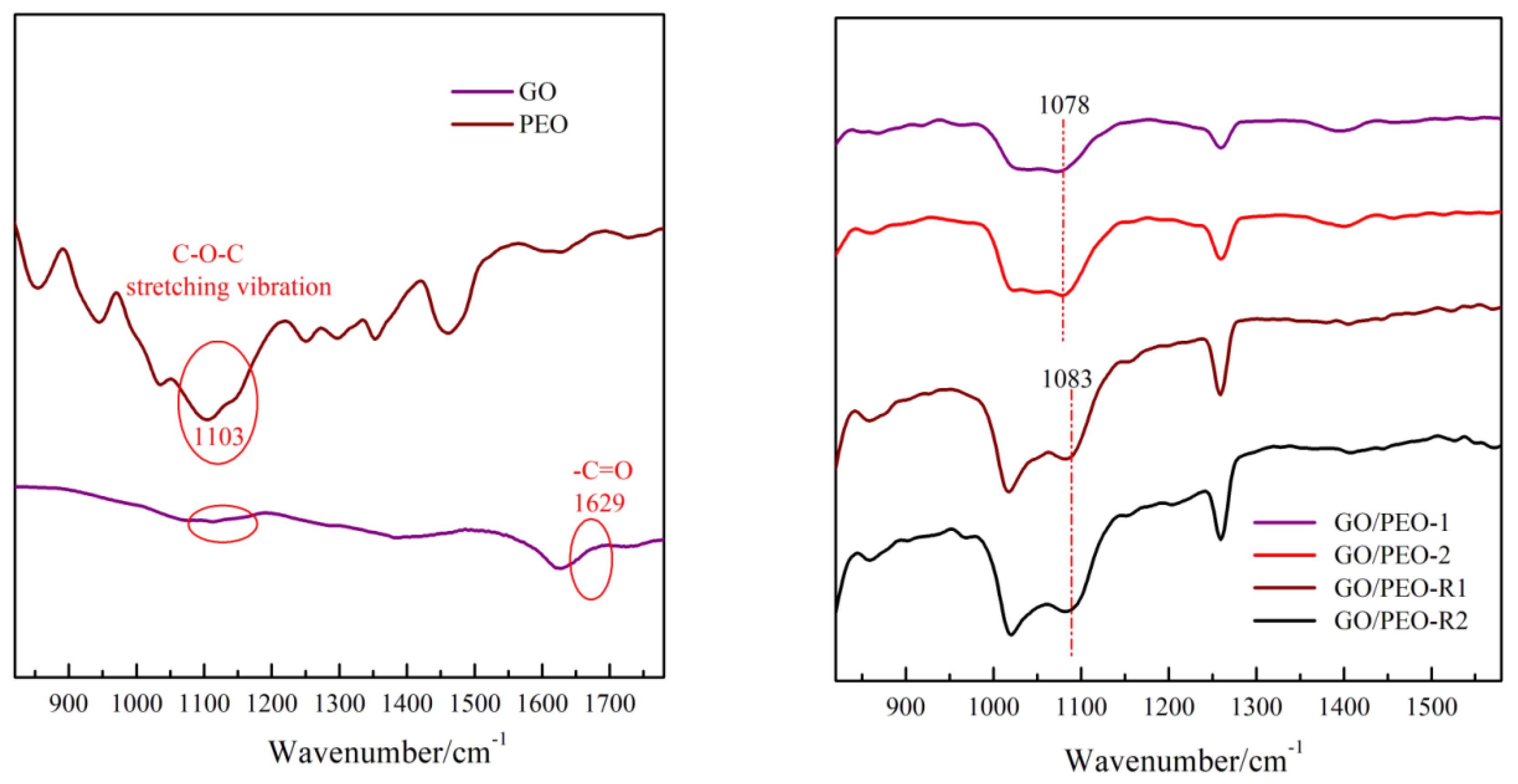
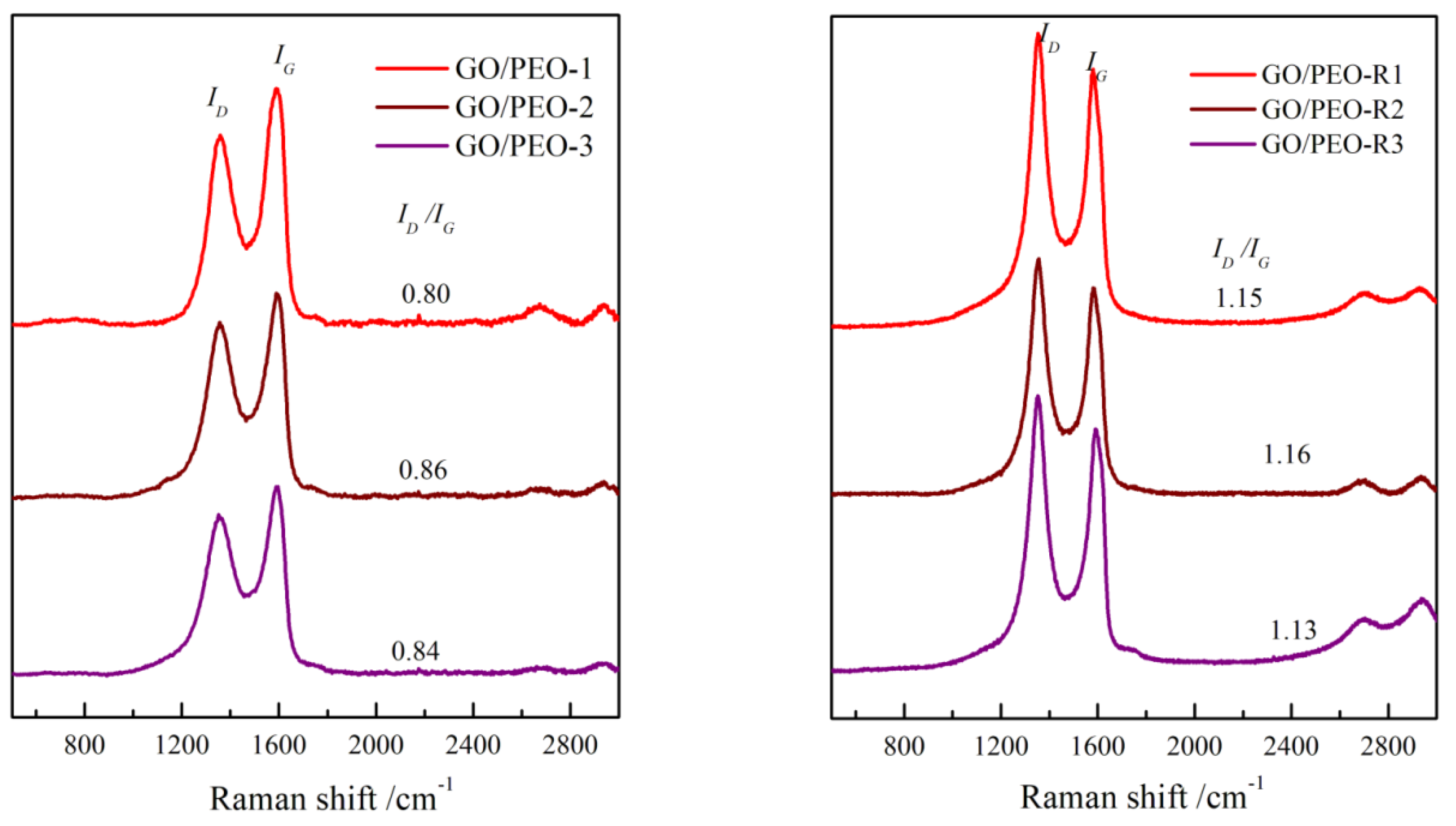
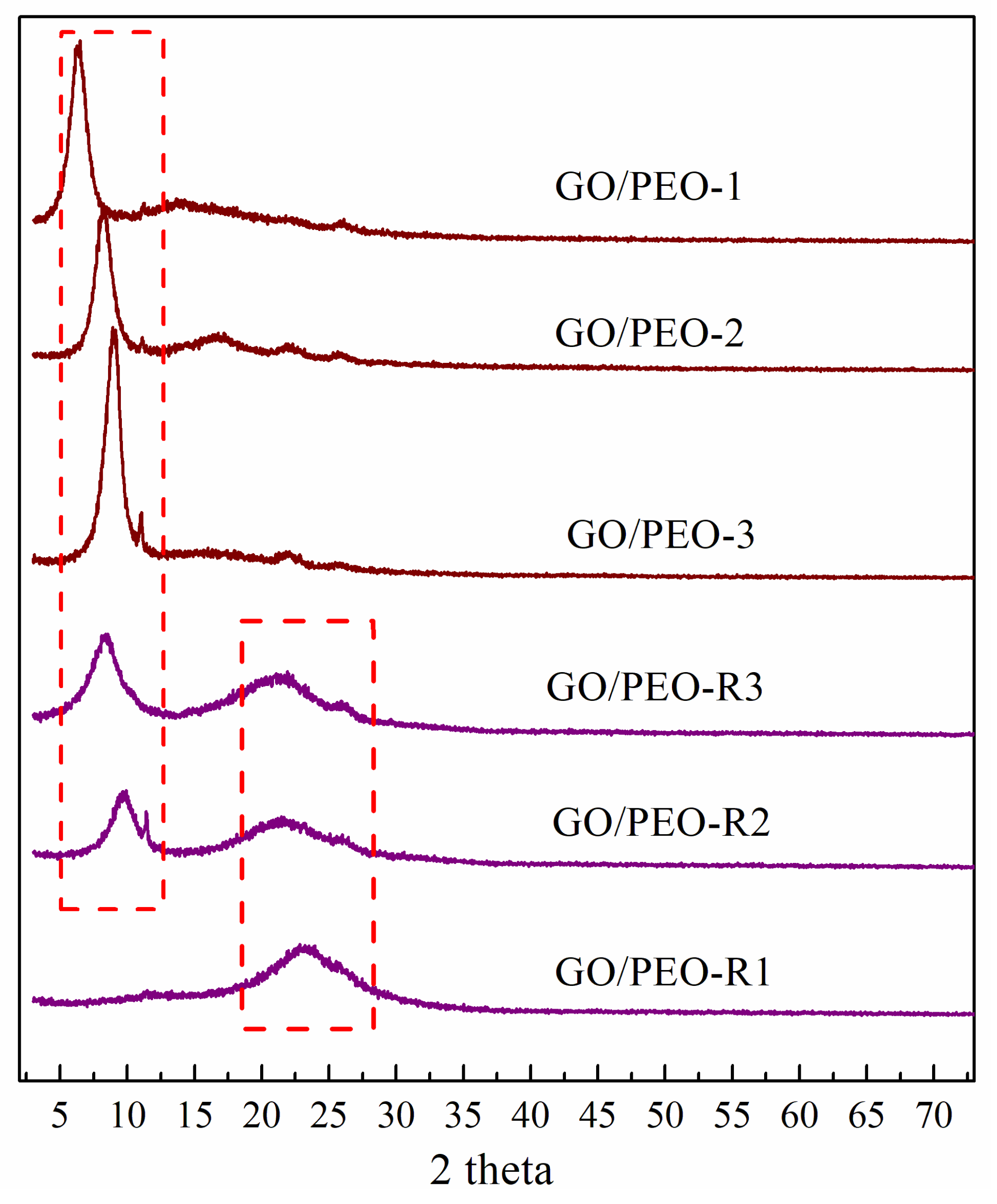
3.2. Raman and XRD Characterization of the Films
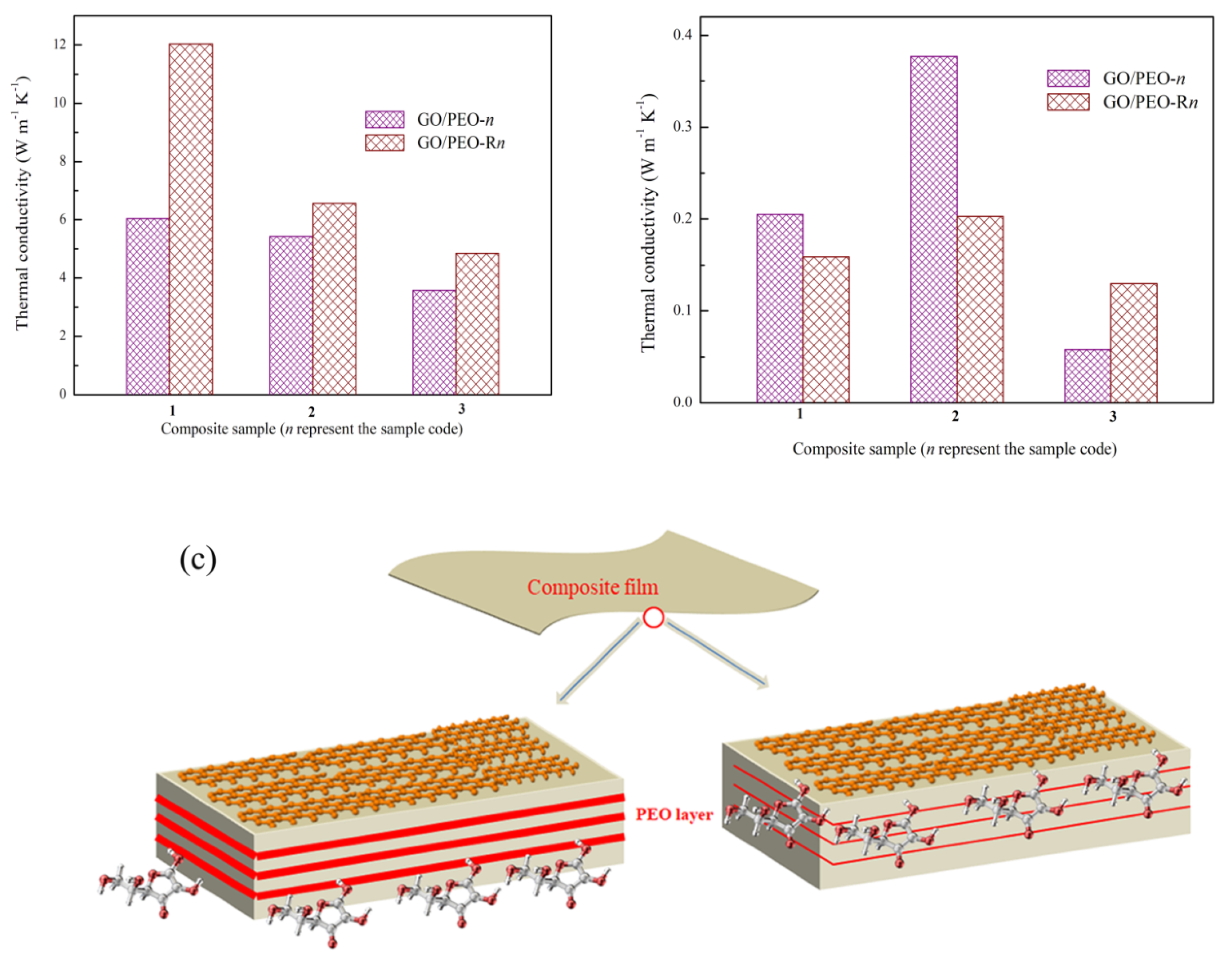
3.3. Thermal Conductivity
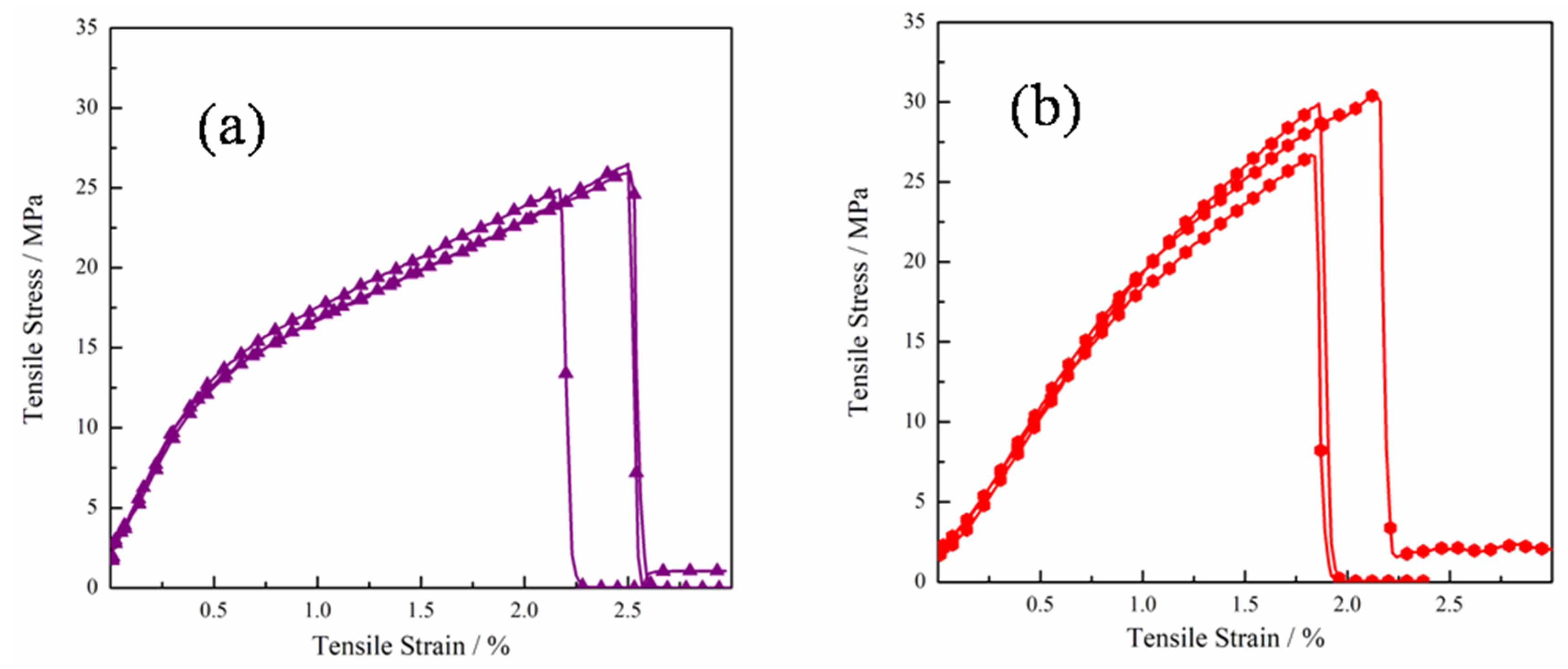
3.4. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Song, N.; Shi, L.Y.; Ding, P. Anisotropic thermally conductive composite with wood-derived carbon scaffolds. Compos. Part A Appl. Sci. Manuf. 2018, 112, 18–24. [Google Scholar] [CrossRef]
- Yang, X.T.; Guo, Y.Q.; Luo, X.; Zheng, N.; Ma, T.B.; Tan, J.J.; Li, C.M.; Zhang, Q.Y.; Gu, J.W. Self-healing, recoverable epoxy elastomers and their composites with desirable thermal conductivities by incorporating BN fillers via in-situ polymerization. Compos. Sci. Technol. 2018, 164, 59–64. [Google Scholar] [CrossRef]
- Dai, W.; Yu, J.H.; Wang, Y.; Song, Y.Z.; Alam, F.E.; Nishimura, K.; Lin, C.T.; Jiang, N. Enhanced thermal conductivity for polyimide composites with a three-dimensional silicon carbide nanowire@graphene sheets filler. J. Mater. Chem. A 2015, 3, 4884–4891. [Google Scholar] [CrossRef]
- Luo, F.B.; Wu, K.; Guo, H.L.; Zhao, Q.; Lu, M.G. Anisotropic thermal conductivity and flame retardancy of nanocomposite based on mesogenic epoxy and reduced graphene oxide bulk. Compos. Sci. Technol. 2016, 132, 1–8. [Google Scholar] [CrossRef]
- Fu, C.J.; Li, Q.; Lu, J.B.; Mateti, S.; Cai, Q.R.; Zeng, X.L.; Du, G.P.; Sun, R.; Chen, Y.; Xu, J.B.; et al. Improving thermal conductivity of polymer composites by reducing interfacial thermal resistance between boron nitride nanotubes. Compos. Sci. Technol. 2018, 165, 322–330. [Google Scholar] [CrossRef]
- Yao, Y.M.; Sun, J.J.; Zeng, X.L.; Sun, R.; Xu, J.B.; Wong, C.P. Construction of 3D Skeleton for Polymer Composites Achieving a High Thermal Conductivity. Small 2018, 14, 1704044. [Google Scholar] [CrossRef]
- Bartlett, M.D.; Kazem, N.; Powell-Palm, M.J.; Huang, X.; Sun, W.; Malen, J.A.; Majidi, C. High thermal conductivity in soft elastomers with elongated liquid metal inclusions. Proc. Natl. Acad. Sci. USA 2017, 114, 2143–2148. [Google Scholar] [CrossRef]
- Gu, J.; Lv, Z.; Wu, Y.; Guo, Y.; Tian, L.; Qiu, H.; Li, W.; Zhang, Q. Dielectric thermally conductive boron nitride/polyimide composites with outstanding thermal stabilities via in-situ polymerization-electrospinning-hot press method. Compos. Part A Appl. Sci. Manuf. 2017, 94, 209–216. [Google Scholar] [CrossRef]
- Jiang, F.; Cui, S.Q.; Song, N.; Shi, L.Y.; Ding, P. Hydrogen Bond-Regulated Boron Nitride Network Structures for Improved Thermal Conductive Property of Polyamide-imide Composites. ACS Appl. Mater. Interfaces 2018, 10, 16812–16821. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, J. BN-MWCNT/PPS core-shell structured composite for high thermal conductivity with electrical insulating via particle coating. Polymer 2016, 101, 168–175. [Google Scholar] [CrossRef]
- Du, B.X.; Kong, X.X.; Cui, B.; Li, J. Improved ampacity of buried HVDC cable with high thermal conductivity LDPE/BN insulation. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 2667–2676. [Google Scholar] [CrossRef]
- Gao, Z.; Zhao, L. Effect of nano-fillers on the thermal conductivity of epoxy composites with micro-Al2O3 particles. Mater. Des. 2015, 66, 176–182. [Google Scholar] [CrossRef]
- Kwon, O.H.; Ha, T.; Kim, D.-G.; Kim, B.G.; Kim, Y.S.; Shin, T.J.; Koh, W.-G.; Lim, H.S.; Yoo, Y. Anisotropy-Driven High Thermal Conductivity in Stretchable Poly(vinyl alcohol)/Hexagonal Boron Nitride Nanohybrid Films. ACS Appl. Mater. Interfaces 2018, 10, 34625–34633. [Google Scholar] [CrossRef]
- Alam, F.E.; Dai, W.; Yang, M.H.; Du, S.Y.; Li, X.M.; Yu, J.H.; Jiang, N.; Lin, C.T. In situ formation of a cellular graphene framework in thermoplastic composites leading to superior thermal conductivity. J. Mater. Chem. A 2017, 5, 6164–6169. [Google Scholar] [CrossRef]
- Dai, L. Functionalization of graphene for efficient energy conversion and storage. Acc. Chem. Res. 2012, 46, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Abdelsayed, V.; Moussa, S.; Hassan, H.M.; Aluri, H.S.; Collinson, M.M.; Elshall, M.S. Photothermal Deoxygenation of Graphite Oxide with Laser Excitation in Solution and Graphene-Aided Increase in Water Temperature. J. Phys. Chem. Lett. 2010, 1, 283–298. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.; Erni, R.; Lee, Z.; Alem, N.; Gannett, W.; Zettl, A. Determination of the local chemical structure of graphene oxide and reduced graphene oxide. Adv. Mater. 2010, 22, 4467–4472. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Xu, G.J.; Yang, X.T.; Ruan, K.P.; Ma, T.B.; Zhang, Q.Y.; Gu, J.W.; Wu, Y.L.; Liu, H.; Guo, Z. Significantly enhanced and precisely modeled thermal conductivity in polyimide nanocomposites with chemically modified graphene via in situ polymerization and electrospinning-hot press technology. J. Mater. Chem. C 2018, 6, 3004–3015. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- He, Q.; Wu, S.; Gao, S.; Cao, X.; Yin, Z.; Li, H.; Chen, P.; Zhang, H. Transparent, flexible, all-reduced graphene oxide thin film transistors. ACS Nano 2011, 5, 5038–5044. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene oxide: Preparation, functionalization, and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.Y.; Song, W.L.; Meziani, M.J.; Tackett, K.N.; Cao, L.; Farr, A.J.; Anderson, A.; Sun, Y.P. Supercritical fluid conversion of graphene oxides. J. Supercrit. Fluids 2012, 61, 206–211. [Google Scholar] [CrossRef]
- Yao, Y.; Zeng, X.; Wang, F.; Sun, R.; Xu, J.B.; Wong, C.P. Significant Enhancement of Thermal Conductivity in Bioinspired Freestanding Boron Nitride Papers Filled with Graphene Oxide. Chem. Mater. 2016, 80, 1049–1057. [Google Scholar] [CrossRef]
- Renteria, J.D.; Ramirez, S.; Malekpour, H.; Alonso, B.; Centeno, A.; Zurutuza, A.; Cocemasov, A.I.; Nika, D.L.; Balandin, A.A. Anisotropy of Thermal Conductivity of Free-Standing Reduced Graphene Oxide Films Annealed at High Temperature. Adv. Funct. Mater. 2015, 25, 4664–4672. [Google Scholar] [CrossRef]
- De Silva, K.K.; Huang, H.H.; Yoshimura, M. Progress of reduction of graphene oxide by ascorbic acid. Appl. Surf. Sci. 2018, 447, 338–346. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Naicker, S.; Barman, A.; Roy, S.S.; Thundat, T.; Waghmare, P.R. Fabrication of free-standing graphene oxide films using a facile approach toluene swollen paraffin peeling and green reduction of these films into highly conductive reduced graphene oxide films. Chem. Eng. J. 2018, 354, 149–161. [Google Scholar] [CrossRef]
- Sadak, O.; Sundramoorthy, A.K.; Gunasekaran, S. Facile and green synthesis of highly conducting graphene paper. Carbon 2018, 138, 108–117. [Google Scholar] [CrossRef]
- Luo, F.B.; Wu, K.; Guo, H.L.; Zhao, Q.; Lu, M.G. Simultaneous reduction and surface functionalization of graphene oxide for enhancing flame retardancy and thermal conductivity of mesogenic epoxy composites. Polym. Int. 2017, 66, 98–107. [Google Scholar] [CrossRef]
- Zong, P.; Chen, X.; Zhu, Y.; Liu, Z.; Zeng, Y.; Chen, L. Construction of a 3D-rGO network-wrapping architecture in a YbyCo4Sb12/rGO composite for enhancing the thermoelectric performance. J. Mater. Chem. A 2015, 3, 8643–8649. [Google Scholar] [CrossRef]
- Pan, T.W.; Kuo, W.S.; Tai, N.H. Tailoring anisotropic thermal properties of reduced graphene oxide/multi-walled carbon nanotube hybrid composite films. Compos. Sci. Technol. 2017, 151, 44–51. [Google Scholar] [CrossRef]
- Huang, Y.; Gong, Q.; Zhang, Q.; Shao, Y.; Wang, J.; Jiang, Y.; Zhao, M.; Zhuang, D.; Liang, J. Fabrication and molecular dynamics analyses of highly thermal conductive reduced graphene oxide films at ultra-high temperatures. Nanoscale 2017, 9, 2340–2347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, D.; Tan, X.; Zhang, B.; Ruan, X.; Liu, H.; Pan, C.; Liao, L.; Zhai, T.; Bando, Y. High quality graphene sheets from graphene oxide by hot-pressing. Carbon 2013, 54, 143–148. [Google Scholar] [CrossRef]
- Cui, X.L.; Ding, P.; Zhuang, N.; Shi, L.Y.; Song, N.; Tang, S.F. Thermal Conductive and Mechanical properties of Polymeric Composites Based on Solution-Exfoliated Boron Nitride and Graphene Nanosheets: A Morphology-Promoted Synergistic Effect. ACS Appl. Mater. Interfaces 2015, 7, 19068–19075. [Google Scholar] [CrossRef]
- Wang, W.; Wang, C.; Wang, T.; Li, W.; Chen, L.; Zou, R.; Zheng, J.; Li, X. Enhancing the thermal conductivity of n-eicosane/silica phase change materials by reduced graphene oxide. Mater. Chem. Phys. 2014, 147, 701–706. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Ma, C.C.; Chiang, J.C.; Ho, K.K.; Chou, T.Y.; Xie, X.; Tsai, C.H.; Chang, L.H.; Hsieh, C.K. Thermally conductive and electrically insulating epoxy nanocomposites with thermally reduced graphene oxide-silica hybrid nanosheets. Nanoscale 2013, 5, 5863–5871. [Google Scholar] [CrossRef]
- Kumar, P.; Yu, S.; Shahzad, F.; Hong, S.M.; Kim, Y.H.; Chong, M.K. Ultrahigh electrically and thermally conductive self-aligned graphene/polymer composites using large-area reduced graphene oxides. Carbon 2016, 101, 120–128. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Chen, C.; Yang, Q.H.; Yang, Y.; Lv, W.; Wen, Y.; Hou, P.X.; Wang, M.; Cheng, H.M. Self-Assembled Free-Standing Graphite Oxide Membrane. Adv. Mater. 2010, 21, 3007–3011. [Google Scholar] [CrossRef]
- Luo, F.; Wu, K.; Shi, J.; Du, X.; Li, X.; Yang, L.; Lu, M. Green reduction of graphene oxide by polydopamine to a construct flexible film: Superior flame retardancy and high thermal conductivity. J. Mater. Chem. A 2017, 5, 18542–18550. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’Homme, R.K.; Aksay, I.A.; Car, R. Raman Spectra of Graphite Oxide and Functionalized Graphene Sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Iliut, M.; Leordean, C.; Canpean, V.; Teodorescu, C.M.; Astilean, S. A new green, ascorbic acid-assisted method for versatile synthesis of Au-graphene hybrids as efficient surface-enhanced Raman scattering platforms. J. Mater. Chem. C 2013, 1, 4094–4104. [Google Scholar] [CrossRef]
- Kim, G.H.; Lee, D.; Shanker, A.; Shao, L.; Kwon, M.S.; Gidley, D.; Kim, J.; Pipe, K.P. High thermal conductivity in amorphous polymer blends by engineered interchain interactions. Nat. Mater. 2015, 14, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Li, D.; Tsai, T.H.; Liu, J.; Braun, P.V.; Cahill, D.G. Thermal Conductivity, Heat Capacity, and Elastic Constants of Water-Soluble Polymers and Polymer Blends. Macromolecules 2016, 49, 972–978. [Google Scholar] [CrossRef]
| Sample Code | Mass Ratio GO/PEO | Reduction |
|---|---|---|
| GO/PEO-1 | 20:1 | without |
| GO/PEO-2 | 20:4 | without |
| GO/PEO-3 | 20:10 | without |
| GO/PEO-R1 | 20:1 | with |
| GO/PEO-R2 | 20:4 | with |
| GO/PEO-R3 | 20:10 | with |
| Sample | Average Tensile Strength (MPa) | Average Elongation at Break (%) | Average Modulus (GPa) |
|---|---|---|---|
| GO/PEO-1 | 25.8 | 2.37 | 2.43 |
| GO/PEO-R1 | 28.7 | 1.90 | 1.46 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, F.; Yan, P.; Qian, Q.; Li, H.; Huang, B.; Chen, Q. Preparation of Layered Polyethylene Oxide/rGO Composite: Flexible Lateral Heat Spreaders. Polymers 2019, 11, 532. https://doi.org/10.3390/polym11030532
Luo F, Yan P, Qian Q, Li H, Huang B, Chen Q. Preparation of Layered Polyethylene Oxide/rGO Composite: Flexible Lateral Heat Spreaders. Polymers. 2019; 11(3):532. https://doi.org/10.3390/polym11030532
Chicago/Turabian StyleLuo, Fubin, Pinping Yan, Qingrong Qian, Hongzhou Li, Baoquan Huang, and Qinghua Chen. 2019. "Preparation of Layered Polyethylene Oxide/rGO Composite: Flexible Lateral Heat Spreaders" Polymers 11, no. 3: 532. https://doi.org/10.3390/polym11030532
APA StyleLuo, F., Yan, P., Qian, Q., Li, H., Huang, B., & Chen, Q. (2019). Preparation of Layered Polyethylene Oxide/rGO Composite: Flexible Lateral Heat Spreaders. Polymers, 11(3), 532. https://doi.org/10.3390/polym11030532






