Preparation of a Hydrophobic-Associating Polymer with Ultra-High Salt Resistance Using Synergistic Effect
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis
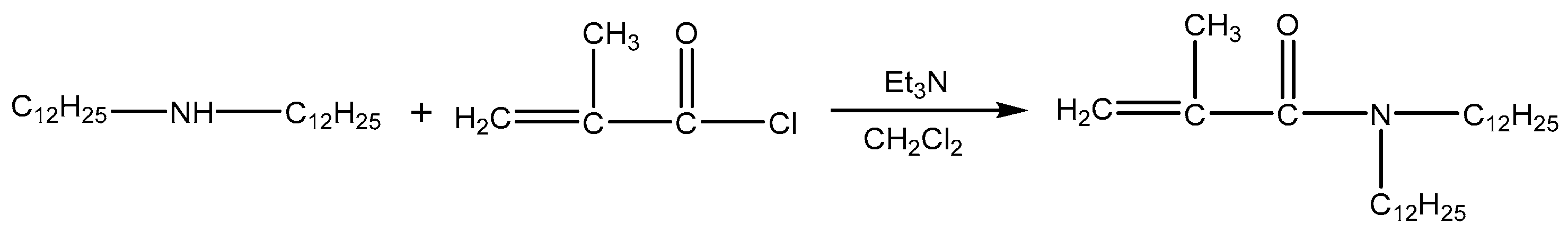
2.2.1. Preparation of Monomer
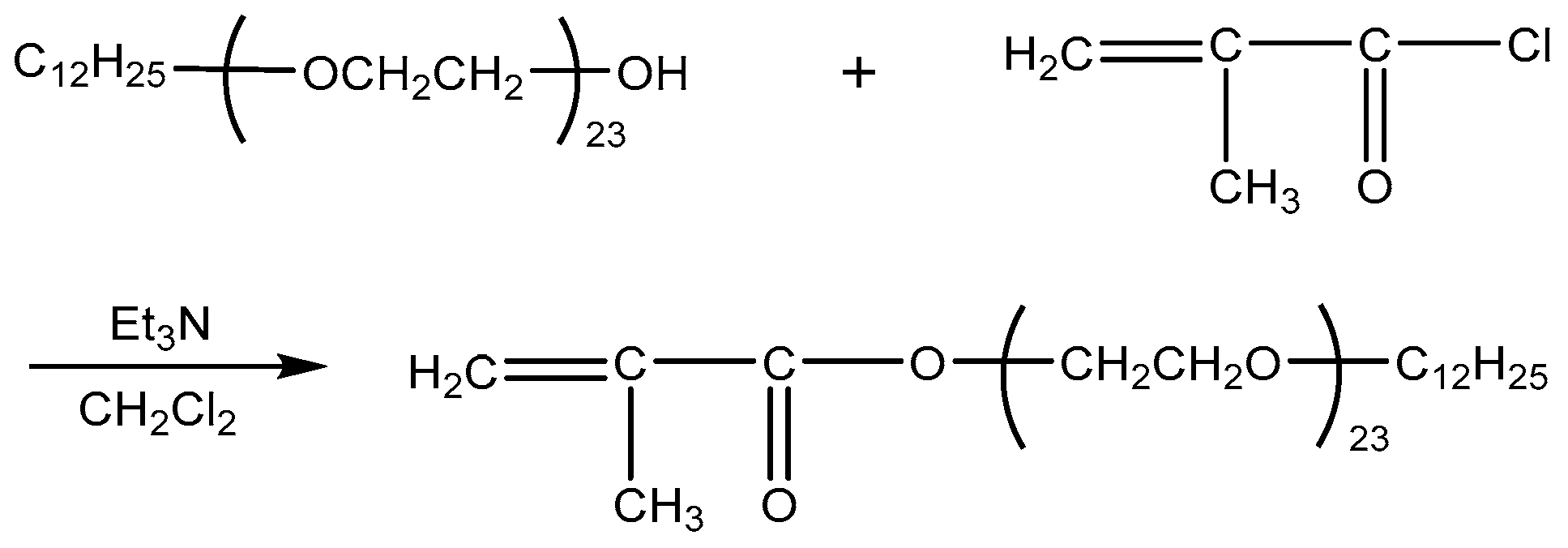
2.2.2. Synthesis of Copolymer
2.3. 1H NMR Characterization
2.4. Water Solubility
2.5. Thickening Performance
2.6. Salt Tolerance
2.7. Viscoelasticity
2.8. Dynamic Light Scattering (DLS) Measurement
2.9. Morphological Observation
2.10. Shear Tolerance
3. Results and Discussion
3.1. 1H NMR of Copolymers
3.2. Intrinsic Viscosity and Molecular Weight of Copolymers
3.3. Water Solubility
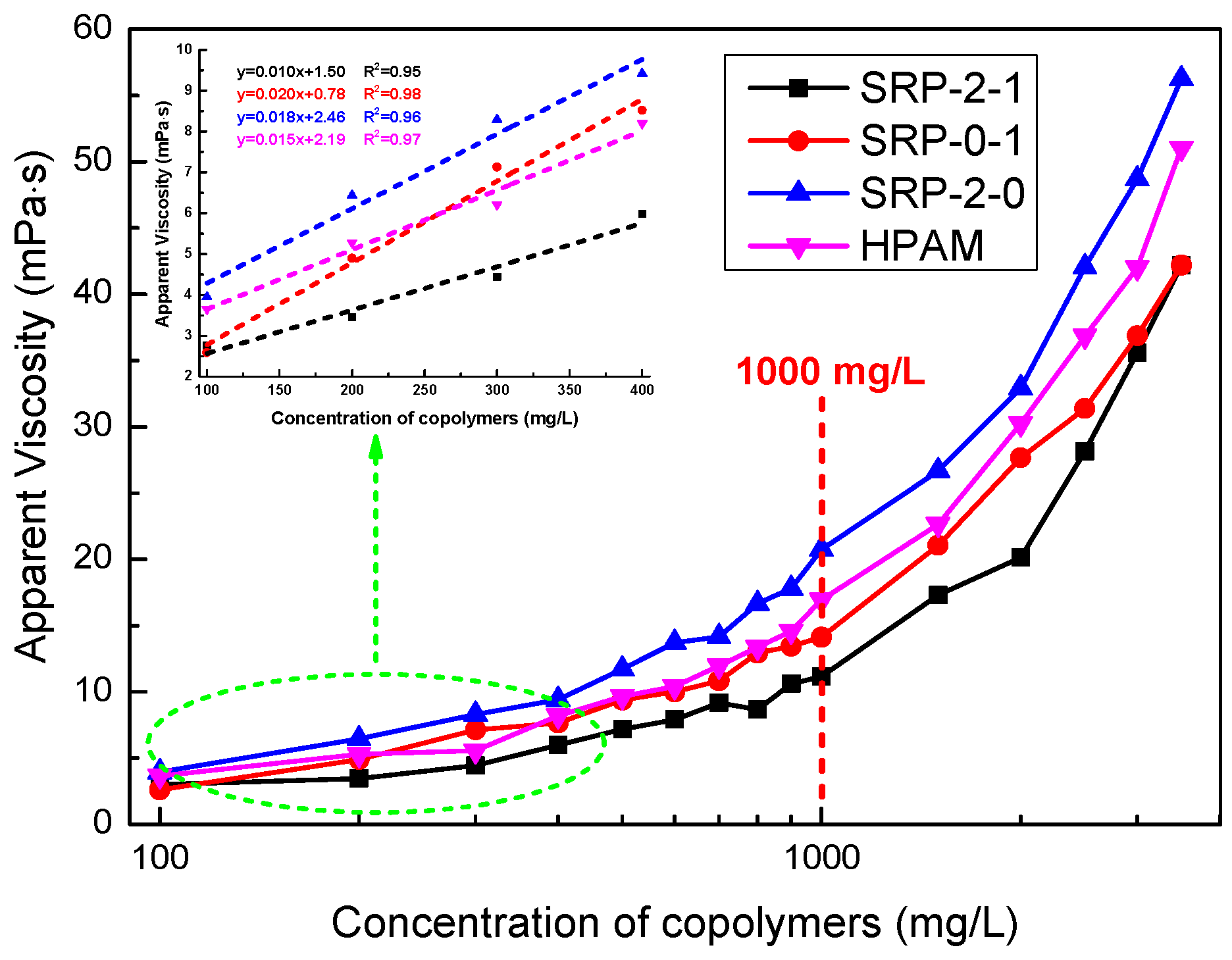
3.4. Thickening Performance
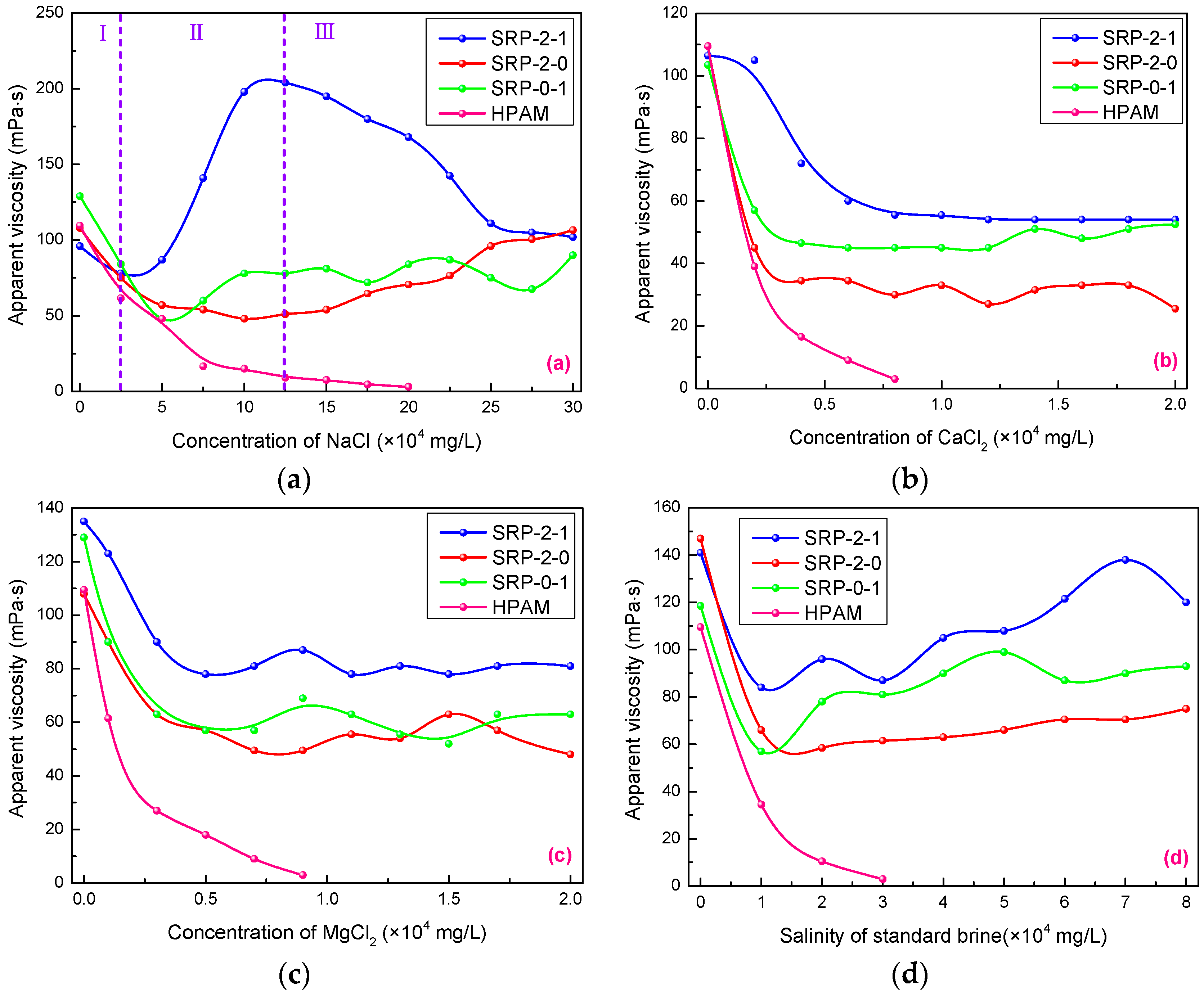
3.5. Salt Tolerance
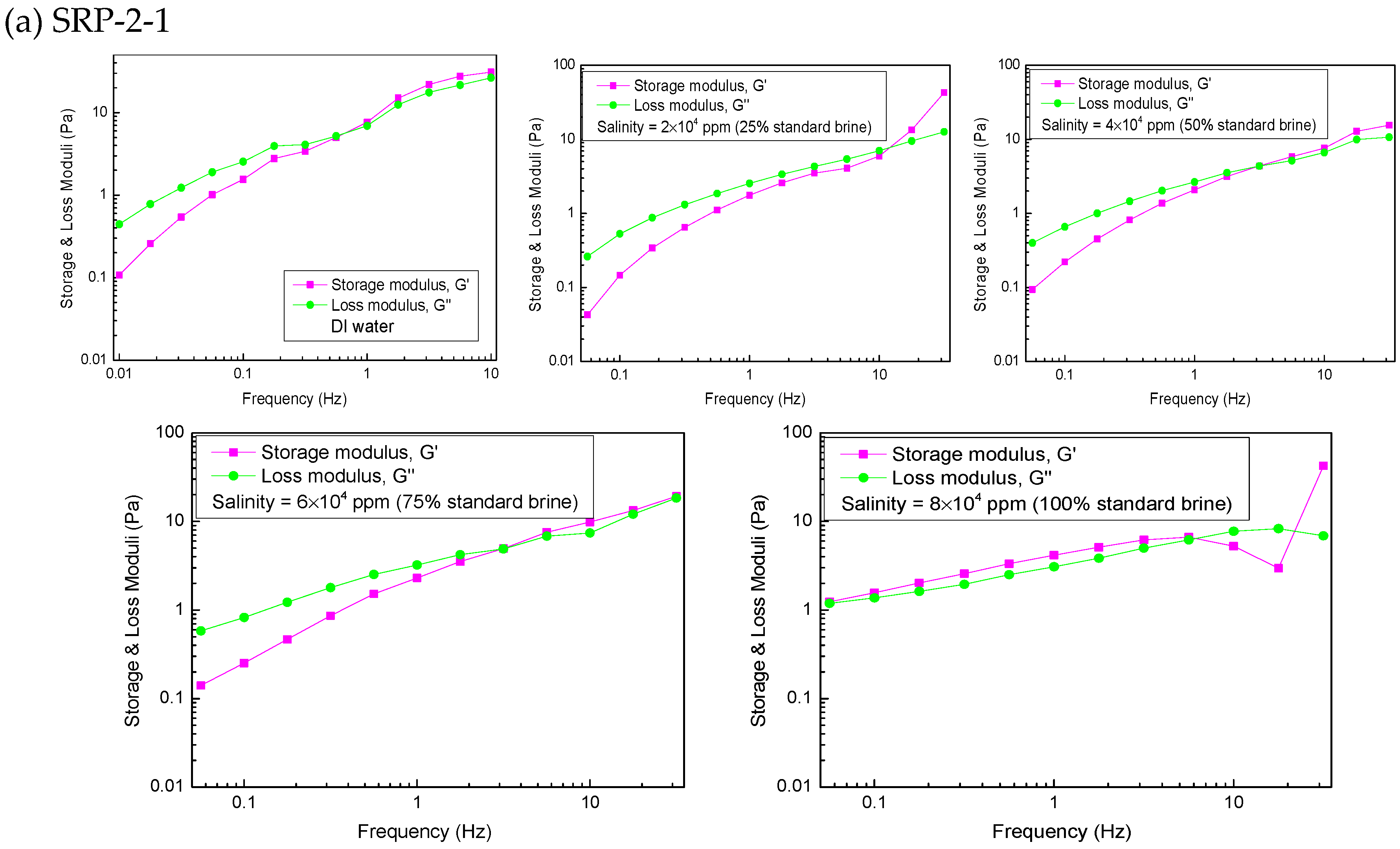
3.6. Viscoelasticity
3.7. Molecular Dimensions of SRP-2-1
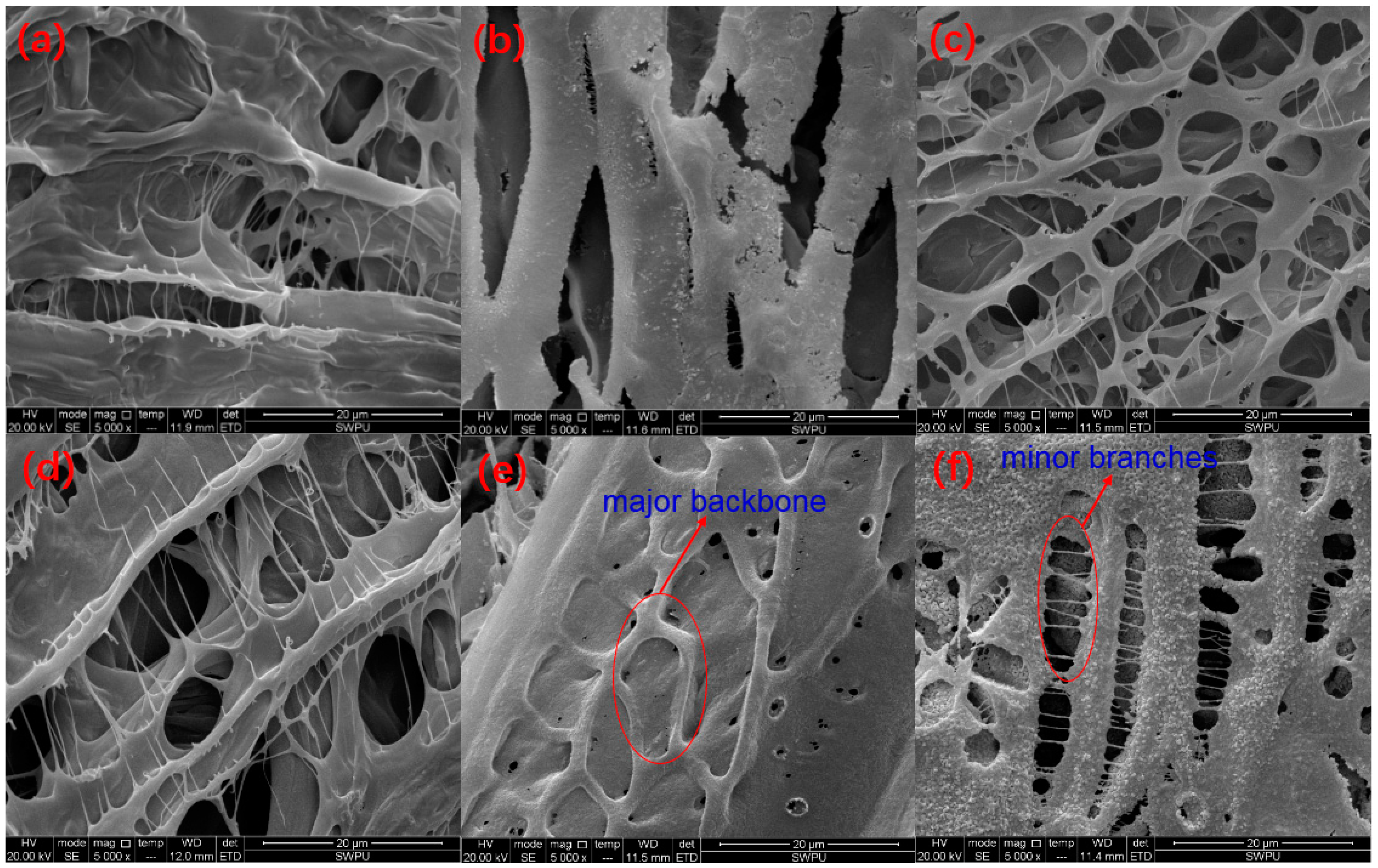
3.8. Microstructure Analysis
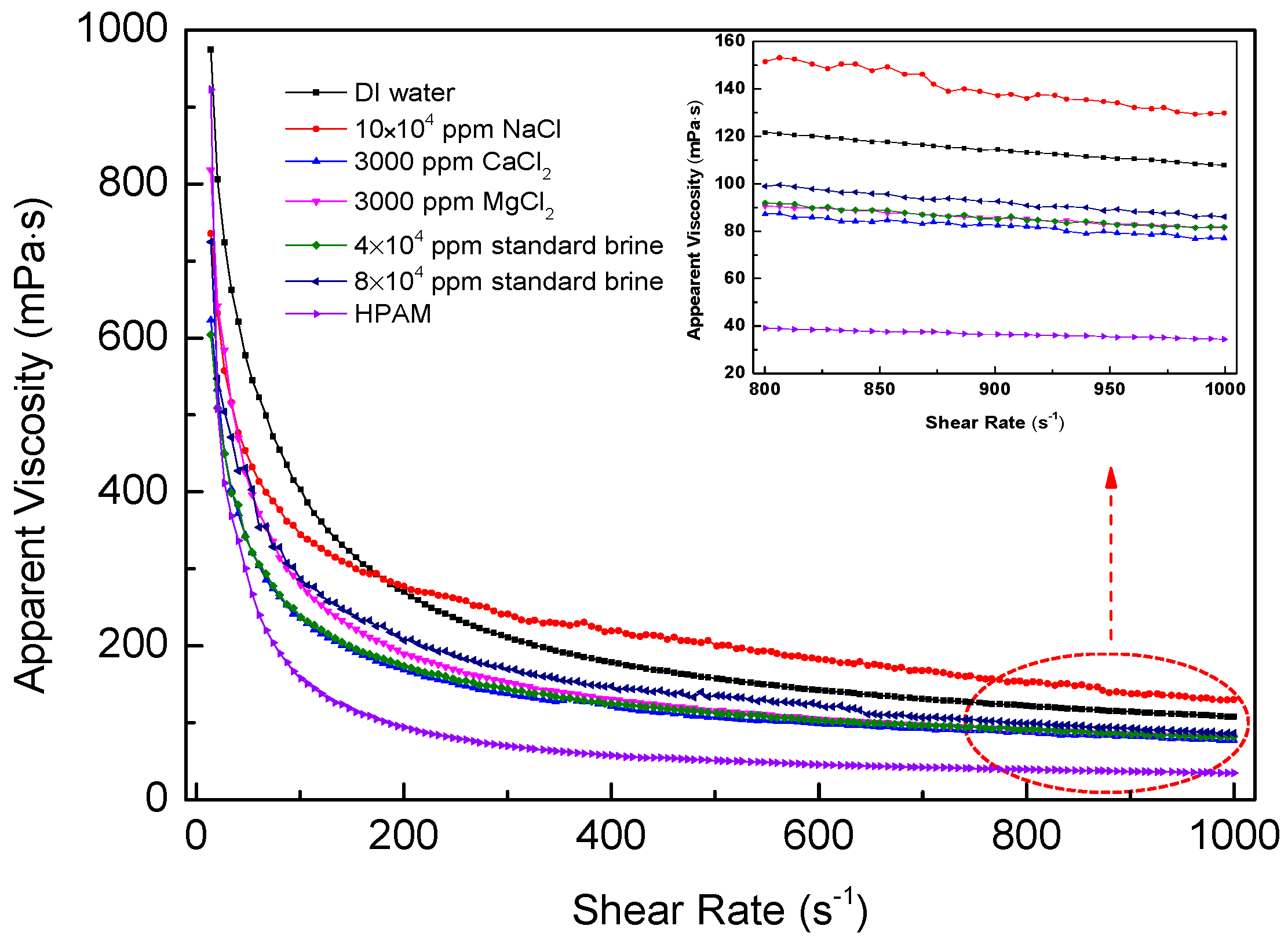
3.9. Shear Tolerance
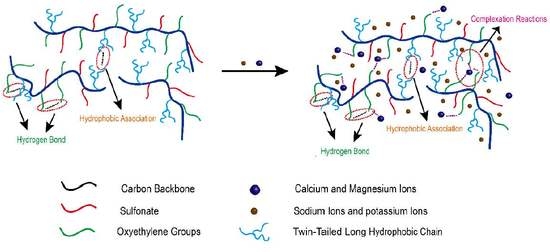
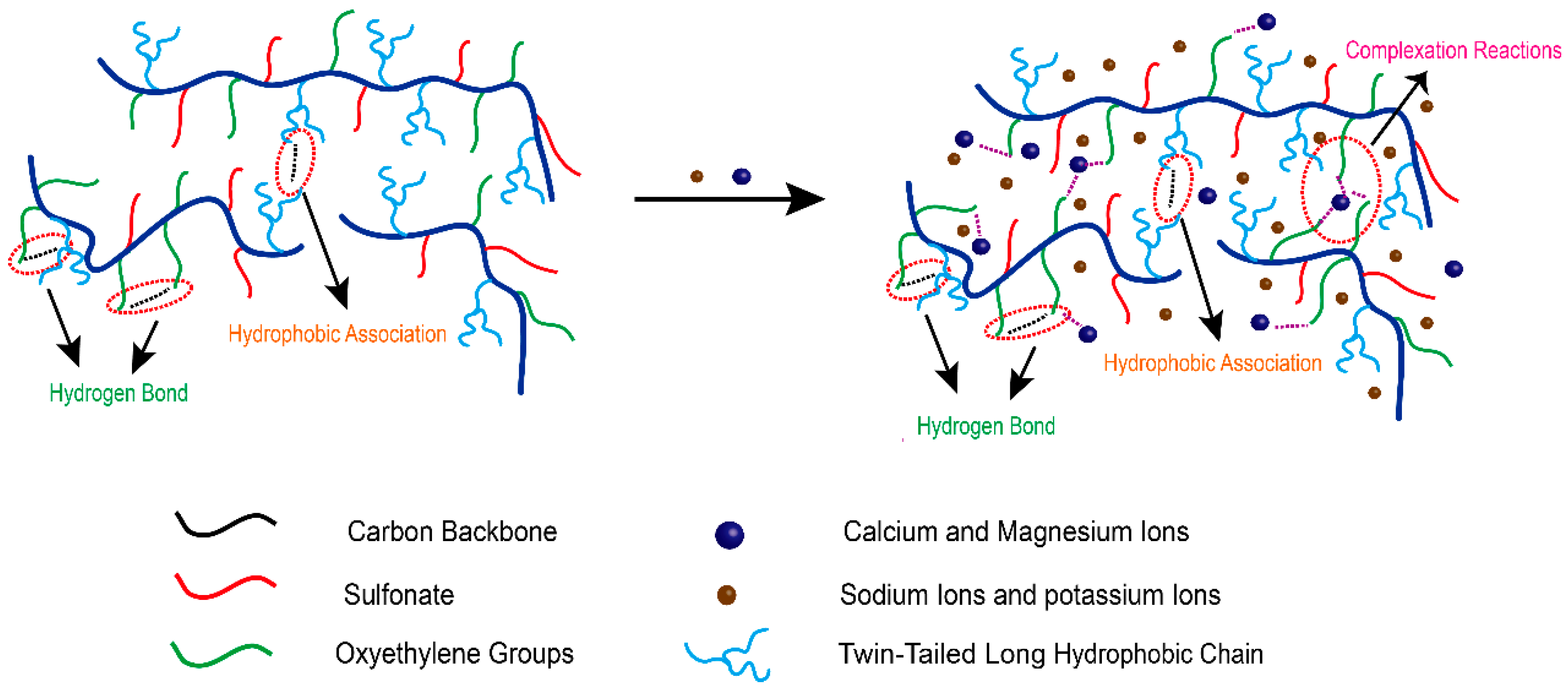
3.10. Salt Tolerance Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wevera, D.A.Z.; Picchionia, F.; Broekhuisa, A.A. Polymers for enhanced oil recovery: A paradigm for structure property relationship in aqueous solution. Prog. Polym. Sci. 2011, 36, 1558–1628. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, J.; Zhang, X.; Feng, R.; Li, H.; Zhang, J.; Lv, X.; Luo, P. Solution property investigation of combination flooding systems consisting of gemini–non-ionic mixed surfactant and hydrophobically associating polyacrylamide for enhanced oil recovery. Energy Fuels 2012, 26, 2116–2123. [Google Scholar]
- Kulawardana, E.; Koh, H.; Kim, D.H.; Liyanage, P.; Upamali, K.; Huh, C.; Weerasooriya, U.; Pope, G.; Kulawardana, E.; Koh, H. Rheology and transport of improved EOR polymers under harsh reservoir conditions. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 14–18 April 2012. [Google Scholar]
- Pu, W.; Liu, R.; Wang, K.; Li, K.; Yan, Z.; Li, B.; Zhao, L. Water-soluble core–shell hyperbranched polymers for enhanced oil recovery. Ind. Eng. Chem. Res. 2015, 54, 798–807. [Google Scholar]
- Godwin Uranta, K.; Rezaei-Gomari, S.; Russell, P.; Hamad, F. Studying the effectiveness of polyacrylamide (pam) application in hydrocarbon reservoirs at different operational conditions. Energies 2018, 11, 2201. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, J.; Zhao, J.; Yang, X.; Zhang, Z.; Yang, B.; Zhang, W.; Zhang, H. Preparation of a novel ultra-high temperature low-damage fracturing fluid system using dynamic crosslinking strategy. Chem. Eng. J. 2018, 354, 913–921. [Google Scholar] [CrossRef]
- Huang, F.; Li, J.; Zhang, C.; Xiao, L.; Tang, J.; Lv, X.; Han, C. The synthes of heatresistant and salttolerant copolymer AMPS/AM/n-penylaleimide. Chin. J. Colloid Polym. 2008, 26, 4–6. [Google Scholar]
- Cao, X.; Liu, K.; Han, Y.; He, D. Synthesis and properties of heat-tolerance dating water-soluble polymer. Pet. Geol. Recovery Effic. 2014, 21, 10–14. [Google Scholar]
- Zhang, Y.; Mao, J.; Xu, T.; Zhang, Z.; Yang, B.; Mao, J.; Yang, X. Preparation of a novel fracturing fluid with good heat and shear resistance. RSC Adv. 2019, 9, 1199–1207. [Google Scholar] [CrossRef]
- Chen, X.; Huang, F. Research development of heat-resistant and salt-tolerant polymer for enhanced oil recovery. Petrochem. Technol. 2009, 38, 1132–1137. [Google Scholar]
- Pu, W.; Jiang, F.; He, Y.; Wei, B.; Tang, Y. Synthesis of a novel comb micro-block hydrophobically associating copolymer for Ca2+/Mg2+ resistance. RSC Adv. 2016, 6, 43634–43637. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, A.; Song, X. Research progress of temperature resistant and salt resistant polymers as flooding agent in tertiary oil recovery. Guangdong Chem. Ind. 2009, 36, 92–94. [Google Scholar]
- Geoffrey, L.; McCormick, C. Water-soluble polymers. 80. rheological and photophysical studies of ph-responsive terpolymers containing hydrophobic twin-tailed acrylamide monomers. Macromolecules 2001, 34, 5579–5586. [Google Scholar]
- Luo, J.; Zhang, Y.; Liu, Y.; Bai, F.; Zhu, H.; Wang, P. Laboratory study on salts tolerant comb-shape polymer with braided side chain for EOR. Oilfield Chem. 2006, 23, 59–62. [Google Scholar]
- Yu, Z.; Xia, Y.; Li, Y. Newest research development of acrylamide based polymer flooding agent with temperature resistance and salt tolerance. Fine Chem. 2012, 29, 59–62. [Google Scholar]
- Peng, B.; Peng, S.; Long, B.; Miao, Y.; Guo, W. Properties of high-temperature-resistant drilling fluids incorporating acrylamide/(acrylic acid)/(2-acrylamido-2-methyl-1-propane sulfonic acid) terpolymer and aluminum citrate as filtration control agents. J. Vinyl Addit. Technol. 2010, 16, 84–89. [Google Scholar] [CrossRef]
- Yin, X. Design, Synthesis and Solution Properties of a New Temperature-Resistant and Salt-Resistant Polymer for Oil and Gas Exploitation; Southwest Petroleum of University: Chengdu, China, 2010. [Google Scholar]
- Li, B. Synthesis and performance evaluation on a new type of salt resistant polymer. J. Yangtze Univ. Nat. Sci. Ed. 2015, 12, 8–10. [Google Scholar]
- Lara-Ceniceros, A.C.; Rivera-Vallejo, C.; Jiménez-Regalado, E.J. Synthesis, characterization and rheological properties of three different associative polymers obtained by micellar polymerization. Polym. Bull. 2007, 58, 425–433. [Google Scholar] [CrossRef]
- Hill, A.; Candau, F.; Selb, J. Properties of hydrophobically associating polyacrylamides: Influence of the method of synthesis. Macromolecules 1993, 26, 4521–4532. [Google Scholar] [CrossRef]
- Ye, Z.; Jiang, J.; Zhang, X.; Chen, H.; Han, L.; Song, J.; Xian, J.; Chen, W. Synthesis and characterizations of hydrophobically associating water-soluble polymer with nonionic surfmer. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Yang, Q. Synthesis of water-soluble high molecular weight polyacrylamide and determination of its viscosity. Adv. Fine Petrochem. 2002, 3, 46–48. [Google Scholar]
- Pu, W.; Yang, Y.; Wei, B.; Yuan, C. Potential of a β-cyclodextrin/adamantane modified copolymer in enhancing oil recovery through host–guest interactions. Ind. Eng. Chem. Res. 2016, 55, 8679–8689. [Google Scholar]
- Wang, W.; Yang, H.; Yan, Y.; Wang, M. Study on characterization of heat-resistant and salt-tolerant polymer pama and properties of its salting solution. Pet. Process. Petrochem. 2014, 45, 31–34. [Google Scholar]
- Mao, J.; Tan, H.; Yang, B.; Zhang, W.; Yang, X.; Zhang, Y.; Zhang, H. Novel hydrophobic associating polymer with good salt tolerance. Polymers 2018, 10, 849. [Google Scholar] [CrossRef]
- Lei, C.; Clark, P.E. Crosslinking of guar and guar derivatives. Spe J. 2007, 12, 316–321. [Google Scholar] [CrossRef]
- Li, Y.; Ren, Q. Synthesis, characterization, and solution properties of a surface-active hydrophobically associating polymer. J. Appl. Polym. Sci. 2018, 135, 46569. [Google Scholar] [CrossRef]
- Mccormick, C.L.; Johnson, C.B. Structurally Tailored Macromolecules for Mobility Control in Enhanced Oil Recovery; Springer: Boston, MA, USA, 1988; pp. 161–180. [Google Scholar]
- Pu, W.; Liu, R.; Peng, Q.; Du, D.; Zhao, Q. Amphiphilically modified chitosan copolymer for enhanced oil recovery in harsh reservoir condition. J. Ind. Eng. Chem. 2016, 37, 216–223. [Google Scholar] [CrossRef]
- Pu, W.; Jiang, F.; Wei, B.; Tang, Y.; He, Y. Influences of structure and multi-intermolecular forces on rheological and oil displacement properties of polymer solutions in the presence of Ca2+/Mg2+. RSC Adv. 2017, 7, 4430–4436. [Google Scholar] [CrossRef]







| Polymers | c (mg/L) | t (s) | t0 (s) | ηr (t/t0) | [η] (dL/g) | Mw |
|---|---|---|---|---|---|---|
| SRP-2-1 | 300 | 141.7 | 113.2 | 1.252 | 7.721 | 345.71 × 104 |
| SRP-2-0 | 250 | 142.2 | 1.256 | 9.416 | 466.91 × 104 | |
| SRP-0-1 | 250 | 138.9 | 1.227 | 8.418 | 394.07 × 104 |
| Copolymers | Apparent Viscosity (mPa·s) | Viscosity Growth Rate | |
|---|---|---|---|
| μ1 (at 1000 mg/L) | μ2 (at 3500 mg/L) | ||
| SRP-2-1 | 11.15 | 42.17 | 278% |
| SRP-0-1 | 14.12 | 42.19 | 199% |
| SRP-2-0 | 20.74 | 56.24 | 171% |
| HPAM | 16.92 | 51.02 | 202% |
| Salinity | 0 | 2 | 4 | 6 | 8 |
|---|---|---|---|---|---|
| Crossover frequency (Hz) | 0.66 | 21.27 | 3.13 | 3.08 | - |
| Longest relaxation time (s) | 1.512 | 0.047 | 0.319 | 0.325 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Mao, J.; Zhao, J.; Yang, X.; Xu, T.; Lin, C.; Mao, J.; Tan, H.; Zhang, Z.; Yang, B.; et al. Preparation of a Hydrophobic-Associating Polymer with Ultra-High Salt Resistance Using Synergistic Effect. Polymers 2019, 11, 626. https://doi.org/10.3390/polym11040626
Zhang Y, Mao J, Zhao J, Yang X, Xu T, Lin C, Mao J, Tan H, Zhang Z, Yang B, et al. Preparation of a Hydrophobic-Associating Polymer with Ultra-High Salt Resistance Using Synergistic Effect. Polymers. 2019; 11(4):626. https://doi.org/10.3390/polym11040626
Chicago/Turabian StyleZhang, Yang, Jincheng Mao, Jinzhou Zhao, Xiaojiang Yang, Tao Xu, Chong Lin, Jinhua Mao, Hongzhong Tan, Zhaoyang Zhang, Bo Yang, and et al. 2019. "Preparation of a Hydrophobic-Associating Polymer with Ultra-High Salt Resistance Using Synergistic Effect" Polymers 11, no. 4: 626. https://doi.org/10.3390/polym11040626