Synthesis and Properties of Side-Chain Functionalized Polytetrahydrofuran Derivatives via the Blue-Light Photocatalytic Thiol-Ene Reaction
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Unsaturated Polytetrahydrofuran (UPTHF)
2.3. Synthesis of Side-Chain Functionalized PTHFs
2.3.1. Synthesis of PTHFs with a Hydroxy Branching Unit (PTHFOH)
2.3.2. Synthesis of PTHFs with a Carboxyl Branching Unit (PTHFbut)
2.3.3. Synthesis of PTHF Derivative with a Carboxyl Branching Unit (PTHFacid)
2.3.4. Synthesis of PTHF Derivative with a mPEG Branching Unit (PTHFmPEG)
2.4. Measurements
3. Results and Discussion
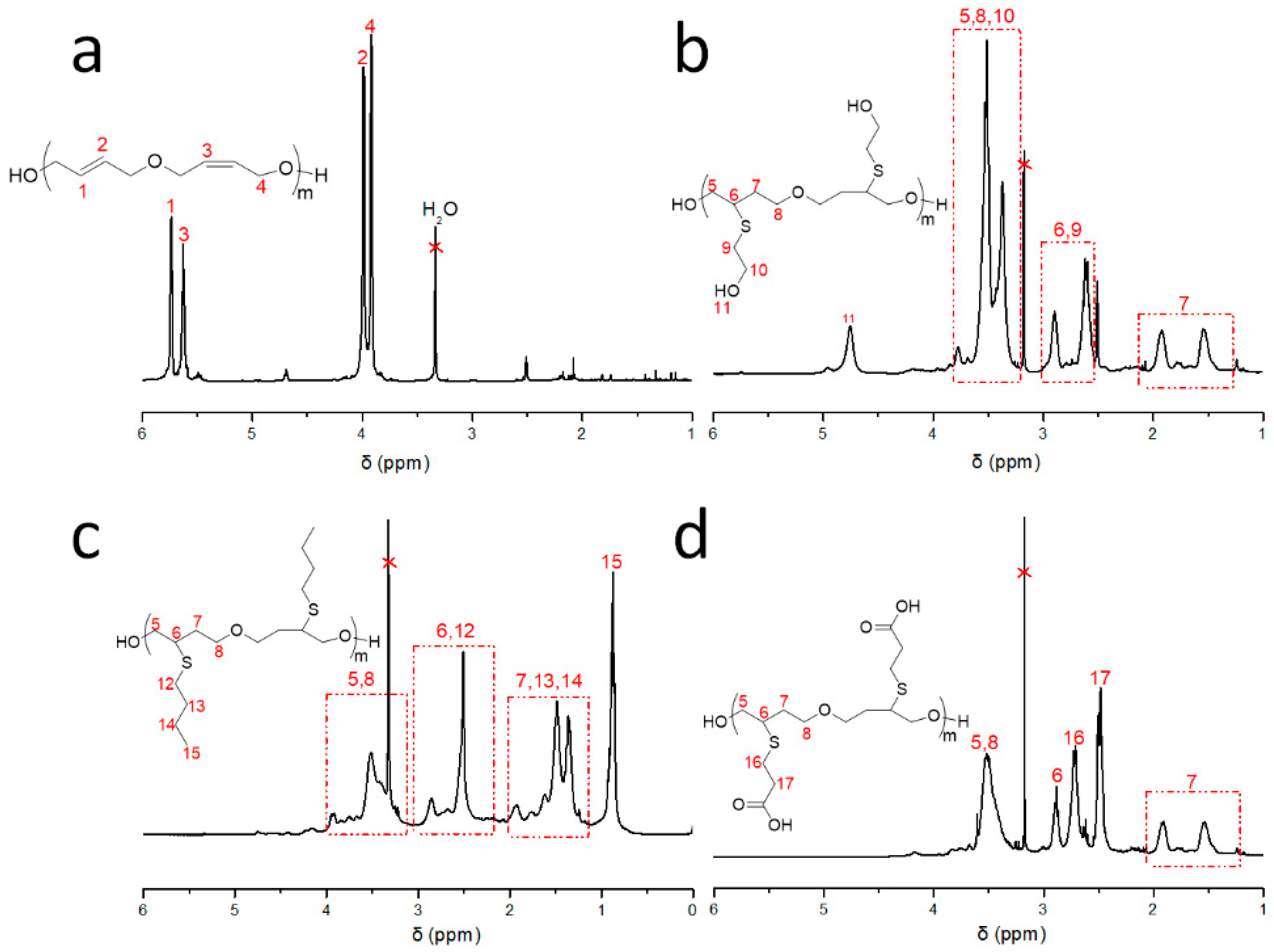
3.1. Synthesis of UPTHF
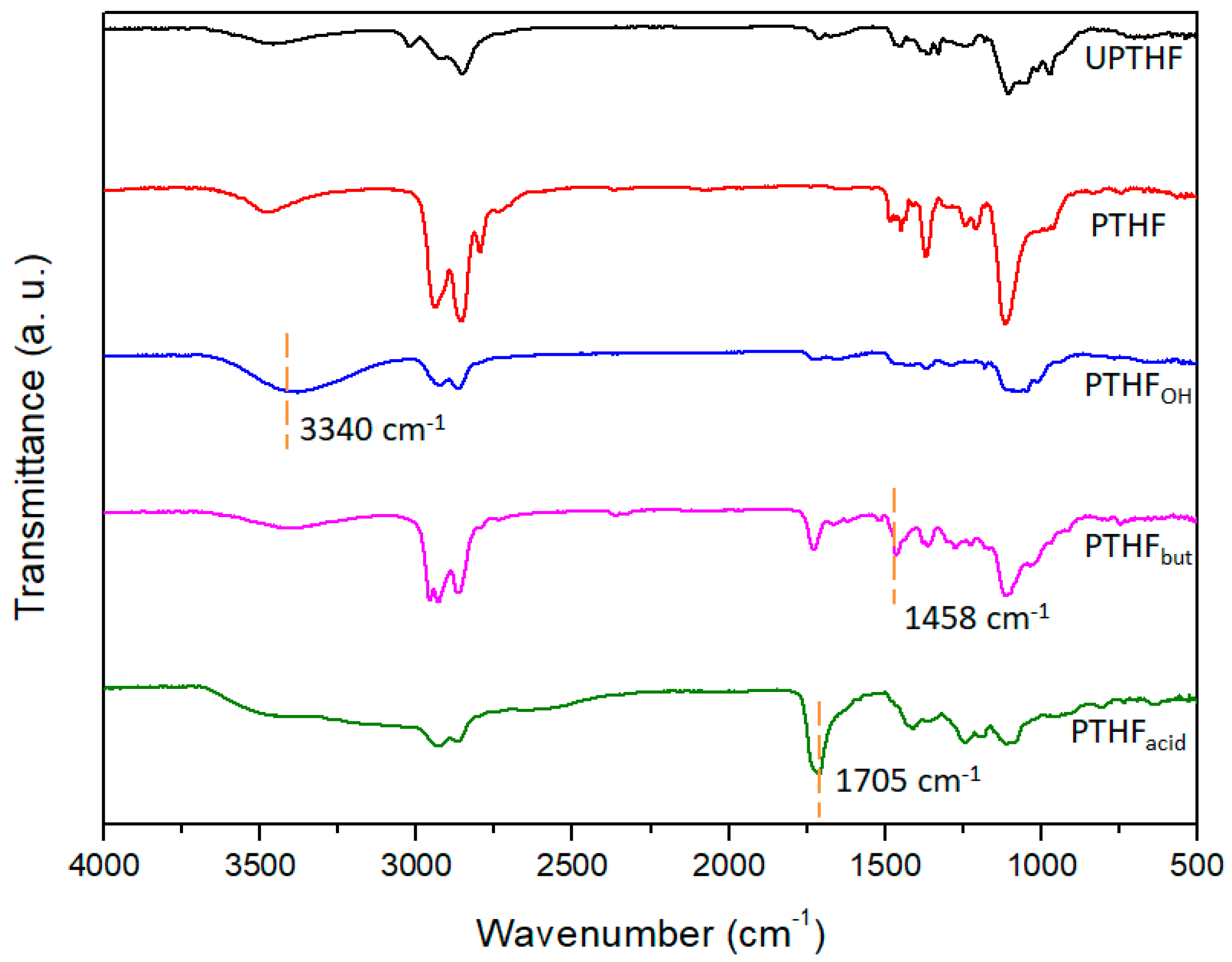
3.2. Synthesis of the Side-Chain Functionalized PTHFs
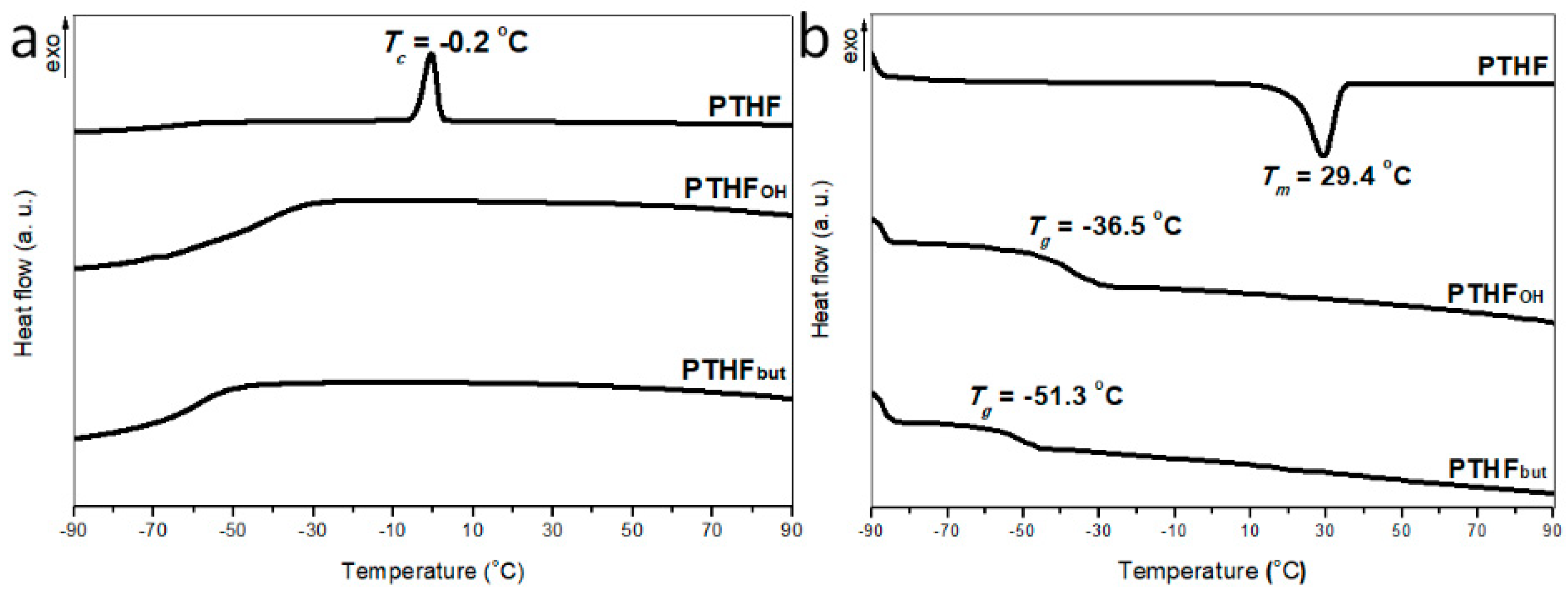
3.3. Thermal Properties of PTHFOH and PTHFbut
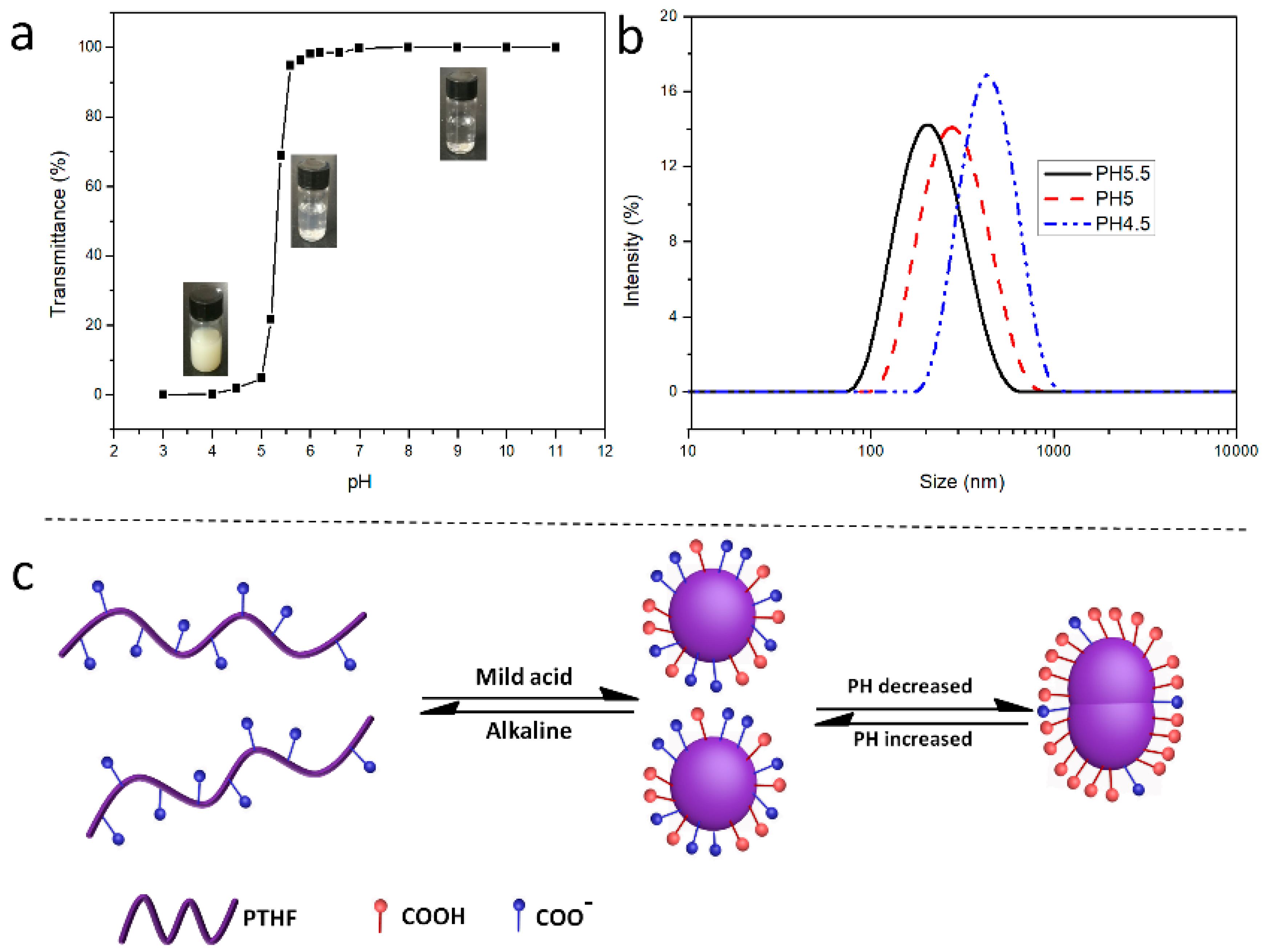
3.4. pH-Responsive Behavior of PTHFacid
3.5. Backbone-Thermoresponsive Behavior of PTHFmPEG
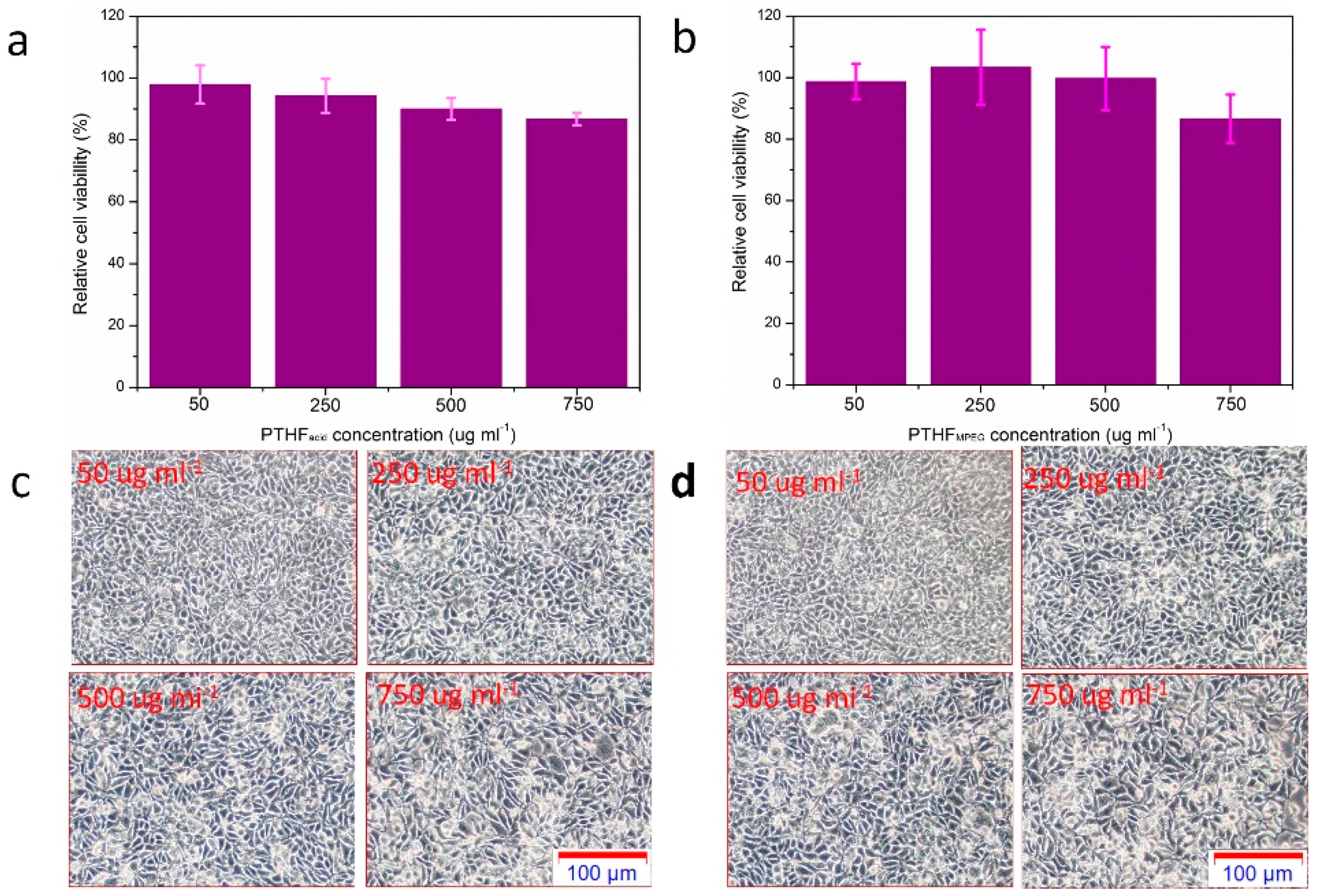
3.6. In Vitro Cytotoxicity of PTHFacid and PTHFmPEG
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jiang, T.; Kai, D.; Liu, J.; Huang, Y.; Heng, J.; Zhao, M.; Chen, B.Q.Y.; Loh, X.J.; Zhu, Y.; Mao, C.; et al. Mechan-ically cartilage-mimicking poly(PCL-PTHF urethane)/collagennanofibers induce chondrogenesis by blocking NFe-kappa B signaling pathway. Biomaterials 2018, 178, 281–292. [Google Scholar] [CrossRef]
- Bai, Y.; Xie, F.Y.; Tian, W. Controlled Self-assembly of Thermo-responsive Amphiphilic H-shaped Polymer for Adjustable Drug Release. Chin. J. Polym. Sci. 2018, 36, 406–416. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Lin, Y.; Fang, X.; Xu, Y.; Ruan, Y.; Weng, W. Spiropyran as a Mechanochromic Probe in Dual Cross-Linked Elastomers. Macromolecules 2014, 47, 6783–6790. [Google Scholar] [CrossRef]
- Balko, J.; Fernandez-d’Arlas, B.; Poselt, E.; Dabbous, R.; Muller, A.J.; Thurn-Albrecht, T. Clarifying the Origin of Multiple Melting of Segmented Thermoplastic Polyurethanes by Fast Scanning Calorimetry. Macromolecules 2017, 50, 7672–7680. [Google Scholar] [CrossRef]
- Sagara, Y.; Karman, M.; Verde-Sesto, E.; Matsuo, E.; Kim, Y.; Tamaoki, N.; Weder, C. Rotaxanes as Mechanochromic Fluorescent Force Transducers in Polymers. J. Am. Chem. Soc. 2018, 140, 1584–1587. [Google Scholar] [CrossRef] [Green Version]
- Zeitsch, K.J. The Chemistry and Technology of Furfural and its Many By-products; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Malkappa, K.; Jana, T. Hydrophobic, Water-Dispersible Polyurethane: Role of Polybutadiene Diol Structure. Ind. Eng. Chem. Res. 2015, 54, 7423–7435. [Google Scholar] [CrossRef]
- Dubreuil, M.F.; Farcy, N.C.; Goethals, E.J. Influence of the alkyl group of triflate esters on their initiation ability for the cationic ring-opening polymerization of tetrahydrofuran. Macromol. Rapid Commun. 1999, 20, 383–386. [Google Scholar] [CrossRef]
- Konig, N.F.; Ouahabi, A.A.; Poyer, S.; Charles, L.; Lutz, J.F. A Simple Post-Polymerization Modification Method for Controlling Side-Chain Information in Digital Polymers. Angew. Chem. Int. Ed. 2017, 56, 7297–7301. [Google Scholar] [CrossRef] [PubMed]
- Oakdale, J.S.; Kwisnek, L.; Fokin, V.V. Selective and Orthogonal Post-Polymerization Modifi cation using Sulfur(VI) Fluoride Exchange (SuFEx) and Copper-Catalyzed Azide-Alkyne Cycloaddition (CuAAC) Reactions. Macromolecules 2016, 49, 4473–4479. [Google Scholar] [CrossRef]
- Kubo, T.; Swartz, J.L.; Scheutz, G.M.; Sumerlin, B.S. Synthesis of Multifunctional Homopolymers through Using Thiazolidine Chemistry and Post-Polymerization Modification. Macromol. Rapid Commun. 2018, 1800590. [Google Scholar] [CrossRef] [PubMed]
- Hyvl, J.; Autenrieth, B.; Schrock, R.R. Proof of Tacticity of Stereoregular ROMP Polymers through Post Polymerization Modification. Macromolecules 2015, 48, 3148–3152. [Google Scholar] [CrossRef]
- Xiao, Z.; Bennett, C.W.; Connal, L.A. Facile and Versatile Platform for the Preparation of Functional Polyethylenes via Thiol-Ene Chemistry. J. Polym. Sci. Part A: Polym. Chem. 2015, 53, 1957–1960. [Google Scholar] [CrossRef]
- Yokozawa, T.; Suzuki, Y.; Hiraoka, S. Aromatic Polyethers with Low Polydispersities from Chain-Growth Polycondensation. J. Am. Chem. Soc. 2001, 123, 9902–9903. [Google Scholar] [CrossRef] [PubMed]
- Yokozawa, T.; Yokoyama, A. Chain-Growth Condensation Polymerization for the Synthesis of Well-Defined Condensation Polymers and π-Conjugated Polymers. Chem. Rev. 2009, 109, 5595–5619. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, L.; Du, X.; Feng, W.; Welle, A.; Trapp, O.; Grunze, M.; Hirtz, M.; Levkin, P.A. Reactive Superhydrophobic Surface and Its Photoinduced Disulfideene and Thiol-ene (Bio) functionalization. Nano Lett. 2015, 15, 675–681. [Google Scholar] [CrossRef]
- Tucker-Schwartz, A.K.; Farrell, R.A.; Garrell, R.L. Thiol-ene Click Reaction as a General Route to Functional Trialkoxysilanes for Surface Coating Applications. J. Am. Chem. Soc. 2011, 133, 11026–11029. [Google Scholar] [CrossRef]
- Li, L.; Li, S.; Cui, D. Highly Cis-1,4-Selective Living Polymerization of 3-Methylenehepta-1,6-diene and Its Subsequent Thiol-Ene Reaction: An Efficient Approach to Functionalized Diene-Based Elastomer. Macromolecules 2016, 49, 1242–1251. [Google Scholar] [CrossRef]
- Kwon, J.S.; Park, H.W.; Kim, D.H.; Kwark, Y. Solvent-Free Processable and Photo-Patternable Hybrid Gate Dielectric for Flexible Top-Gate Organic Field-Effect Transistors. ACS Appl. Mater. Interfaces. 2017, 9, 5366–5374. [Google Scholar] [CrossRef]
- Zuo, Y.; Gou, Z.; Zhang, J.; Feng, S. Color-Transformable Silicone Elastomers Prepared by Thiol-Ene Reaction with Potential Application in UV-LEDs. Macromol. Rapid Commun. 2016, 37, 597–604. [Google Scholar] [CrossRef]
- Zuo, Y.; Gou, Z.; Zhang, C.; Feng, S. Polysiloxane-Based Autonomic Self-Healing Elastomers Obtained through Dynamic Boronic Ester Bonds Prepared by Thiol-Ene “Click” Chemistry. Macromol. Rapid Commun. 2016, 37, 1052–1059. [Google Scholar] [CrossRef]
- Bai, J.; Li, H.; Shi, Z.; Yin, J. An Eco-Friendly Scheme for the Cross-Linked Polybutadiene Elastomer via Thiol-Ene and Diels-Alder Click Chemistry. Macromolecules 2015, 48, 3539–3546. [Google Scholar] [CrossRef]
- Berthold, A.; Sagar, K.; Ndoni, S. Patterned Hydrophilization of Nanoporous 1,2-PB by Thiol-ene Photochemistry. Macromol. Rapid Commun. 2011, 32, 1259–1263. [Google Scholar] [CrossRef] [PubMed]
- Brummelhuis, N.; Diehl, C.; Schlaad, H. Thiol-Ene Modification of 1,2-Polybutadiene Using UV Light or Sunlight. Macromolecules 2008, 41, 9946–9947. [Google Scholar] [CrossRef]
- Wolfberger, A.; Rupp, B.; Kern, W.; Griesser, T.; Slugovc, C. Ring Opening Metathesis Polymerization Derived Polymers as Photoresists: Making Use of Thiol-ene Chemistry. Macromol. Rapid Commun. 2011, 32, 518–522. [Google Scholar] [CrossRef]
- Gauthier, M.A.; Gibson, M.I.; Klok, H.A. Synthesis of Functional Polymers by Post-Polymerization Modification. Angew. Chem. Int. Ed. 2009, 48, 48–58. [Google Scholar] [CrossRef]
- Silverstein, J.S.; Casey, B.J.; Natoli, M.E.; Dair, B.J.; Kofinas, P. Rapid Modular Synthesis and Processing of Thiol-Ene Functionalized Styrene-Butadiene Block Copolymers. Macromolecules 2012, 45, 3161–3167. [Google Scholar] [CrossRef]
- Tyson, E.L.; Niemeyer, L.Z.; Yoon, T.P. Redox Mediators in Visible Light Photocatalysis: Photocatalytic Radical Thiol-Ene Additions. J. Org. Chem. 2014, 79, 1427–1436. [Google Scholar] [CrossRef]
- Tyson, E.L.; Ament, M.S.; Yoon, T.P. Transition Metal Photoredox Catalysis of Radical Thiol-Ene Reac-tions. J. Org. Chem. 2013, 78, 2046–2050. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chung, H. Thiol-Ene Reaction on Natural Lignin. ACS Sustainable Chem. Eng. 2017, 5, 9160–9168. [Google Scholar] [CrossRef]
- Santandrea, J.; Kairouz, V.; Collins, S.K. Flow Science in an Undergraduate Teaching Laboratory: Photocatalytic Thiol-Ene Reaction Using Visible Light. J. Chem. Educ. 2018, 95, 1073–1077. [Google Scholar] [CrossRef]
- Xu, J.; Boyer, C. Visible Light Photocatalytic Thiol-Ene Reaction: An Elegant Approach for Fast Polymer Postfunctionalization and Step-Growth Polymerization. Macromolecules 2015, 48, 520–529. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, G.; Du, L.; Zhang, C.; Li, L.; Zhu, J.; Pei, J. Synthesis of hydroxyl-terminated polybutadiene bearing pendant carboxyl groups by combination of anionic polymerization and blue light photocatalytic thiol-ene reaction and its pH-triggered self-assemble behavior. React. Funct. Polym. 2018, 127, 161–167. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, J.; Chen, H.; Song, Z.; Wu, Y.; Dai, X.; Kong, J. Acid-Cleavable Unimolecular Micelles from Amphiphilic Star Co-polymers for Triggered Release of Anticancer Drugs. Macromol. Biosci. 2017, 17, 1600258. [Google Scholar] [CrossRef]
- Bai, T.; Du, J.; Chen, J.; Duan, X.; Zhuang, Q.; Chen, H.; Kong, J. Reduction-responsive dithiomaleimide-based polymeric micelles for controlled anti-cancer drug delivery and bioimaging. Polym. Chem. 2017, 8, 7160–7168. [Google Scholar] [CrossRef]
- Ilg, A.D.; Price, C.J.; Miller, S.A. Linear Low-Density Polyoxymethylene versus Linear Low-Density Polyethylene. Macromolecules 2007, 40, 7739–7741. [Google Scholar] [CrossRef]
- Jia, Z.; Chen, H.; Zhu, X.; Yan, D. Backbone-Thermoresponsive Hyperbranched Polyethers. J. Am. Chem. Soc. 2006, 128, 8144–8145. [Google Scholar] [CrossRef]
- Fan, W.; Fan, X.; Tian, W.; Zhang, X.; Wang, G.; Zhang, W.; Bai, Y.; Zhu, X. Phase transition dynamics and mechanism for backbone-thermoresponsive hyperbranched polyethers. Polym. Chem. 2014, 5, 4022–4031. [Google Scholar] [CrossRef]
- Cao, G.; Li, G.; Yang, Q.; Liu, Z.; Liu, Z.; Jiang, J. LCST-Type Hyperbranched Poly(oligo(ethylene glycol) with Thermo- and CO2-Responsive Backbone. Macromol. Rapid Commun. 2018, 39, 1700684. [Google Scholar] [CrossRef] [PubMed]




| Sample | Solvent | Temperature (°C) | Catalyst | Reaction Time (h) | Mn (g mol−1) | Mw/Mn |
|---|---|---|---|---|---|---|
| 1 | acetone | reflux | NaH | 48 | 423 | 1.31 |
| 2 | acetone | reflux | KOH | 72 | 4306 | 1.48 |
| 3 | acetone | reflux | KOH | 48 | 1806 | 1.72 |
| 4 | acetone | reflux | K2CO3 | 72 | 279 | 1.18 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, X.; Bai, T.; Wang, Z.; Liu, J.; Min, X.; Wang, T.; Zhang, W.; Fan, X. Synthesis and Properties of Side-Chain Functionalized Polytetrahydrofuran Derivatives via the Blue-Light Photocatalytic Thiol-Ene Reaction. Polymers 2019, 11, 583. https://doi.org/10.3390/polym11040583
Zhu X, Bai T, Wang Z, Liu J, Min X, Wang T, Zhang W, Fan X. Synthesis and Properties of Side-Chain Functionalized Polytetrahydrofuran Derivatives via the Blue-Light Photocatalytic Thiol-Ene Reaction. Polymers. 2019; 11(4):583. https://doi.org/10.3390/polym11040583
Chicago/Turabian StyleZhu, Xiuzhong, Ting Bai, Zichao Wang, Jie Liu, Xin Min, Tong Wang, Wanbin Zhang, and Xiaodong Fan. 2019. "Synthesis and Properties of Side-Chain Functionalized Polytetrahydrofuran Derivatives via the Blue-Light Photocatalytic Thiol-Ene Reaction" Polymers 11, no. 4: 583. https://doi.org/10.3390/polym11040583






