Preparation and Characterization of Magadiite–Magnetite Nanocomposite with Its Sorption Performance Analyses on Removal of Methylene Blue from Aqueous Solutions
Abstract
:1. Introduction
2. Experimental
2.1. Materials Collection
2.2. Preparation of MAG-Fe3O4
2.3. Instruments
2.4. Characterization Study
2.4.1. Batch Adsorption Experiments
2.4.2. Adsorption Kinetics Study
Pseudo-First-Order Kinetic Model
Pseudo-Second-Order Kinetic Model
2.4.3. Adsorption Isotherms
Langmuir Isotherm Model
Freundlich Isotherm Model
3. Results and Discussion
3.1. Characterization and Structure Analysis of MAG–Fe3O4
3.1.1. XRD Analyses
3.1.2. FTIR Analyses
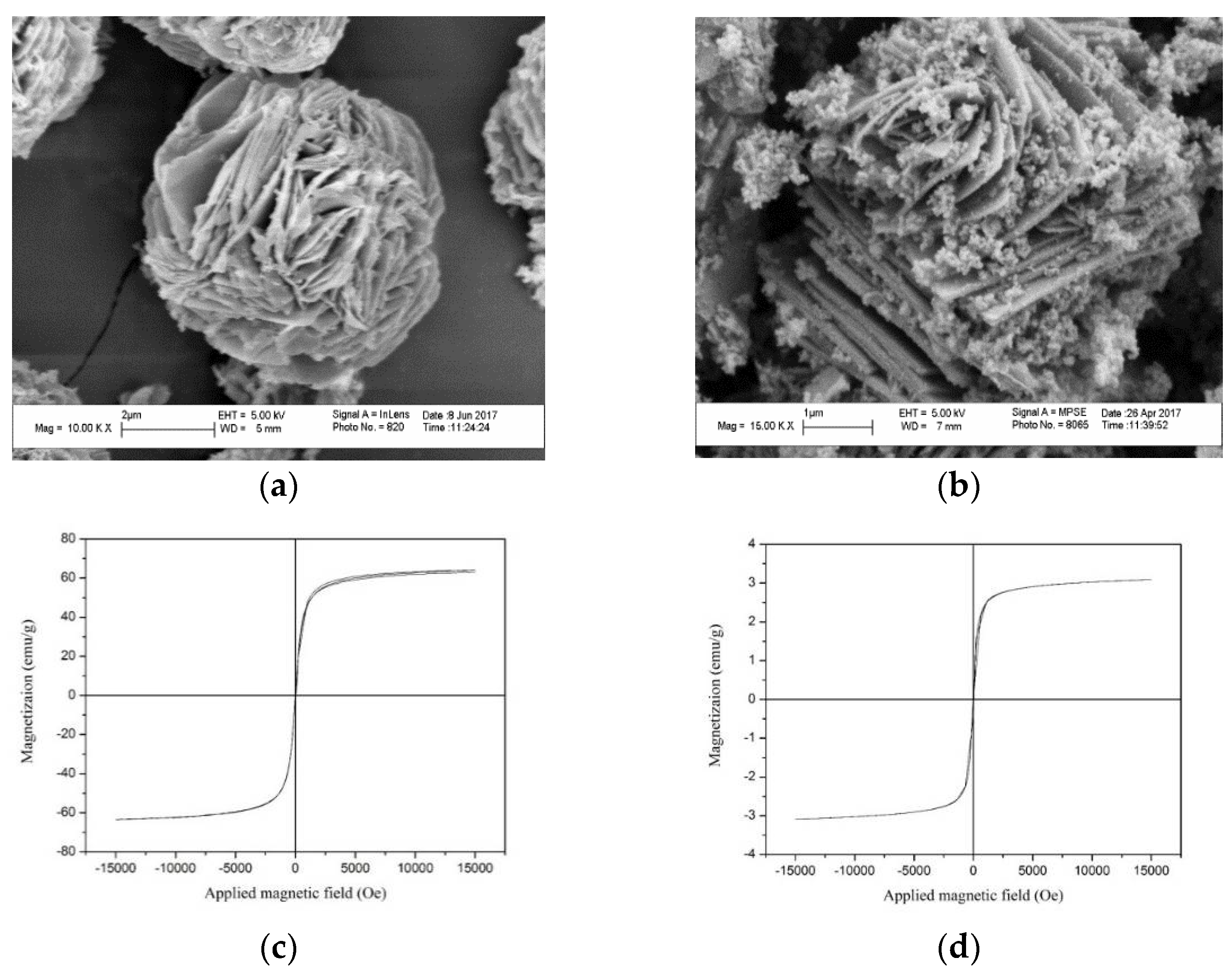
3.1.3. SEM Images Analyses
3.1.4. Magnetism Analyses
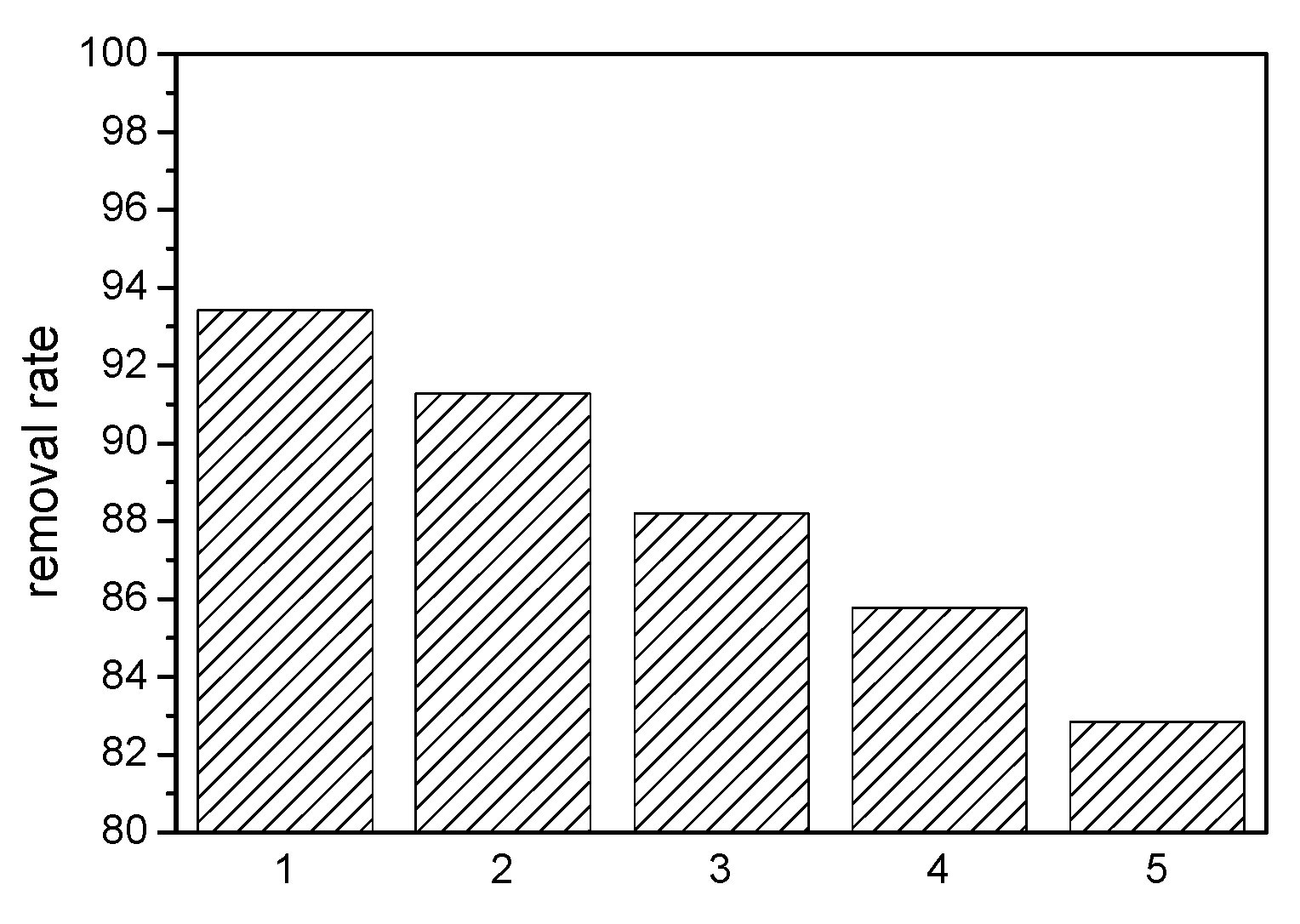
3.2. Adsorption Properties of MB on MAG–Fe3O4
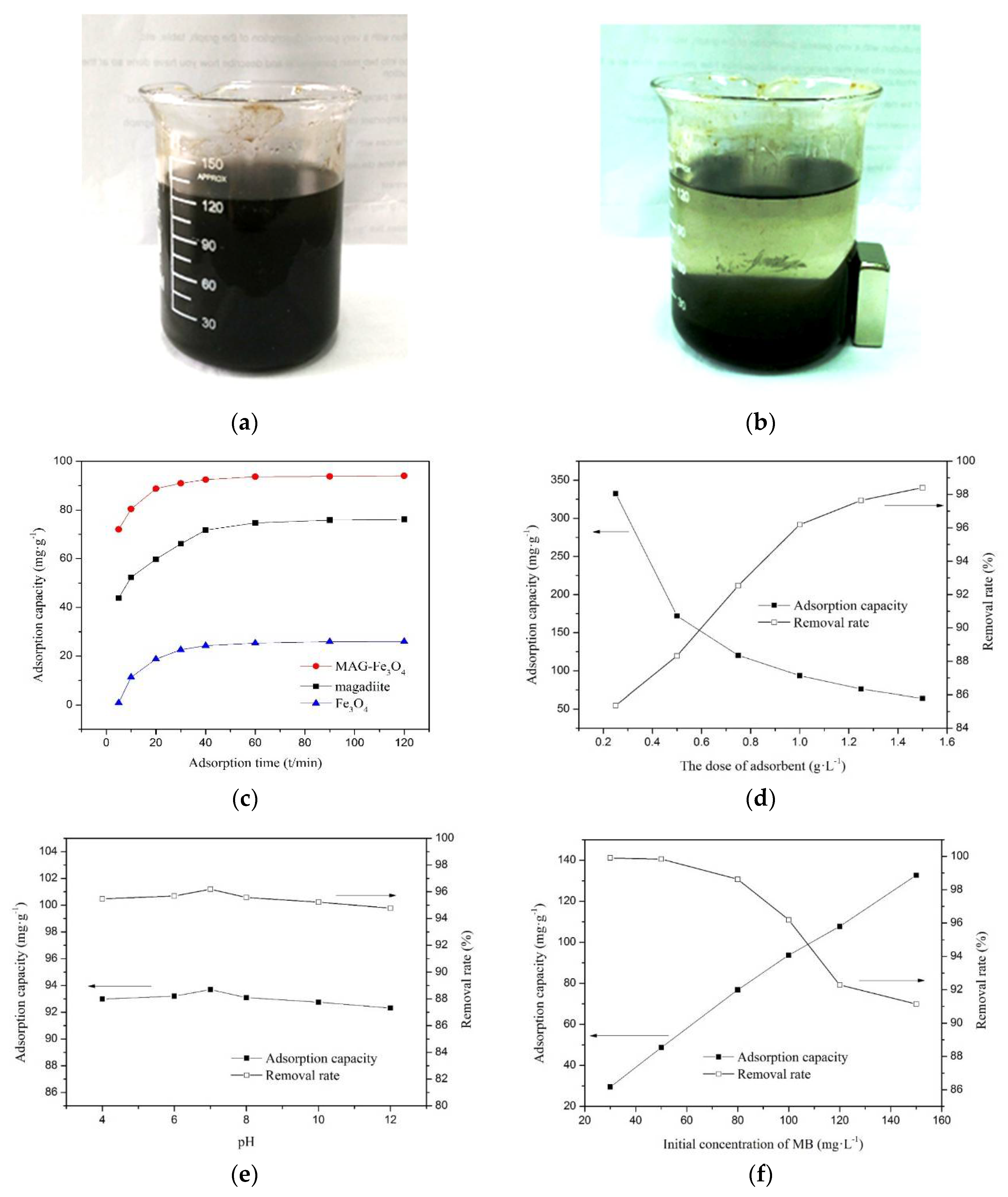
3.2.1. Adsorption Capacity Analyses of MAG, Fe3O4, and MAG–Fe3O4
3.2.2. Effect of Adsorbent Dosage on the Adsorption
3.2.3. Effect of the Solution pH on Adsorption
3.2.4. Effect of Initial Concentration of MB Solutions on the Adsorption
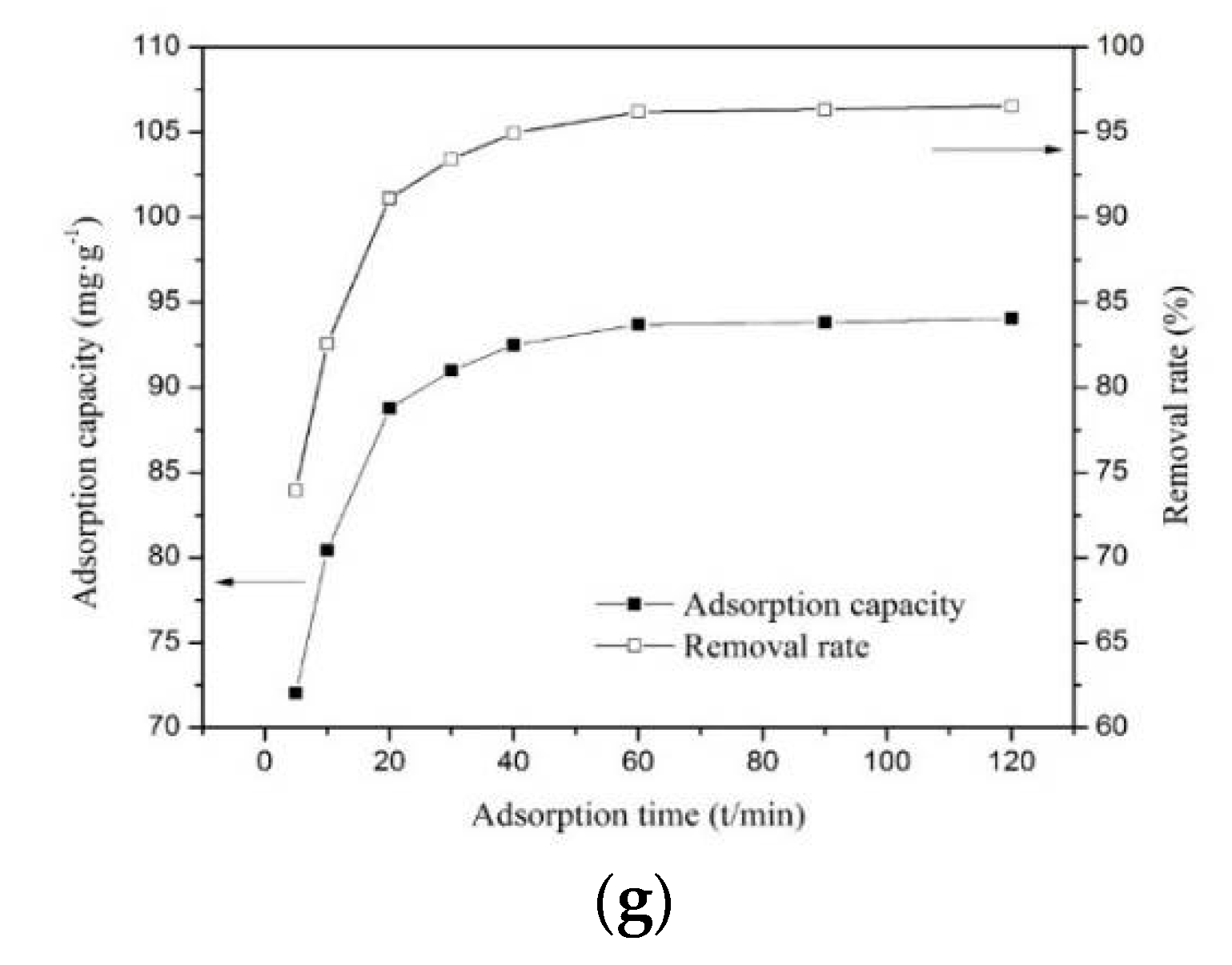
3.2.5. Effect of Contact Time on the Adsorption
3.2.6. Adsorption Kinetics Analyses
3.2.7. Adsorption Isotherm Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, P.; Kazlauciunas, A.; Menzel, R.; Lin, L. Determining the Mechanism and Efficiency of Industrial Dye Adsorption through Facile Structural Control of Organo-montmorillonite Adsorbents. ACS Appl. Mater. Interfaces 2017, 9, 26383–26391. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, P. Adsorption behavior of methylene blue on halloysite nanotubes. Microporous Mesoporous Mater. 2008, 112, 419–424. [Google Scholar] [CrossRef]
- Weng, C.-H.; Pan, Y.-F. Adsorption of a cationic dye (methylene blue) onto spent activated clay. J. Hazard. Mater. 2007, 144, 355–362. [Google Scholar] [CrossRef]
- Gong, J.L.; Wang, B.; Zeng, G.M.; Yang, C.P.; Niu, C.G.; Niu, Q.Y.; Zhou, W.J.; Liang, Y. Removal of cationic dyes from aqueous solution using magnetic multi-wall carbon nanotube nanocomposite as sorbent. J. Hazard. Mater. 2009, 164, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Fatimah, S.; Wang, D. Wulandari, ZnO/montmorillonite for photocatalytic and photochemical degradation of methylene blue. Appl. Clay Sci. 2011, 53, 553–560. [Google Scholar] [CrossRef]
- Cottet, L.; Almeida, C.A.P.; Naidek, N.; Viante, M.F.; Lopes, M.C.; Debacher, N.A. Adsorption characteristics of montmorillonite clay modified with iron oxide with respect to methylene blue in aqueous media. Appl. Clay Sci. 2014, 95, 25–31. [Google Scholar] [CrossRef]
- Peng, Y.G.; Chen, D.J.; Ji, J.L.; Kong, Y.; Wan, H.X.; Yao, C. Chitosan-modified palygorskite: Preparation, characterization and reactive dye removal. Appl. Clay Sci. 2013, 74, 81–86. [Google Scholar] [CrossRef]
- Zhou, C.-H.; Zhang, D.; Tong, D.-S.; Wu, L.-M.; Yu, W.-H.; Ismadji, S. Paper-like composites of cellulose acetate–organo-montmorillonite for removal of hazardous anionic dye in water. Chem. Eng. J. 2012, 209, 223–234. [Google Scholar] [CrossRef] [Green Version]
- Sivakumar, P.; Palanisamy, P.N. Adsorption studies of basic red 29 by a nonconventional activated carbon prepared from Euphorbia antiquorum L. J. Chem. Tech. Res. 2009, 1, 502–510. [Google Scholar]
- Eren, E.; Afsin, B. Investigation of a basic dye adsorption from aqueous solution onto raw and pre-treated sepiolite surfaces. Dyes Pigment. 2007, 73, 162–167. [Google Scholar] [CrossRef]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Li, K.; Gao, Q.; Yadavalli, G.; Shen, X.; Lei, H.; Han, B.; Xia, K.; Zhou, C. Selective Adsorption of Gd3+ on a Magnetically Retrievable Imprinted Chitosan/Carbon Nanotube Composite with High Capacity. ACS Appl. Mater. Interfaces 2015, 7, 21047–21055. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Song, Z.; Che, G.; Ren, A.; Li, P.; Liu, C.; Zhang, J. Adsorption behavior of metal–organic frameworks for methylene blue from aqueous solution. Microporous Mesoporous Mater. 2014, 193, 27–34. [Google Scholar] [CrossRef]
- Demey, H.; Tria, S.A.; Soleri, R.; Guiseppi-Elie, A.; Bazin, I. Sorption of his-tagged Protein G and Protein G onto chitosan/divalent metal ion sorbent used for detection of microcystin-LR. Environ. Sci. Pollut. Res. 2017, 24, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Demey, H.; Marchand, M.; Lapo, B.; Ruiz, M.; Fortuny, A.; Sastre, A. Neodymium Recovery by Chitosan/Iron(III) Hydroxide [ChiFer(III)] Sorbent Material: Batch and Column Systems. Polymers 2018, 10, 204. [Google Scholar] [CrossRef]
- Awadh, S.M.; Abdulla, F.H. Purification of aqueous solutions from Pb (II) by natural bentonite: An empirical study on chemical adsorption. Environ. Earth Sci. 2017, 76, 386. [Google Scholar] [CrossRef]
- Drweesh, S.A.; Fathy, N.A.; Wahba, M.A.; Hanna, A.A.; Akarish, A.I.; Elzahany, E.A.; El-Sherif, I.Y.; Abou-El-Sherbini, K.S.; Wahba, M.A. Equilibrium, kinetic and thermodynamic studies of Pb(II) adsorption from aqueous solutions on HCl-treated Egyptian kaolin. J. Environ. Chem. Eng. 2016, 4, 1674–1684. [Google Scholar] [CrossRef]
- Deng, L.; Yuan, P.; Liu, D.; Annabi-Bergaya, F.; Zhou, J.; Chen, F.; Liu, Z. Effects of microstructure of clay minerals, montmorillonite, kaolinite and halloysite, on their benzene adsorption behaviors. Appl. Clay Sci. 2017, 143, 184–191. [Google Scholar] [CrossRef]
- Belleville, P.; Popall, M.; Nicole, L.; Sanchez, C. Applications of advanced hybrid organic–inorganic nanomaterials: From laboratory to market. Chem. Soc. Rev. 2011, 40, 696. [Google Scholar]
- Eugster, H.P. Hydrous sodium silicates from Lake Magadi, Kenya: Precurous of bedded chert. Science 1967, 157, 1177–1180. [Google Scholar] [CrossRef]
- Thiesen, P.H.; Beneke, K.; Lagaly, G. Silylation of a crystalline silicic acid: An MAS NMR and porosity study. J. Mater. Chem. 2002, 12, 3010–3015. [Google Scholar] [CrossRef]
- Wang, S.; Lin, M.; Shieh, Y.; Wang, Y.; Wang, S. Organic modification of synthesized clay-magadiite. Ceram. Int. 2007, 33, 681–685. [Google Scholar] [CrossRef]
- Ge, M.; Zhu, C.; Wang, Y. Preparation and properties of organic quaternary phosphonium salts modified nano-magaiite/nylon6 composites. Acta Mater. Compos. Sin. 2017, 34, 33–52. [Google Scholar]
- Ge, M.; Du, M.; Wang, Y. Adsorption Performance and Mechanism of Pb2+ on Mesoporous Material Magadiite. J. Chin. Ceram. Soc. 2017, 45, 37–45. [Google Scholar]
- Attar, K.; Demey, H.; Bouazza, D.; Sastre, A.M. Sorption and Desorption Studies of Pb(II) and Ni(II) from Aqueous Solutions by a New Composite Based on Alginate and Magadiite Materials. Polymers 2019, 11, 340. [Google Scholar] [CrossRef]
- Attar, K.; Bouazza, D.; Miloudi, H.; Tayeb, A.; Boos, A.; Sastre, A.M.; Demey, H. Cadmium removal by a low-cost magadiite-based material: Characterization and sorption applications. J. Environ. Chem. Eng. 2018, 6, 5351–5360. [Google Scholar] [CrossRef]
- Joussein, E.; Petit, S.; Churchman, J. Halloysite clay minerals—A review. Clay Miner. 2005, 40, 383–426. [Google Scholar] [CrossRef]
- Rawtani, D. Multifarious applications of halloysite nano tubes: A review. Rev. Adv. Mater. Sci. 2012, 30, 282–295. [Google Scholar]
- Ouyang, J.; Zhou, Z.; Zhang, Y.; Yang, H. High morphological stability and structural transition of halloysite (Hunan, China) in heat treatment. Appl. Clay Sci. 2014, 101, 16–22. [Google Scholar] [CrossRef]
- Barrientos-Ramírez, S.; Oca-Ramírez, G.M.; Ramos-Fernández, E.V.; Sepúlveda-Escribano, A.; Pastor-Blas, M.M.; González-Montiel, A. Surface modification of natural halloysite clay nanotubes with aminosilanes. Application as catalyst supports in the atom transfer radical polymerization of methyl methacrylate. Appl. Catal. A Gen. 2011, 406, 22–33. [Google Scholar] [CrossRef]
- Wan, D.; Li, W.; Wang, G.; Chen, K.; Lu, L.; Hu, Q. Adsorption and heterogeneous degradation of rhodamine B on the surface of magnetic bentonite material. Appl. Surf. Sci. 2015, 349, 988–996. [Google Scholar] [CrossRef]
- Xie, Y.; Qian, D.; Wu, D.; Ma, X. Magnetic halloysite nanotubes/iron oxide composites for the adsorption of dyes. Chem. Eng. J. 2011, 168, 959–963. [Google Scholar] [CrossRef]
- Chang, J.; Ma, J.; Ma, Q.; Zhang, D.; Qiao, N.; Hu, M.; Ma, H. Adsorption of methylene blue onto Fe3O4/activated montmorillonite nanocomposite. Appl. Clay Sci. 2016, 119, 132–140. [Google Scholar] [CrossRef]
- Kalantari, K.; Ahmad, M.B.; Masoumi, H.R.F.; Shameli, K.; Basri, M.; Khandanlou, R. Rapid and high capacity adsorption of heavy metals by Fe3O4/montmorillonite nanocomposite using response surface methodology: Preparation, characterization, optimization, equilibrium isotherms, and adsorption kinetics study. J. Taiwan Inst. Chem. Eng. 2015, 49, 192–198. [Google Scholar] [CrossRef]
- Ge, M.; Chen, M. Preparation and characterization of magadiite. J. Chin. Ceram Soc. 2013, 41, 1704–1708. [Google Scholar]
- Wang, X.; Wang, H.; Lu, M.; Teng, R.; Du, X. Facile synthesis of phenyl-modified magnetic graphene/mesoporous silica with hierarchical bridge-pore structure for efficient adsorption of pesticides. Mater. Chem. Phys. 2017, 198, 393–400. [Google Scholar] [CrossRef]
- Meng, A.; Xiang, J.; Li, Z.; Li, Q. Cr-Doped ZnO Nanoparticles: Synthesis, Characterization, Adsorption Property, and Recyclability. ACS Appl. Mater. Interfaces 2015, 7, 27449–27457. [Google Scholar] [CrossRef]
- Mall, I.D.; Srivastava, V.C.; Agarwal, N.K. Removal of Orange-G and Methyl Violet dyes by adsorption onto bagasse fly ash—Kinetic study and equilibrium isotherm analyses. Dyes Pigment. 2006, 69, 210–223. [Google Scholar] [CrossRef]
- Almond, G.G.; Harris, R.K.; Franklin, K.R. A structural consideration of kanemite, octosilicate, magadiite and kenyaite. J. Mater. Chem. 1997, 7, 681–687. [Google Scholar] [CrossRef]
- Xu, J.; Liu, X.; Lowry, G.V.; Cao, Z.; Zhao, H.; Zhou, J.L.; Xu, X. Dechlorination Mechanism of 2,4-Dichlorophenol by Magnetic MWCNTs Supported Pd/Fe Nanohybrids: Rapid Adsorption, Gradual Dechlorination, and Desorption of Phenol. ACS Appl. Mater. Interfaces 2016, 8, 7333–7342. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Feng, C.; Gao, Y.; Ma, X.; Wu, Q.; Wang, Z. Preparation of a graphene-based magnetic nanocomposite for the removal of an organic dye from aqueous solution. Chem. Eng. J. 2011, 173, 92–97. [Google Scholar] [CrossRef]
- Guo, B.; Ouyang, J.; Yang, H. Adsorption Performance to Methylene Blue by Nano-Fe3O4 Assembled in Lumen of Halloysite Nanotubes. J. Chin. Ceram Soc. 2016, 44, 1655–1661. [Google Scholar]
- Wang, Y.; Shang, Y.; Zhu, J.; Wu, J.; Ji, S.; Meng, C. Synthesis of magadiite using a natural diatomite material. J. Chem. Technol. Biotechnol. 2009, 84, 1894–1898. [Google Scholar] [CrossRef]
- Zheng, P.; Du, Y.; Ma, X. Selective fabrication of iron oxide particles in halloysite lumen. Mater. Chem. Phys. 2015, 151, 14–17. [Google Scholar] [CrossRef]
- Mu, B.; Wang, W.; Zhang, J.; Wang, A. Superparamagnetic sandwich structured silver/halloysite nanotube/Fe3O4 nanocomposites for 4-nitrophenol reduction. RSC Adv. 2014, 4, 39439–39445. [Google Scholar] [CrossRef]
- Rehman, M.A.; Yusoff, I.; Alias, Y. Fluoride adsorption by doped and un-doped magnetic ferrites CuCexFe2−xO4: Preparation, characterization, optimization and modeling for effectual remediation technologies. J. Hazard. Mater. 2015, 299, 316–324. [Google Scholar] [CrossRef]
- Wang, W.; Feng, Q.; Dong, F.; Li, H.; Zhao, X. Preparation and properties of magnetic bentonite. J. Chin. Ceram. Soc. 2010, 38, 684–688. [Google Scholar]
- Ge, M.; Cao, L.; Du, M.; Jahangir Alam, S.M. Adsorptive characterization of a pure magadiite and an organic modified magadiite on removal of methylene blue from related aqueous solution. Mater. Chem. Phys. 2018, 217, 533–540. [Google Scholar] [CrossRef]
- Lou, Z.; Zhou, Z.; Zhang, W.; Zhang, X.; Hu, X.; Liu, P.; Zhang, H. Magnetized bentonite by Fe3O4 nanoparticles treated as adsorbent for methylene blue removal from aqueous solution: Synthesis, characterization, mechanism, kinetics and regeneration. J. Taiwan Inst. Chem. E. 2015, 49, 199–205. [Google Scholar] [CrossRef]
- Ge, M.; Du, M.; Zheng, L.; Wang, B.; Zhou, X.; Jia, Z.; Hu, G.; Jahangir Alam, S.M. A maleic anhydride grafted sugarcane bagasse adsorbent and its performance on the removal of methylene blue from related wastewater. Mater. Chem. Phys. 2017, 192, 147–155. [Google Scholar] [CrossRef]
- Ge, M.; Wang, X.; Du, M.; Liang, G.; Hu, G.; Jahangir Alam, S.M. Effects on the Mechanical Properties of Nacre-Like Bio-Hybrid Membranes with Inter-Penetrating Petal Structure Based on Magadiite. Materials 2019, 12, 173. [Google Scholar] [CrossRef]
- Ge, M.; Wang, X.; Du, M.; Liang, G.; Hu, G.; Jahangir Alam, S.M. Adsorption Analyses of Phenol from Aqueous Solutions Using Magadiite Modified with Organo-Functional Groups: Kinetic and Equilibrium Studies. Materials 2019, 12, 96. [Google Scholar] [CrossRef]
- Ge, M.; Cao, L.; Du, M.; Hu, G.; Alam, S.M.J. Competitive adsorption analyses of a pure magadiite and a New silylated magadiite on methylene blue and phenol from related aqueous Solution. Mater. Chem. Phys. 2018, 217, 393–402. [Google Scholar] [CrossRef]
- Ge, M.; Du, M.; Zheng, L.; Zhou, X.; Jia, Z. Solid phase grafting preparation of SCB-g-MMA and its adsorption property. Acta Mater. Compos. Sin. 2016, 33, 2444–2453. [Google Scholar]
- Shen, T.; Wang, Z.; Miao, W.; Zhou, Q.; Cheng, M.M.; Wang, Q.; Chen, Z.E. Study on adsorption of methylene blue on composite attapulgite material. Dev. Appl. Mater. 2009, 24, 44–48. [Google Scholar]
- Huang, Z.; Li, Y.; Chen, W.; Shi, J.; Zhang, N.; Wang, X.; Li, Z.; Gao, L.; Zhang, Y. Modified bentonite adsorption of organic pollutants of dye wastewater. Mater. Chem. Phys. 2017, 202, 266–276. [Google Scholar] [CrossRef]
- Tang, M.; Li, X.; Gao, C.; Li, X.; Qiu, H. Adsorption performance of CuFe2O4/rGO nanocomposites towards organic dye. Mater. Chem. Phys. 2017, 185, 114–121. [Google Scholar] [CrossRef]
- Rehman, M.S.U.; Kim, I.; Han, J.I. Adsorption of methylene blue dye from aqueous solution by sugar extracted spent rice biomass. Carbohydr. Polym. 2012, 90, 1314–1322. [Google Scholar] [CrossRef]
- Mokhtar, M.; Mostafa, M.M.M. Application of Synthetic Layered Sodium Silicate Magadiite Nanosheets for Environmental Remediation of Methylene Blue Dye in Water. Materials 2017, 10, 760. [Google Scholar] [CrossRef]
- Li, C.; Zhong, H.; Wang, S.; Xue, J.; Zhang, Z. Removal of basic dye (methylene blue) from aqueous solution using zeolite synthesized from electrolytic manganese residue. J. Ind. Eng. Chem. 2015, 23, 344–352. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Bajaj, H.C.; Tayade, R.J. Preferential adsorption behavior of methylene blue dye onto surface hydroxyl group enriched TiO2 nanotube and its photocatalytic regeneration. J. Sci. 2014, 433, 104–114. [Google Scholar]



| Model | qeq (mg/g) | qeqC (mg/g) | K | R2 |
|---|---|---|---|---|
| Pseudo-first-order | 94.1 | 16.4976 | 0.04915 | 0.94821 |
| Pseudo-second-order | 94.1 | 95.4198 | 0.00689 | 0.99996 |
| Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|
| R2 | n | R2 | |||
| 128.5347 | 1.591 | 0.971 | 4.6 | 72.057 | 0.95725 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, M.; Xi, Z.; Zhu, C.; Liang, G.; Hu, G.; Jamal, L.; S. M., J.A. Preparation and Characterization of Magadiite–Magnetite Nanocomposite with Its Sorption Performance Analyses on Removal of Methylene Blue from Aqueous Solutions. Polymers 2019, 11, 607. https://doi.org/10.3390/polym11040607
Ge M, Xi Z, Zhu C, Liang G, Hu G, Jamal L, S. M. JA. Preparation and Characterization of Magadiite–Magnetite Nanocomposite with Its Sorption Performance Analyses on Removal of Methylene Blue from Aqueous Solutions. Polymers. 2019; 11(4):607. https://doi.org/10.3390/polym11040607
Chicago/Turabian StyleGe, Mingliang, Zhuangzhuang Xi, Caiping Zhu, Guodong Liang, Guoqing Hu, Lafifa Jamal, and Jahangir Alam S. M. 2019. "Preparation and Characterization of Magadiite–Magnetite Nanocomposite with Its Sorption Performance Analyses on Removal of Methylene Blue from Aqueous Solutions" Polymers 11, no. 4: 607. https://doi.org/10.3390/polym11040607





