Synthesis and Characterization of Phosphorus- and Carborane-Containing Polyoxanorbornene Block Copolymers
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.3. ROMP (Ring Opening Metathesis Polymerization) Block Copolymers
2.4. Deprotection of ROMP (Ring Opening Metathesis Polymerization) Block Copolymer P2
2.5. Preparation of Polymer Coated-Nanoparticles
3. Results and Discussion
3.1. Monomer Synthesis
3.2. Synthesis of Carborane- and Phosphorus-Containing Block Copolymers
3.3. Conversion of the Phosphonate Ester to Phosphonic Acid, P2A
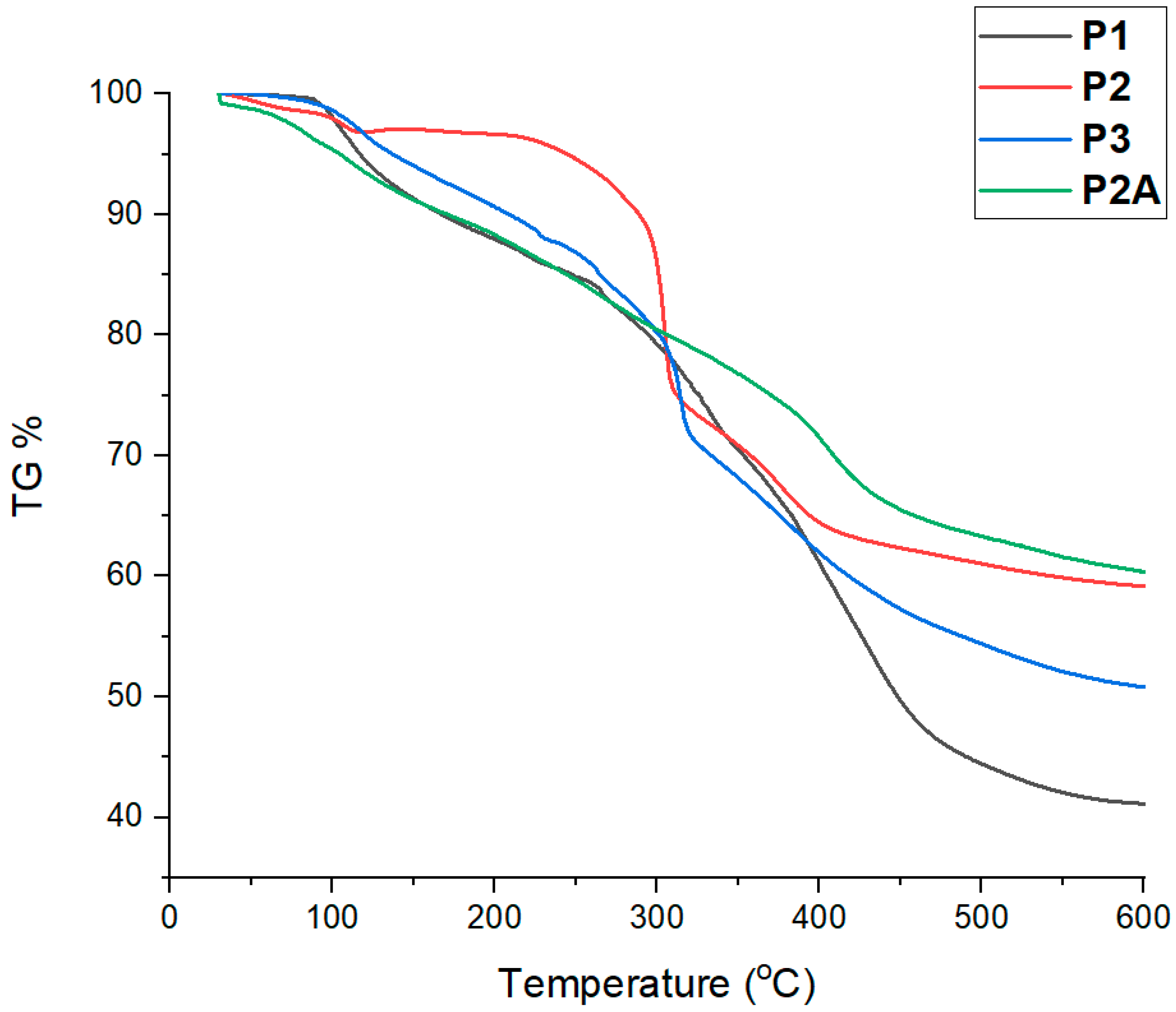
3.4. Thermal Properties of the Polymers
3.5. Stabilization of Magnetic Fe3O4 Nanoparticles with Block Copolymer P2A
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Sanchez, C.; Lebeau, B.; Chaput, F.; Boilot, J.-P. Optical properties of functional hybrid organic–inorganic nanocomposites. Adv. Mater. 2003, 15, 1969–1994. [Google Scholar] [CrossRef]
- Orilall, M.C.; Wiesner, U. Block copolymer based composition and morphology control in nanostructured hybrid materials for energy conversion and storage: Solar cells, batteries, and fuel cells. Chem. Soc. Rev. 2011, 40, 520–535. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Pispas, S.; Floudas, G. Block Copolymers: Synthetic Strategies, Physical Properties, and Applications; Wiley: Hoboken, NJ, USA, 2003; Volume 125, pp. 11453–11454. [Google Scholar] [CrossRef]
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef] [PubMed]
- Schacher, F.H.; Rupar, P.A.; Manners, I. Functional Block Copolymers: Nanostructured Materials with Emerging Applications. Angew. Chem. Int. Ed. 2012, 51, 7898–7921. [Google Scholar] [CrossRef]
- Pu, L.Y.; Wang, J.L.; Li, N.; Chai, Q.X.; Irache, J.M.; Wang, G.; Tang, J.Z.; Gu, Z.W. Synthesis of Electroneutralized Amphiphilic Copolymers with Peptide Dendrons for Intramuscular Gene Delivery. ACS Appl. Mater. Interfaces 2016, 8, 13724–13734. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Eisenberg, A. Polymer vesicles. Science 2002, 297, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.S.; Varshney, S.K.; Wong, S.; Eisenberg, A. Block-Copolymer Crew-Cut Micelles in Water. Macromolecules 1994, 27, 7923–7927. [Google Scholar] [CrossRef]
- Xu, H.; Yang, P.; Ma, H.; Yin, W.; Wu, X.; Wang, H.; Xu, D.; Zhang, X. Amphiphilic block copolymers-based mixed micelles for noninvasive drug delivery. Drug Deliv. 2016, 23, 3063–3071. [Google Scholar] [CrossRef]
- Hayward, R.C.; Pochan, D.J. Tailored Assemblies of Block Copolymers in Solution: It Is All about the Process. Macromolecules 2010, 43, 3577–3584. [Google Scholar] [CrossRef]
- Al-Badri, Z.M.; Maddikeri, R.R.; Zha, Y.P.; Thaker, H.D.; Dobriyal, P.; Shunmugam, R.; Russell, T.P.; Tew, G.N. Room temperature magnetic materials from nanostructured diblock copolymers. Nat. Commun. 2011, 2. [Google Scholar] [CrossRef]
- Belfield, K.D.; Zhang, L. Norbornene-functionalized diblock copolymers via ring-opening metathesis polymerization for magnetic nanoparticle stabilization. Chem. Mater. 2006, 18, 5929–5936. [Google Scholar] [CrossRef]
- Gref, R.; Minamitake, Y.; Peracchia, M.T.; Trubetskoy, V.; Torchilin, V.; Langer, R. Biodegradable Long-Circulating Polymeric Nanospheres. Science 1994, 263, 1600–1603. [Google Scholar] [CrossRef] [PubMed]
- Schöttner, S.; Schaffrath, H.-J.; Gallei, M. Poly(2-hydroxyethyl methacrylate)-Based Amphiphilic Block Copolymers for High Water Flux Membranes and Ceramic Templates. Macromolecules 2016, 49, 7286–7295. [Google Scholar] [CrossRef]
- Rüttiger, C.; Hübner, H.; Schöttner, S.; Winter, T.; Cherkashinin, G.; Kuttich, B.; Stühn, B.; Gallei, M. Metallopolymer-Based Block Copolymers for the Preparation of Porous and Redox-Responsive Materials. ACS Appl. Mater. Interfaces 2018, 10, 4018–4030. [Google Scholar] [CrossRef]
- Chen, Y.; Abdellatif, M.M.; Nomura, K. Olefin metathesis polymerization: Some recent developments in the precise polymerizations for synthesis of advanced materials (by ROMP, ADMET). Tetrahedron 2018, 74, 619–643. [Google Scholar] [CrossRef]
- Leitgeb, A.; Wappel, J.; Slugovc, C. The ROMP toolbox upgraded. Polymer 2010, 51, 2927–2946. [Google Scholar] [CrossRef]
- Eren, T.; Tew, G.N. Phosphonic Acid-Based Amphiphilic Diblock Copolymers Derived from ROMP. J. Polym. Sci. A Polym. Chem. 2009, 47, 3949–3956. [Google Scholar] [CrossRef]
- Simon, Y.C.; Coughlin, E.B. Ring-Opening Metathesis Copolymerization of Cyclooctene and a Carborane-Containing Oxanorbornene. J. Polym. Sci. A Polym. Chem. 2010, 48, 2557–2563. [Google Scholar] [CrossRef]
- Simon, Y.C.; Ohm, C.; Zimny, M.J.; Coughlin, E.B. Amphiphilic carborane-containing diblock copolymers. Macromolecules 2007, 40, 5628–5630. [Google Scholar] [CrossRef]
- Smith, D.; Pentzer, E.B.; Nguyen, S.T. Bioactive and Therapeutic ROMP Polymers. Polym. Rev. 2007, 47, 419–459. [Google Scholar] [CrossRef]
- Kolonko, E.M.; Pontrello, J.K.; Mangold, S.L.; Kiessling, L.L. General Synthetic Route to Cell-Permeable Block Copolymers via ROMP. J. Am. Chem. Soc. 2009, 131, 7327–7333. [Google Scholar] [CrossRef]
- Pawar, G.M.; Lalancette, R.A.; Bonder, E.M.; Sheridan, J.B.; Jäkle, F. ROMP-Derived Pyridylborate Block Copolymers: Self-Assembly, pH-Responsive Properties, and Metal-Containing Nanostructures. Macromolecules 2015, 48, 6508–6515. [Google Scholar] [CrossRef]
- Plesek, J. Potential Applications of the Boron Cluster Compounds. Chem. Rev. 1992, 92, 269–278. [Google Scholar] [CrossRef]
- Grimes, R.N. Carboranes in the chemist’s toolbox. Dalton Trans. 2015, 44, 5939–5956. [Google Scholar] [CrossRef] [PubMed]
- Kokado, K.; Chujo, Y. Emission via Aggregation of Alternating Polymers with o-Carborane and p-Phenylene-Ethynylene Sequences. Macromolecules 2009, 42, 1418–1420. [Google Scholar] [CrossRef]
- Sumitani, S.; Oishi, M.; Nagasaki, Y. Carborane confined nanoparticles for boron neutron capture therapy: Improved stability, blood circulation time and tumor accumulation. React. Funct. Polym. 2011, 71, 684–693. [Google Scholar] [CrossRef]
- Bucca, D.; Keller, T.M. Thermally and oxidatively stable thermosets derived from preceramic monomers. J. Polym. Sci. A Polym. Chem. 1997, 35, 1033–1038. [Google Scholar] [CrossRef]
- Colquhoun, H.M.; Lewis, D.F.; Herbertson, P.L.; Wade, K. Polyetherketones based on para-carborane: Synthesis, sulfonation, and membrane-forming characteristics. Polymer 1997, 38, 4539–4546. [Google Scholar] [CrossRef]
- Fox, M.A.; Wade, K. Model compounds and monomers for phenylene ether carboranylene ketone (PECK) polymer synthesis: Preparation and characterization of boron-arylated ortho-carboranes bearing carboxyphenyl, phenoxyphenyl or benzoylphenyl substituents. J. Mater. Chem. 2002, 12, 1301–1306. [Google Scholar] [CrossRef]
- Cheng, S.L.; Han, J.H.; Wang, X.; Yuan, K.Y.; Jian, X.G.; Wang, J.Y. Oxidatively stable thermosets derived from thermal copolymerization of acetylene-terminated imide monomer with an acetylenic monomer containing carborane. Polymer 2017, 115, 96–105. [Google Scholar] [CrossRef]
- Ahrens, V.M.; Frank, R.; Stadlbauer, S.; Beck-Sickinger, A.G.; Hey-Hawkins, E. Incorporation of ortho-Carbaboranyl-N-epsilon-Modified L-Lysine into Neuropeptide Y Receptor Y-1- and Y-2-Selective Analogues. J. Med. Chem. 2011, 54, 2368–2377. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M.; Kaluderovic, G.N.; Kommera, H.; Paschke, R.; Will, J.; Sheldrick, W.S.; Hey-Hawkins, E. Carbaboranes as pharmacophores: Similarities and differences between aspirin and asborin. Eur. J. Med. Chem. 2011, 46, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.L.; Yuan, K.Y.; Wang, X.; Han, J.H.; Jian, X.G.; Wang, J.Y. Poly(phenylene-carborane) for boron-carbide/carbon ceramic precursor synthesized via nickel catalysis. Polymer 2017, 115, 224–231. [Google Scholar] [CrossRef]
- Davis, A.R.; Peterson, J.J.; Carter, K.R. Effect of o-Carborane on the Optoelectronic and Device-Level Properties of Poly(fluorene)s. ACS Macro Lett. 2012, 1, 469–472. [Google Scholar] [CrossRef]
- Malenfant, P.R.L.; Wan, J.L.; Taylor, S.T.; Manoharan, M. Self-assembly of an organic-inorganic block copolymer for nano-ordered ceramics. Nat. Nanotechnol. 2007, 2, 43–46. [Google Scholar] [CrossRef]
- Yeniad, B.; Albayrak, A.Z.; Olcum, N.C.; Avci, D. Synthesis and photopolymerizations of new phosphonated monomers for dental applications. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 2290–2299. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.Z. Aryl Polyphosphonates: Useful Halogen-Free Flame Retardants for Polymers. Materials 2010, 3, 4746–4760. [Google Scholar] [CrossRef]
- Velencoso, M.M.; Battig, A.; Markwart, J.C.; Schartel, B.; Wurm, F.R. Molecular Firefighting—How Modern Phosphorus Chemistry Can Help Solve the Challenge of Flame Retardancy. Angew. Chem. Int. Ed. 2018, 57, 10450–10467. [Google Scholar] [CrossRef]
- Attenberger, B.; Peresypkina, E.V.; Scheer, M. Novel Two- and Three-Dimensional Organometallic-Organic Hybrid Materials Based on Polyphosphorus Complexes. Inorg. Chem. 2015, 54, 7021–7029. [Google Scholar] [CrossRef]
- Bauer, K.N.; Tee, H.T.; Velencoso, M.M.; Wurm, F.R. Main-chain poly(phosphoester)s: History, syntheses, degradation, bio-and flame-retardant applications. Prog. Polym. Sci. 2017, 73, 61–122. [Google Scholar] [CrossRef]
- Monge, S.; Canniccioni, B.; Graillot, A.; Robin, J.-J. Phosphorus-Containing Polymers: A Great Opportunity for the Biomedical Field. Biomacromolecules 2011, 12, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.C.; Zhang, L.F.; Cheng, Z.P.; Zhu, X.L. Recent advances in “living”/controlled radical polymerization of phosphorus-containing monomers and their potential applications. Sci. China. Chem. 2015, 58, 1633–1640. [Google Scholar] [CrossRef]
- Seo, J.H.; Matsuno, R.; Takai, M.; Ishihara, K. Cell adhesion on phase-separated surface of block copolymer composed of poly(2-methacryloyloxyethyl phosphorylcholine) and poly(dimethylsiloxane). Biomaterials 2009, 30, 5330–5340. [Google Scholar] [CrossRef] [PubMed]
- Hsiue, G.H.; Lo, C.L.; Cheng, C.H.; Lin, C.P.; Huang, C.K.; Chen, H.H. Preparation and characterization of poly (2-methacryloyloxyethyl phosphorylcholine)-block-poly(D,L-lactide) polymer nanoparticles. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 688–698. [Google Scholar] [CrossRef]
- Queffelec, C.; Petit, M.; Janvier, P.; Knight, D.A.; Bujoli, B. Surface Modification Using Phosphonic Acids and Esters. Chem. Rev. 2012, 112, 3777–3807. [Google Scholar] [CrossRef]
- Boyer, C.; Whittaker, M.R.; Bulmus, V.; Liu, J.Q.; Davis, T.P. The design and utility of polymer-stabilized iron-oxide nanoparticles for nanomedicine applications. NPG Asia Mater. 2010, 2, 23–30. [Google Scholar] [CrossRef]
- Basuki, J.S.; Jacquemin, A.; Esser, L.; Li, Y.; Boyer, C.; Davis, T.P. A block copolymer-stabilized co-precipitation approach to magnetic iron oxide nanoparticles for potential use as MRI contrast agents. Polym. Chem. 2014, 5, 2611–2620. [Google Scholar] [CrossRef]
- Markiewicz, K.; Seiler, L.; Misztalewska-Turkowicz, I.; Winkler, K.; Harrisson, S.; Wilczewska, A.; Destarac, M.; Marty, J.-D. Advantages of poly(vinyl phosphonic acid)-based double hydrophilic block copolymers for the stabilization of iron oxide nanoparticles. Polym. Chem. 2016, 7, 6391–6399. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Xie, C.; Gao, A.; Chang, Z.; Kwon Oh, J.; Yang, P.; Li, P. Phosphonated homopolymers and copolymers via ring opening metathesis polymerization: Tg tuning, flame resistance, and photolithography. J. Polym. Sci. A Polym. Chem. 2016, 54, 1396–1408. [Google Scholar] [CrossRef]
- Bingöl, B.; Kroeger, A.; Jannasch, P. Well-defined phosphonated homo-and copolymers via direct ring opening metathesis polymerization. Polymer 2013, 54, 6676–6688. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, J.; Wang, L.; Wang, C. Ladder- and bridge-like polynorbornenes with phosphate linkers: Facile one-pot synthesis and excellent properties. RSC Adv. 2015, 5, 81986–81993. [Google Scholar] [CrossRef]
- Turgut, G.; Isiksel, E.; Kahraman, G.; Eren, T.; Ozkoc, G. Synthesis of phosphorus- and phenyl-based ROMP polymers and investigation of their effects on the thermomechanical and flammabilityproperties of a polypropylene-IFR system. J. Appl. Polym. Sci. 2018, 135, 45998. [Google Scholar] [CrossRef]
- Jiang, D.D.; Yao, Q.; McKinney, M.A.; Wilkie, C.A. TGA/FTIR studies on the thermal degradation of some polymeric sulfonic and phosphonic acids and their sodium salts. Polym. Degrad. Stabil. 1999, 63, 423–434. [Google Scholar] [CrossRef]
- N’Guyen, T.T.T.; Duong, H.T.T.; Basuki, J.; Montembault, V.; Pascual, S.; Guibert, C.; Fresnais, J.; Boyer, C.; Whittaker, M.R.; Davis, T.P.; et al. Functional Iron Oxide Magnetic Nanoparticles with Hyperthermia-Induced Drug Release Ability by Using a Combination of Orthogonal Click Reactions. Angew. Chem. Int. Ed. 2013, 52, 14152–14156. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, Y.; Pizem, H.; Fried, T.; Golodnitsky, D.; Burstein, L.; Sukenik, C.N.; Markovich, G. Alkyl phosphonate/phosphate coating on magnetite nanoparticles: A comparison with fatty acids. Langmuir 2001, 17, 7907–7911. [Google Scholar] [CrossRef]
- Krekic, K.; Klintuch, D.; Pietschnig, R. Facile access to efficiently luminescent Ln(3+) phosphonic ester coordination polymers (Ln = Eu, Tb, Dy). Chem. Commun. 2017, 53, 11076–11079. [Google Scholar] [CrossRef]






| Polymer | m a | n b | Mol Ratio Th (m/n) c | Mol Ratio Obs (m/n) d | Mn/block Th (g/mol) e | Mn/block NMR (g/mol) f | Đg | Cis/Trans Ratio h |
|---|---|---|---|---|---|---|---|---|
| P1 | 31.75 | 86.33 | 0.37 | 0.42 | 50,000 | 56,756 | 1.06 | 1.48 |
| P2 | 127 | 21.59 | 5.88 | 6.48 | 50,000 | 55,102 | 1.09 | 1.43 |
| P3 | 79.365 | 53.95 | 1.47 | 1.59 | 50,000 | 54,081 | 1.03 | 1.44 |
| Polymer | Char Residue (under N2)% | Char Residue (in Air)% |
|---|---|---|
| P1 | 41.2 | 62.4 |
| P2 | 59.3 | 48.4 |
| P3 | 51.1 | 56.8 |
| P2A | 60.4 | 60.6 |
| Polymer | Fe Amount Before Dialysis | Fe Amount After Dialysis |
|---|---|---|
| P2 | 858 ppm | 61 ppm |
| P2A | 758 ppm | 338 ppm |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kahraman, G.; Wang, D.-Y.; von Irmer, J.; Gallei, M.; Hey-Hawkins, E.; Eren, T. Synthesis and Characterization of Phosphorus- and Carborane-Containing Polyoxanorbornene Block Copolymers. Polymers 2019, 11, 613. https://doi.org/10.3390/polym11040613
Kahraman G, Wang D-Y, von Irmer J, Gallei M, Hey-Hawkins E, Eren T. Synthesis and Characterization of Phosphorus- and Carborane-Containing Polyoxanorbornene Block Copolymers. Polymers. 2019; 11(4):613. https://doi.org/10.3390/polym11040613
Chicago/Turabian StyleKahraman, Gizem, De-Yi Wang, Jonas von Irmer, Markus Gallei, Evamarie Hey-Hawkins, and Tarik Eren. 2019. "Synthesis and Characterization of Phosphorus- and Carborane-Containing Polyoxanorbornene Block Copolymers" Polymers 11, no. 4: 613. https://doi.org/10.3390/polym11040613
APA StyleKahraman, G., Wang, D.-Y., von Irmer, J., Gallei, M., Hey-Hawkins, E., & Eren, T. (2019). Synthesis and Characterization of Phosphorus- and Carborane-Containing Polyoxanorbornene Block Copolymers. Polymers, 11(4), 613. https://doi.org/10.3390/polym11040613








