Effect of a Modified Silicone as a Thickener on Rheology of Liquid CO2 and Its Fracturing Capacity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
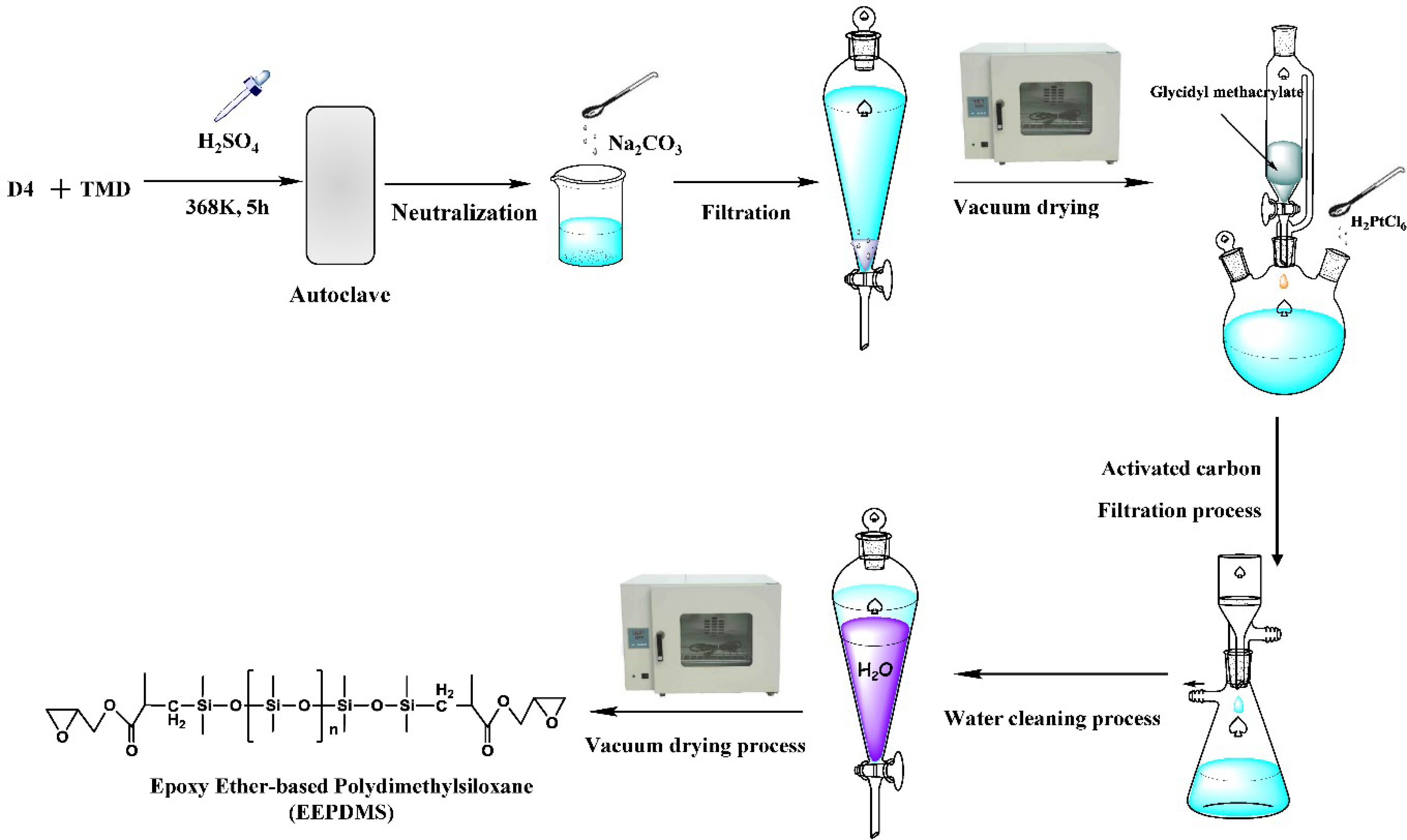
2.2. Preparation of EEPDMS
2.3. EEPDMS Characterization
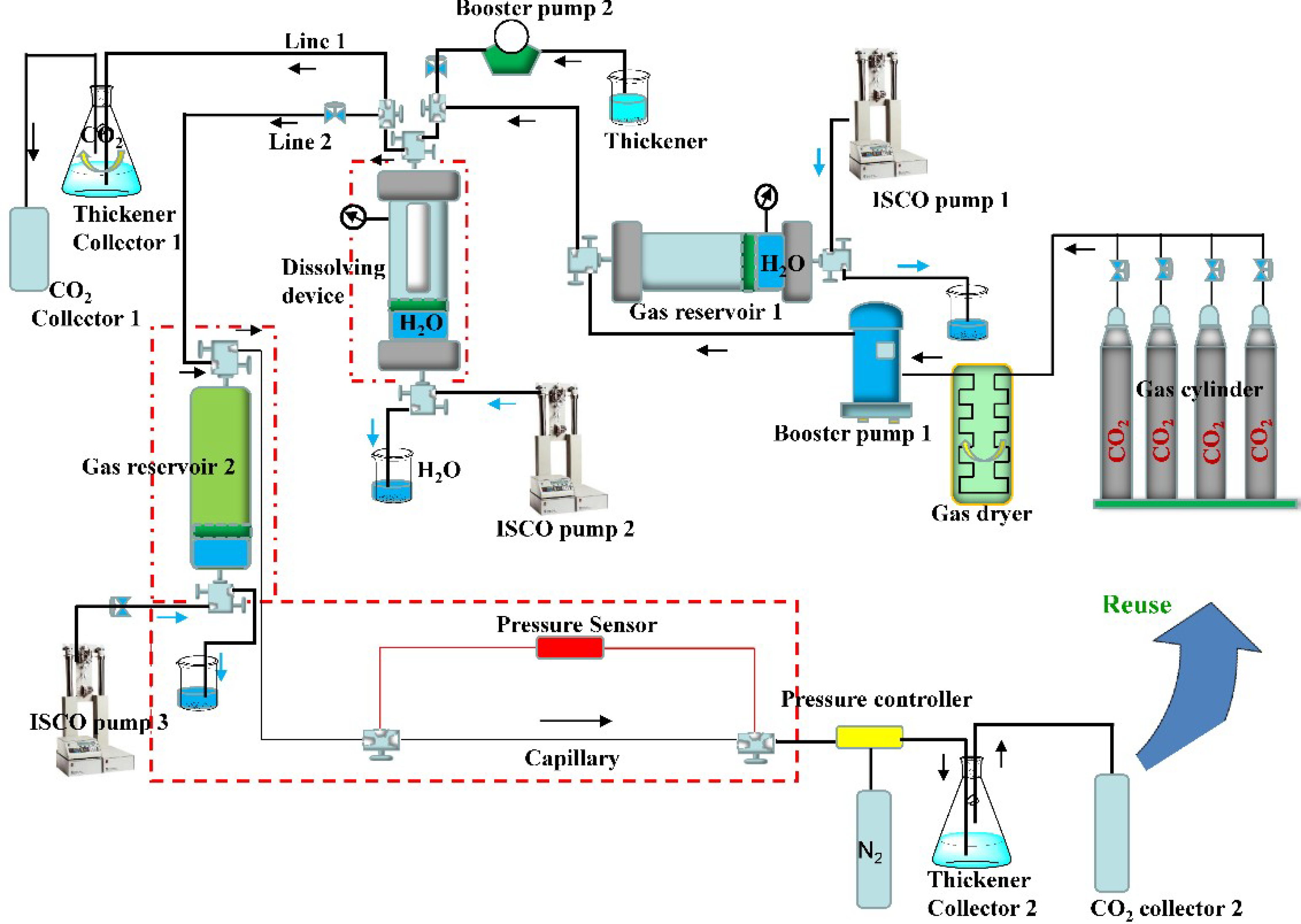
2.4. Measurement Device and Calculation Method
2.5. Cosolvent Screening
2.6. Phase Behavior for the Thickened Liquid CO2
2.7. Numerical Modeling and CO2 Fracturing Simulation
3. Results and Discussion
3.1. Structural Characterization of EEPDMS
3.2. Solubility and Phase Behavior of EEPDMS in Organic Solvents
3.3. Solubility and Phase Behavior of EEPDMS in Liquid CO2
3.4. Apparent Viscosity and Rheology of the Thickened Liquid CO2
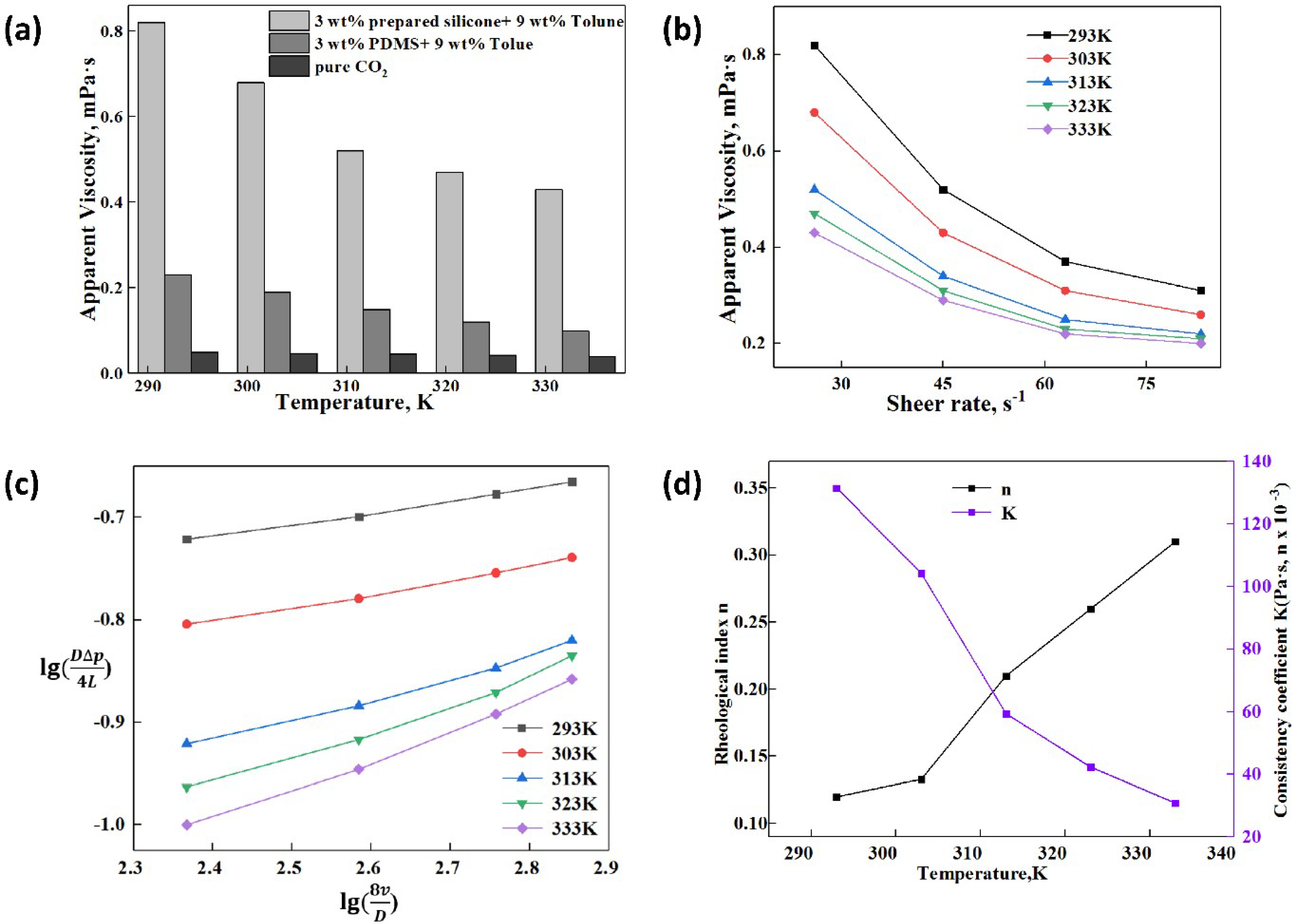
3.4.1. Effect of Temperature on the Apparent Viscosity and Rheology
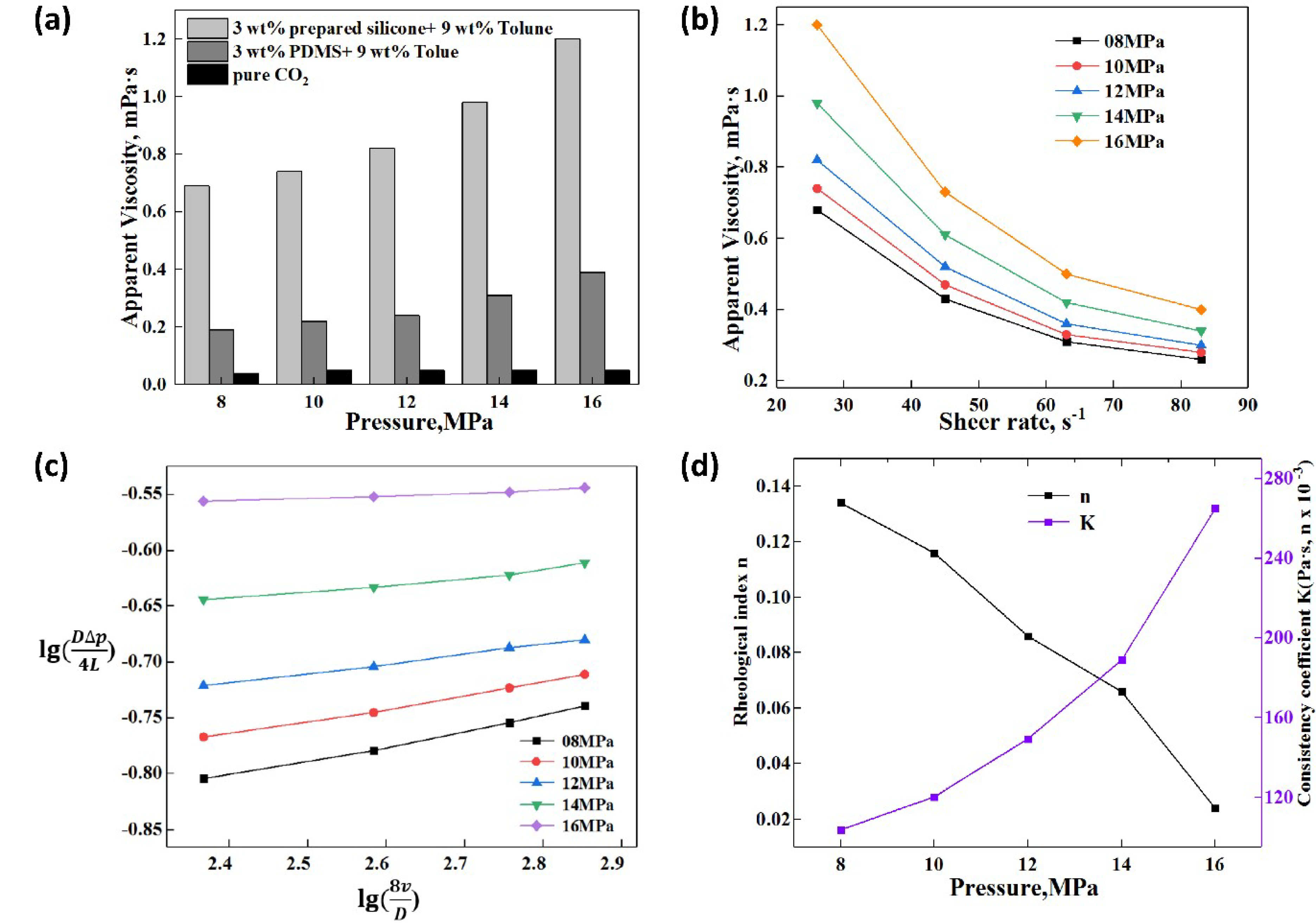
3.4.2. Effect of Pressure on the Apparent Viscosity and Rheology
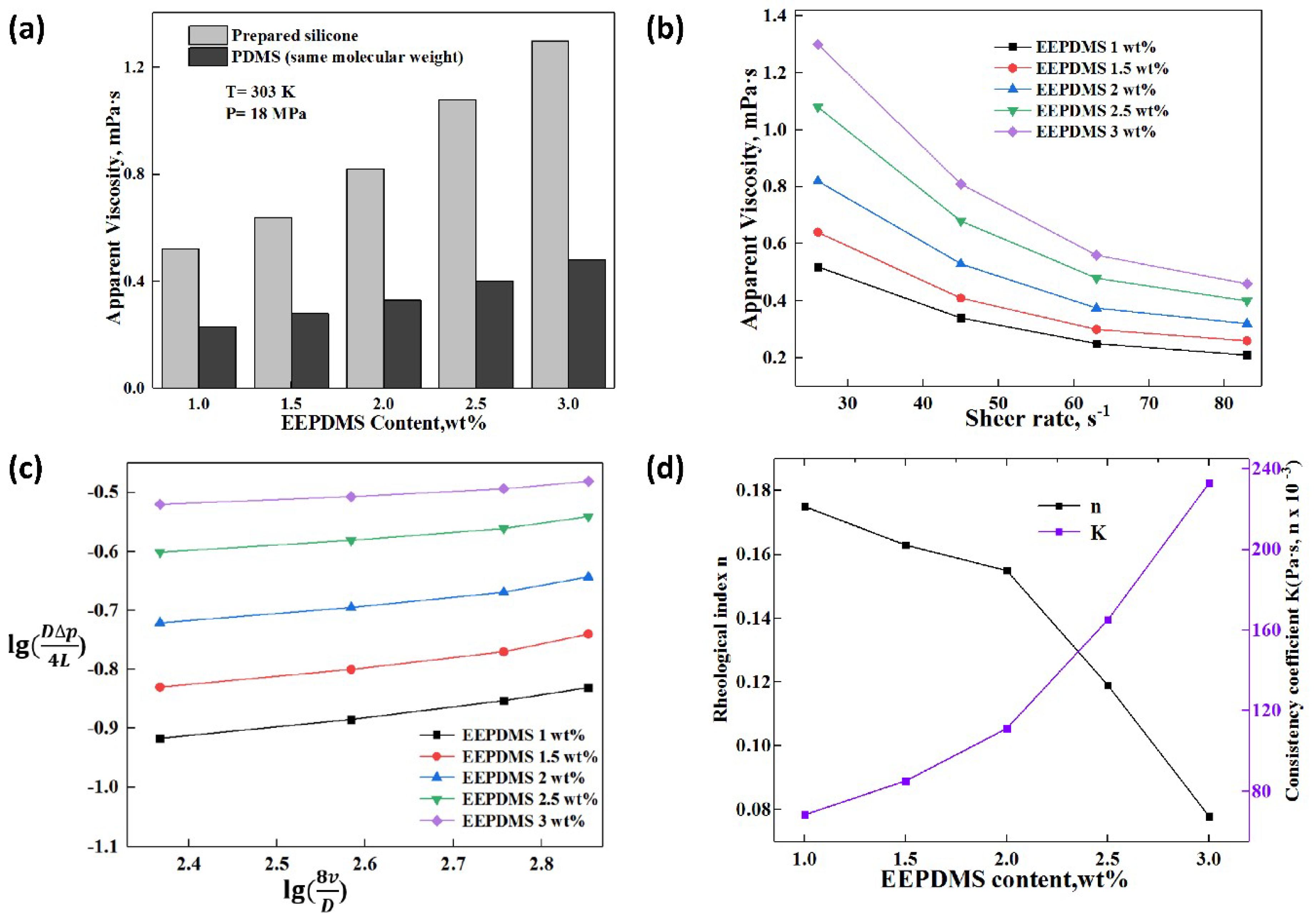
3.4.3. Effect of Thickener Content on the Apparent Viscosity and Rheology
3.4.4. Effect of Shear Rate on the Apparent Viscosity and Rheology
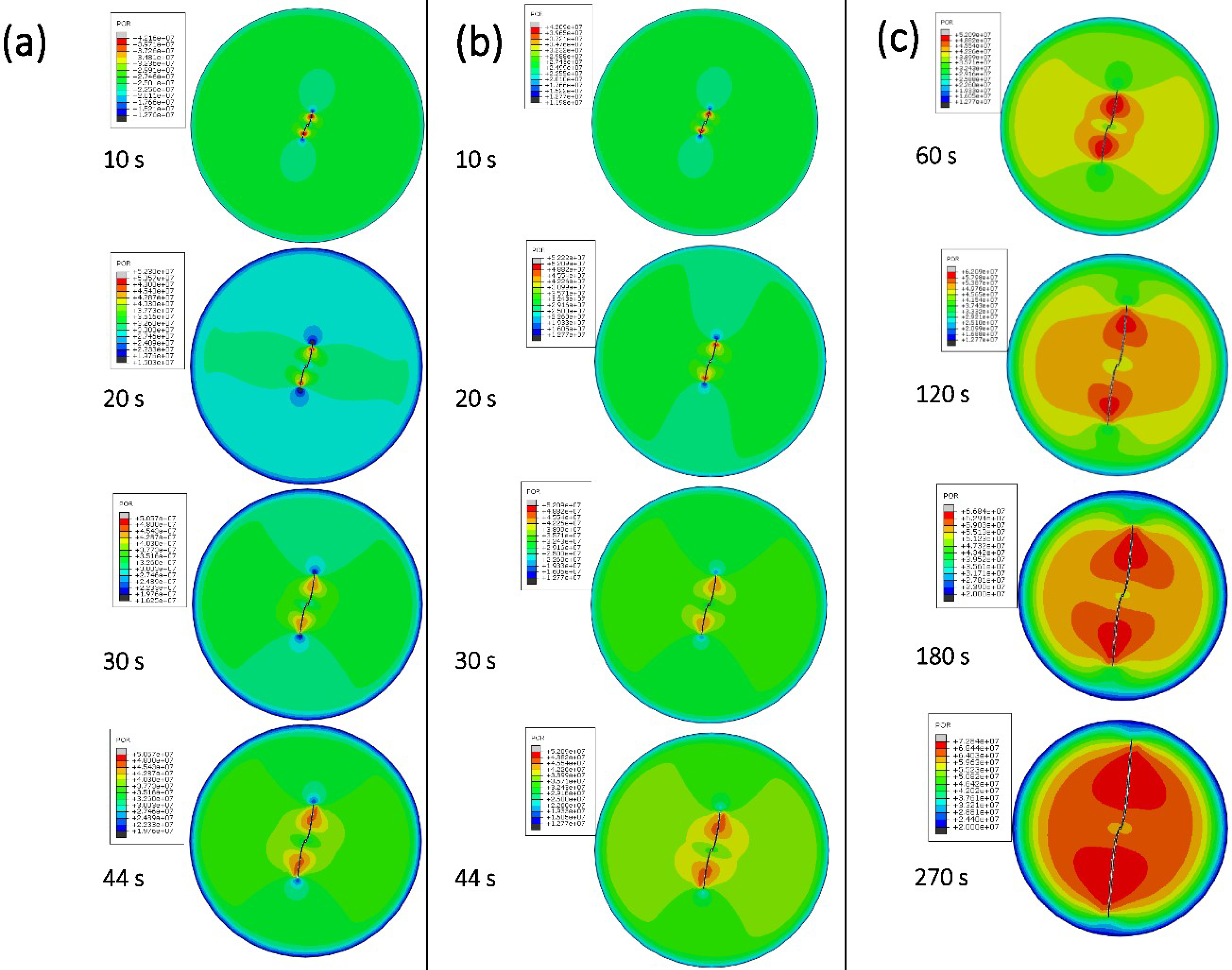
3.5. Numerical Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| σh | Minimum horizontal principal stress |
| σv | Minimum vertical principal stress |
| Pf | Injection pressure of CO2 fracturing fluid |
References
- Lv, Q.; Li, Z.; Li, B.; Zhang, C.; Shi, D.; Zheng, C.; Zhou, T. Experimental study on the dynamic filtration control performance of N2/liquid CO2 foam in porous media. Fuel 2017, 202, 435–445. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Wang, X.; Yu, H.; Li, Q.; Wang, F.; Bai, H.; Kobina, F. An Application of Thickener to Increase Viscosity of Liquid CO2 and the Assessment of the Reservoir Geological Damage and CO2 Utilization. Energy Source Part A 2019, 41, 368–377. [Google Scholar]
- Ayatollahi, S.; Hemmati-Sarapardeh, A.; Roham, M.; Hajirezaie, S. A rigorous approach for determining interfacial tension and minimum miscibility pressure in paraffin-CO2 systems: Application to gas injection processes. J. Taiwan. Inst. Chem. Eng. 2016, 63, 107–115. [Google Scholar] [CrossRef]
- Rassenfoss, S. More carbon dioxide means more oil. J. Petrol. Sci. Eng. 2014, 66, 38–50. [Google Scholar] [CrossRef]
- Zhao, F.; Hao, H.; Hou, J.; Hou, L.; Song, Z. CO2 mobility control and sweep efficiency improvement using starch gel or ethylenediamine in ultra-low permeability oil layers with different types of heterogeneity. J. Petrol. Sci. Eng. 2015, 133, 52–65. [Google Scholar] [CrossRef]
- King, S.R. Liquid CO2 for the stimulation of low permeability reservoirs. In Proceedings of the 1983 SPE/DOE Symposium on Low Permeability, Denver, CO, USA, 14–16 March 1983; pp. 14–16. [Google Scholar]
- Bahrami, H.; Rezaee, R.; Nazhat, D.; Ostojic, J. Evaluation of damage mechanisms and skin factor in tight gas reservoirs. APPEA J. 2011, 51, 639–652. [Google Scholar]
- Cheng, Y. Impact of water dynamics in fractures on the performance of hydraulically fractured wells in gas shale reservoirs. In Proceedings of the SPE International Symposium and Exhibition on Formation Damage Control, Lafayette, LA, USA, 10–12 February 2010. [Google Scholar]
- Luo, X.; Wang, S.; Wang, Z.; Jing, Z.; Lv, M.; Zhai, Z.; Han, T. Experimental investigation on rheological properties and friction performance of thickened CO2 fracturing fluid. J. Petrol. Sci. Eng. 2015, 133, 410–420. [Google Scholar] [CrossRef]
- Doherty, M.D.; Lee, J.J.; Dhuwe, A.; O’Brien, M.J.; Perry, R.J.; Beckman, E.J.; Enick, R.M. Small molecule cyclic amide and urea based thickeners for organic and sc-CO2/organic solutions. Energy Fuels 2016, 30, 5601–5610. [Google Scholar] [CrossRef]
- Cummings, S.; Xing, D.; Enick, R.; Rogers, S.; Heenan, R.; Grillo, I.; Eastoe, Z. Design principles for supercritical CO2 viscosifiers. Soft Matter 2012, 8, 7044–7055. [Google Scholar] [CrossRef]
- Triolo, A.; Celso, F.L.; Triolo, F.; Amenitsch, H.; Steinhart, M. Kinetics of block-copolymer aggregation in super critical CO2. J. Non-Cryst. Solids 2002, 307, 725–730. [Google Scholar] [CrossRef]
- Baysal, C.; Erman, B.; Chu, B. Conformational features of poly (1, 1-dihydroperfluorooctyl acrylate) and poly (vinyl acetate) diblock oligomers in supercritical carbon dioxide. J. Chem. Phys. 2001, 114, 5444–5449. [Google Scholar] [CrossRef]
- Bae, J.H.; Irani, C.A. A laboratory investigation of viscosified CO2 process. SPE Adv. Technol. Ser. 1993, 1, 166–171. [Google Scholar] [CrossRef]
- Williams, L.L.; Rubin, J.B.; Edwards, H.W. Calculation of Hansen solubility parameter values for a range of pressure and temperature conditions, including the supercritical fluid region. Ind. Eng. Chem. Res. 2004, 43, 4967–4972. [Google Scholar] [CrossRef]
- Wang, Y.; Hong, L.; Tapriyal, D.; Kim, I.C.; Paik, I.; Crosthwaite, J.M.; Hamilton, A.D.; Thies, M.C.; Beckenman, E.J.; Enick, R.M.; et al. Design and evaluation of nonfluorous CO2-soluble oligomers and polymers. J. Phys. Chem. B 2009, 113, 14971–14980. [Google Scholar] [CrossRef]
- Kilic, S.; Michalik, S.; Wang, Y.; Johnson, J.K.; Enick, R.M.; Beckenman, E.J. Effect of grafted Lewis base groups on the phase behavior of model poly (dimethyl siloxanes) in CO2. Ind. Eng. Chem. Res. 2003, 42, 6415–6424. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.; Li, Q.; Foster, G.; Lei, C. Study on the optimization of silicone copolymer synthesis and the evaluation of its thickening performance. RSC Adv. 2018, 8, 8770–8778. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Perry, R.J.; Doherty, M.D.; Lee, J.J.; Dhuwe, A.; Beckman, E.J.; Enick, R.M. Anthraquinone siloxanes as thickening agents for supercritical CO2. Energy Fuels 2016, 30, 5990–5998. [Google Scholar] [CrossRef]
- Sun, B.; Sun, W. Research progress and prospectives of supercritical CO2 thickening technology. J. China Univ. Petroleum (Ed. Nat. Sci.) 2015, 39, 76–83. [Google Scholar]
- Sarbu, T.; Styranec, T.J.; Beckman, E.J. Design and synthesis of low cost, sustainable CO2-philes. Ind. Eng. Chem. Res. 2000, 39, 4678–4683. [Google Scholar] [CrossRef]
- Crivello, J.V.; Lee, J.L. The synthesis, characterization, and photoinitiated cationic polymerization of silicon-containing epoxy resins. J. Polym. Sci. Polym. Chem. 1990, 28, 479–503. [Google Scholar] [CrossRef]
- Morita, Y.; Tajima, S.; Suzuki, H.; Sugino, H. Thermally initiated cationic polymerization and properties of epoxy siloxane. J. Appl. Polym. Sci. 2006, 100, 2010–2019. [Google Scholar] [CrossRef]
- Grunlan, M.A.; Lee, N.S.; Cai, G.; Gädda, T.; Mabry, J.M.; Mansfeld, F.; Kus, E.; Wendt, D.E.; Kowalke, G.L.; Finlay, J.A.; et al. Synthesis of α, ω-Bis Epoxy Oligo (1 ‘H, 1 ‘H, 2 ‘H, 2 ‘H-Perfluoroalkyl Siloxane) s and Properties of Their Photo-Acid Cross-Linked Films. Chem. Mater 2004, 16, 2433–2441. [Google Scholar] [CrossRef]
- Cui, L.Z. Synthesis and Analysis of Hydrogen-Terminated Polydimethylsiloxane; Zhejiang University: Hangzhou, China, 2012. [Google Scholar]
- Lewis, L.N.; Lewis, N. Platinum-catalyzed hydrosilylation-colloid formation as the essential step. J. Am. Chem. Soc. 1986, 108, 7228–7231. [Google Scholar] [CrossRef]
- Barry, A.J. Viscometric investigation of dimethylsiloxane polymers. J. Appl. Phys. 1946, 17, 1020–1024. [Google Scholar] [CrossRef]
- Kuo, A.C.M. Poly (dimethylsiloxane). In Polymer Data Handbook; Oxford University Press: Oxfordshire, UK, 1999; pp. 411–435. [Google Scholar]
- Craft, N.E.; Soares, J.H. Relative solubility, stability, and absorptivity of lutein and. beta-carotene in organic solvents. J. Agric. Food. Chem. 1992, 40, 431–434. [Google Scholar] [CrossRef]
- Fink, R.; Beckman, E.J. Phase behavior of siloxane-based amphiphiles in supercritical carbon dioxide. J. Supercrit. Fluid 2000, 18, 101–110. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, Y.; Li, Q.; Ansari, U.; Liu, Y.; Yan, C.; Lei, C. Development and verification of the comprehensive model for physical properties of hydrate sediment. Arab. J. Geosci. 2018, 11, 325. [Google Scholar] [CrossRef]
- Zou, J.; Chen, W.; Jiao, Y.Y. Numerical simulation of hydraulic fracture initialization and deflection in anisotropic unconventional gas reservoirs using XFEM. J. Nat. Gas Sci. Eng. 2018, 55, 466–475. [Google Scholar] [CrossRef]
- Gnanasambandam, R.; Proctor, A. Determination of pectin degree of esterification by diffuse reflectance Fourier transform infrared spectroscopy. Food Chem. 2000, 68, 327–332. [Google Scholar] [CrossRef]
- Yang, H.; Nguyen, Q.T.; Ding, Y.D.; Long, Y.C.; Ping, Z. Investigation of poly (dimethyl siloxane) (PDMS)–solvent interactions by DSC. J. Membrane Sci. 2000, 164, 37–43. [Google Scholar] [CrossRef]
- Wu, C.; Li, J.; Dong, H.; Jiang, J. Solubility of 1, 3-dimethyl-1, 1, 3, 3-tetraphenyldisiloxane in different solvents from (288.15 to 313.15) K. Thermochim. Acta 2012, 543, 24–31. [Google Scholar] [CrossRef]
- Lee, B.C.; Danner, R.P. Prediction of polymer-solvent phase equilibria by a modified group-contribution EOS. AIChE J. 1996, 42, 837–849. [Google Scholar] [CrossRef]
- Bueche, A.M. Interaction of polydimethylsiloxanes with swelling agents. J. Polym. Sci. 1955, 15, 97–103. [Google Scholar] [CrossRef]
- Bae, J.H. Viscosified CO2 Process: Chemical Transport and Other Issues. In Proceedings of the SPE International Symposium on Oilfield Chemistry, San Antonio, TX, USA, 14–17 February 1995. [Google Scholar]
- Raveendran, P.; Wallen, S.L. Cooperative C− H··· O Hydrogen Bonding in CO2− Lewis Base Complexes: Implications for Solvation in Supercritical CO2. J. Am. Chem. Soc. 2002, 124, 12590–12599. [Google Scholar] [CrossRef]
- Tsukahara, T.; Kayaki, Y.; Ikariya, T.; Ikeda, Y. 13C NMR spectroscopic evaluation of the affinity of carbonyl compounds for carbon dioxide under supercritical conditions. Angew. Chem. Int. Edit. 2004, 43, 3719–3722. [Google Scholar] [CrossRef] [PubMed]
- Chandrika, B.; Schnackenberg, L.K.; Raveendran, P.; Raveendran, P.; Wallen, S.L. High resolution 1H NMR structural studies of sucrose octaacetate in supercritical carbon dioxide. Chem. Eur. J. 2005, 11, 6266–6271. [Google Scholar] [CrossRef] [PubMed]
- Pozrikidis, C. Numerical investigation of the effect of surfactants on the stability and rheology of emulsions and foam. J. Eng. Math. 2001, 41, 237–258. [Google Scholar] [CrossRef]
- Shen, Z.; Zhao, Z.; Kang, W. Colloid and Surface Chemistry; Chemical Industry Press: Beijing, China, 2012. [Google Scholar]
- Liu, L.; Li, L.; Elsworth, D.; Zhi, S.; Yu, Y. The Impact of Oriented Perforations on Fracture Propagation and Complexity in Hydraulic Fracturing. Processes 2018, 6, 213. [Google Scholar] [CrossRef]
- Chen, D.; Yao, Y.; Fu, G.; Meng, H.; Xie, S. A new model for predicting liquid loading in deviated gas wells. J. Nat. Gas. Sci. Eng. 2016, 34, 178–184. [Google Scholar] [CrossRef]
- Detournay, E. Propagation regimes of fluid-driven fractures in impermeable rocks. Int. J. Geomech. 2004, 4, 35–45. [Google Scholar] [CrossRef]
- Zhang, X.; Jeffrey, R.G.; Thiercelin, M. Deflection and propagation of fluid-driven fractures at frictional bedding interfaces: A numerical investigation. J. Struct. Geol. 2007, 29, 396–410. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, W.; Wei, C.; Elsworth, D.; Wang, J. Microcrack-based geomechanical modeling of rock-gas interaction during supercritical CO2 fracturing. J. Petrol. Sci. Eng. 2018, 164, 91–102. [Google Scholar] [CrossRef]


| Parameter | Value | Parameter | Value |
|---|---|---|---|
| Elastic Modulus, E/GPa | 20 | Poisson’s ratio, ν | 0.26 |
| Minimum horizontal principal stress, σh/MPa | 31 | Maximum horizontal principal stress, σH/MPa | 37 |
| Tensile strength, C/MPa | 3.5 | Initial pore pressure, Pip/MPa | 18 |
| Initial porosity, ϕ/% | 8 | Permeability, K/m2 | 4 × 10−17 |
| Injection rate, Q/(m3/min) | 4 | Total fracturing time, T/min | 10 |
| Leak-off coefficient | 1 × 10−14 | Viscosity, mPa·s | 0.04 and 1.3 |
| Solvent | EEPDMS Content (wt.%) | Solution State | 283 K | 293 K | 303 K | 313 K | 323 K |
|---|---|---|---|---|---|---|---|
| Toluene | 1 | Clear, Viscous liquid | M | M | M | S | S |
| Hexane | 1 | Clear, Viscous liquid | M | M | M | S | S |
| Pentane | 1 | Clear, Viscous liquid | M | S | S | S | S |
| Nonanol | 1 | Turbid solution | I | I | I | I | S |
| Methanol | 1 | Turbid solution | I | I | I | I | I |
| Ethanol | 1 | Turbid solution | I | I | I | I | I |
| 1,2-Propylene glycol | 1 | Turbid solution | I | I | I | I | I |
| Toluene | 2 | Clear, Viscous liquid | M | M | M | S | S |
| Hexane | 2 | Clear, Viscous liquid | M | M | M | S | S |
| Pentane | 2 | Clear, Viscous liquid | M | S | S | S | S |
| Nonanol | 2 | Turbid solution | I | I | I | I | I |
| Methanol | 2 | Turbid solution | I | I | I | I | I |
| Ethanol | 2 | Turbid solution | I | I | I | I | I |
| 1,2-Propylene glycol | 2 | Turbid solution | I | I | I | I | I |
| Toluene | 4 | Clear, Viscous liquid | M | M | M | M | S |
| Hexane | 4 | Clear, Viscous liquid | M | M | M | M | S |
| Pentane | 4 | Clear, Viscous liquid | M | M | S | S | S |
| Nonanol | 4 | Turbid solution | I | I | I | I | I |
| Methanol | 4 | Turbid solution | I | I | I | I | I |
| Ethanol | 4 | Turbid solution | I | I | I | I | I |
| 1,2-Propylene glycol | 4 | Turbid solution | I | I | I | I | I |
| Fluid type | Viscosity (mPa s) | Injection Rate (m3/min) | Thickener Content (wt.%) | Cosolvent Content (wt.%) |
|---|---|---|---|---|
| Pure liquid CO2 | 0.04 | 4 | 0 | 0 |
| Thickened liquid CO2 | 1.30 | 4 | 3 | 9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Wang, Y.; Wang, F.; Li, Q.; Kobina, F.; Bai, H.; Yuan, L. Effect of a Modified Silicone as a Thickener on Rheology of Liquid CO2 and Its Fracturing Capacity. Polymers 2019, 11, 540. https://doi.org/10.3390/polym11030540
Li Q, Wang Y, Wang F, Li Q, Kobina F, Bai H, Yuan L. Effect of a Modified Silicone as a Thickener on Rheology of Liquid CO2 and Its Fracturing Capacity. Polymers. 2019; 11(3):540. https://doi.org/10.3390/polym11030540
Chicago/Turabian StyleLi, Qiang, Yanling Wang, Fuling Wang, Qingchao Li, Forson Kobina, Hao Bai, and Lin Yuan. 2019. "Effect of a Modified Silicone as a Thickener on Rheology of Liquid CO2 and Its Fracturing Capacity" Polymers 11, no. 3: 540. https://doi.org/10.3390/polym11030540
APA StyleLi, Q., Wang, Y., Wang, F., Li, Q., Kobina, F., Bai, H., & Yuan, L. (2019). Effect of a Modified Silicone as a Thickener on Rheology of Liquid CO2 and Its Fracturing Capacity. Polymers, 11(3), 540. https://doi.org/10.3390/polym11030540





