Deprotonation-Induced Conductivity Shift of Polyethylenedioxythiophenes in Aqueous Solutions: The Effects of Side-Chain Length and Polymer Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Measurements
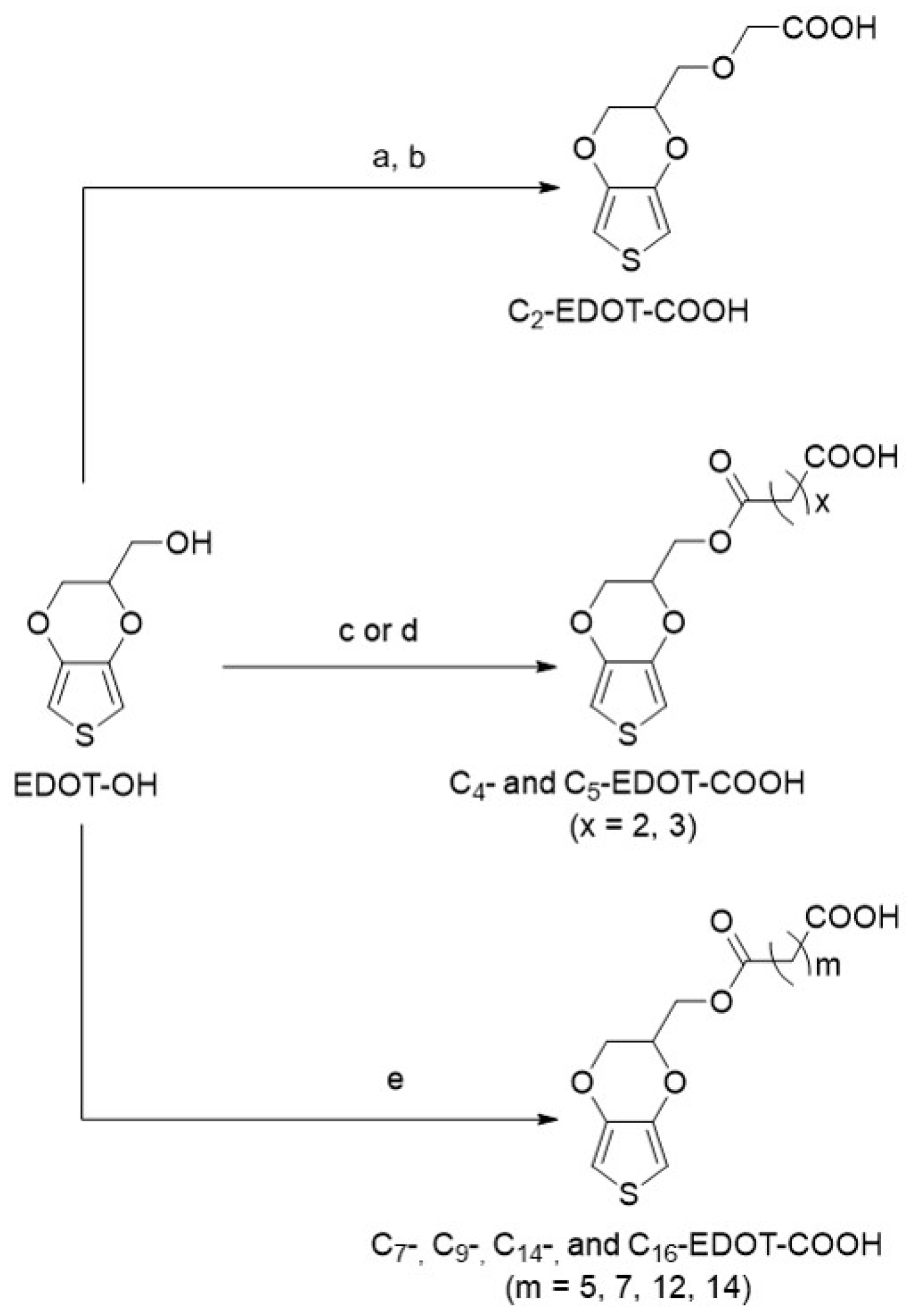
2.2. Synthesis of Cn-EDOT-COOH Monomers
2.2.1. Synthesis of 4-((2,3-dihydrothieno[3,4-b][1,4]dioxin-2-yl)methoxy)-4-oxobutanoic acid (C4-EDOT-COOH) [27]
2.2.2. Typical Synthesis of C7- to C16-EDOT-COOH Monomers
2.2.3. 7-((2,3-dihydrothieno[3,4-b][1,4]dioxin-2-yl)methoxy)-7-oxoheptanoic acid (C7-EDOT-COOH)
2.2.4. 9-((2,3-dihydrothieno[3,4-b][1,4]dioxin-2-yl)methoxy)-9-oxononanoic acid (C9-EDOT-COOH)
2.2.5. 14-((2,3-dihydrothieno[3,4-b][1,4]dioxin-2-yl)methoxy)-14-oxotetradecanoic acid (C14-EDOT-COOH)
2.2.6. 16-((2,3-dihydrothieno[3,4-b][1,4]dioxin-2-yl)methoxy)-16-oxohexadecanoic acid (C16-EDOT-COOH)
2.3. Electrochemical Polymerization of Cn-EDOT-COOHs
2.4. Drain Current Measurement
3. Results and Discussion
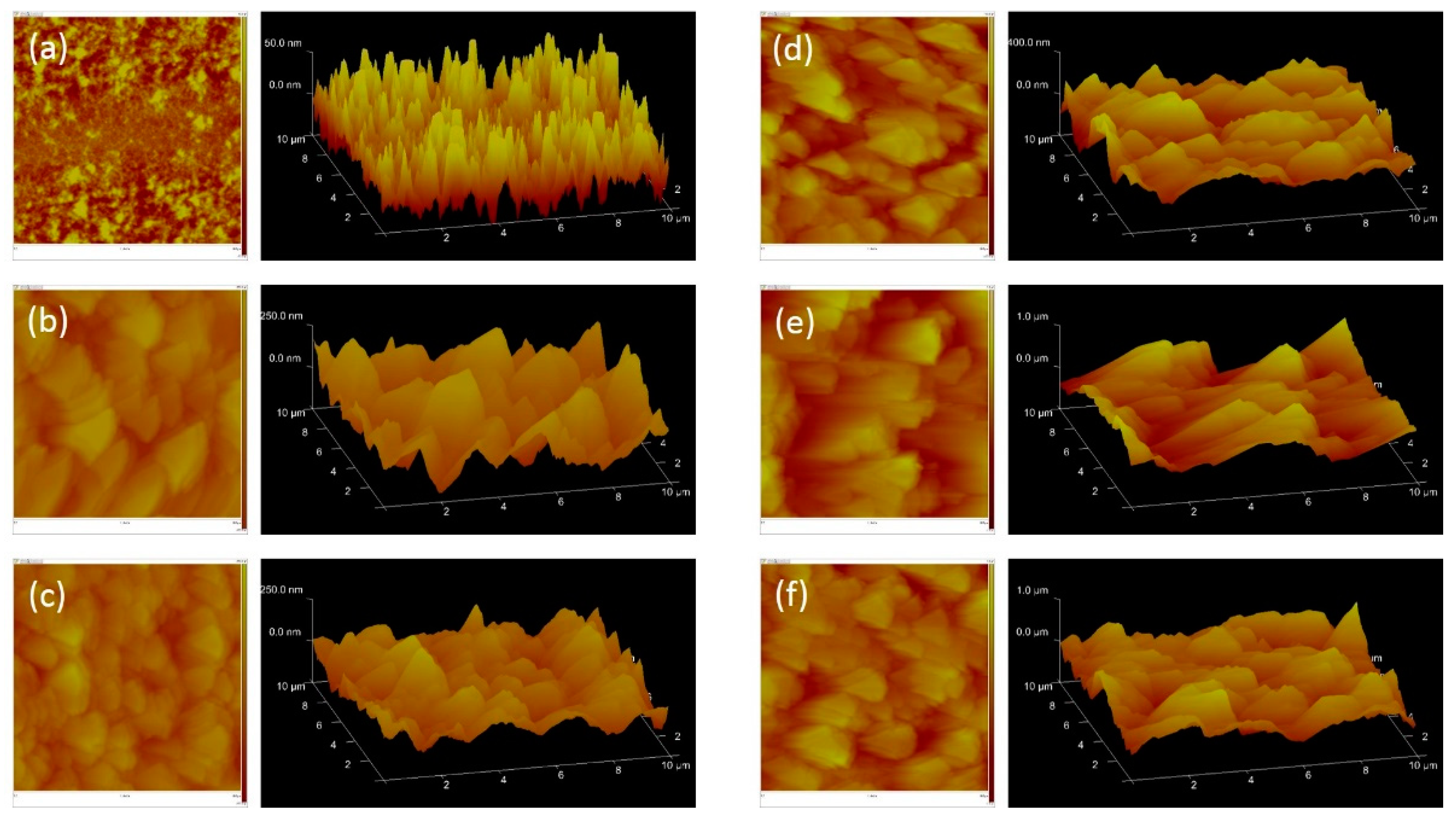
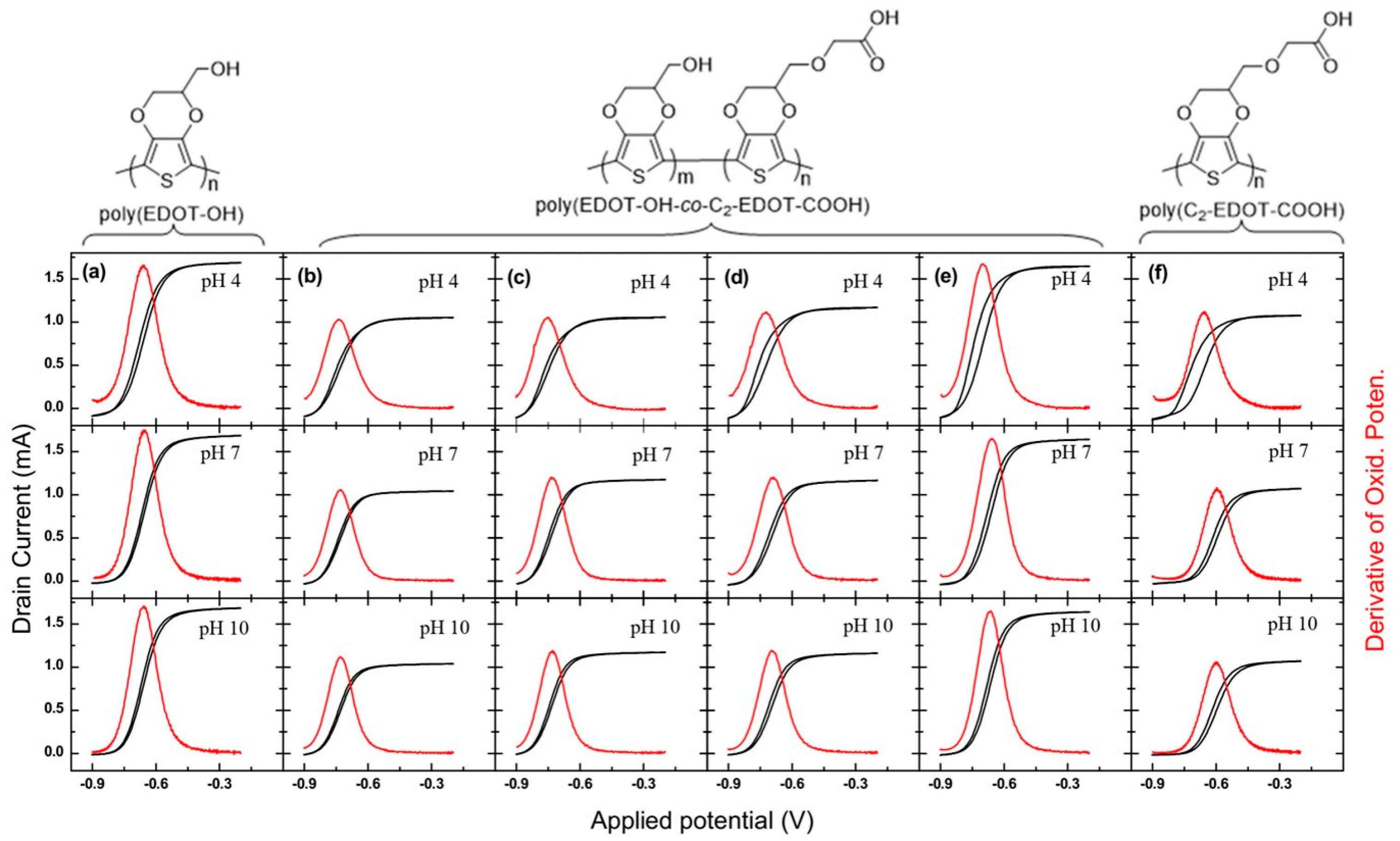
3.1. Synthesis of EDOT-COOH Monomers and Polymers with Different Side-Chain Length
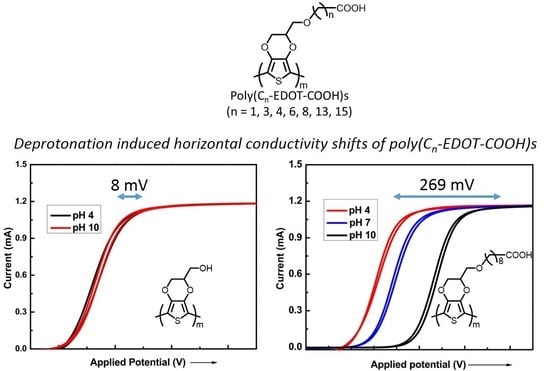
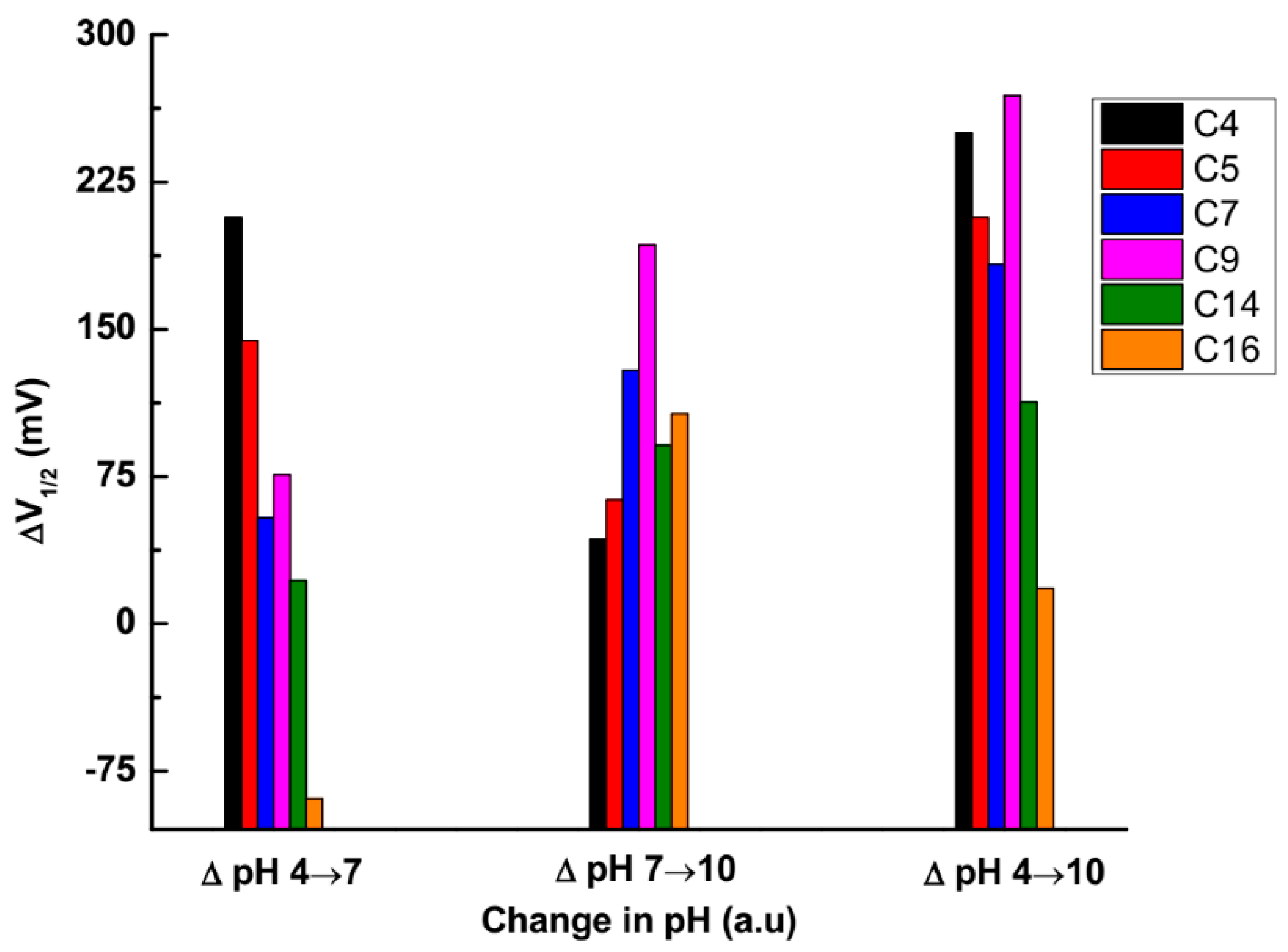
3.2. Effect of Carboxylic Acid Side-Chain Length on the Poly(EDOT-COOH) Conductivity
3.3. The Factors that Affect the Conductivity Shift
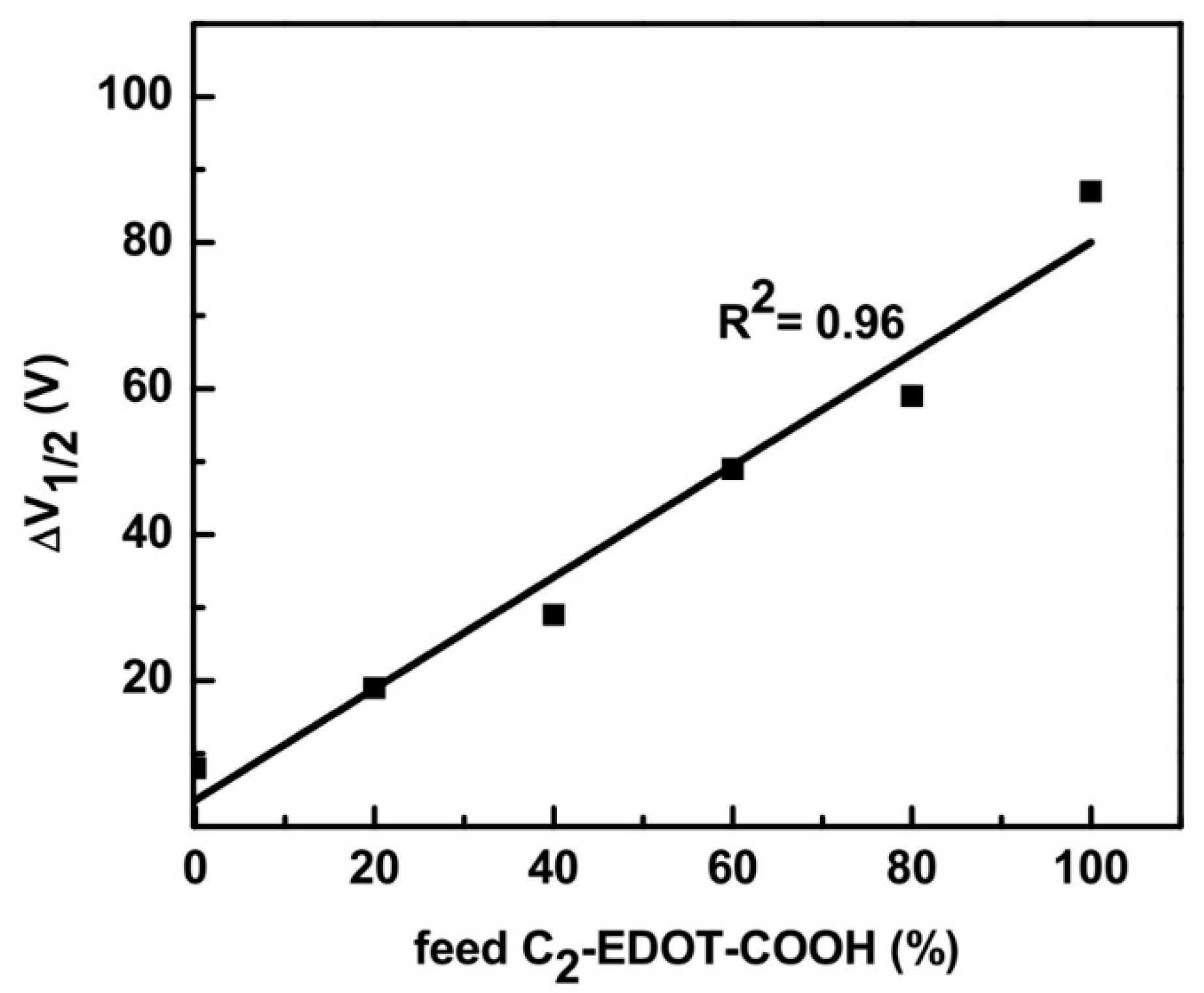
3.4. Compositional Effect on the Copolymer Conductivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jaffrezic-Renault, N.; Dzyadevych, S.V. Conductometric Microbiosensors for Environmental Monitoring. Sensors 2008, 8, 2569–2588. [Google Scholar] [CrossRef] [PubMed]
- Bobacka, J.; Ivaska, A.; Lewenstam, A. Potentiometric Ion Sensors. Chem. Rev. 2008, 108, 329–351. [Google Scholar] [CrossRef] [PubMed]
- Virji, S.; Kaner, R.B.; Weiller, B.H. Hydrogen Sensors Based on Conductivity Changes in Polyaniline Nanofibers. J. Phys. Chem. B 2006, 110, 22266–22270. [Google Scholar] [CrossRef] [PubMed]
- Stetter, J.R.; Li, J. Amperometric Gas Sensors: A Review. Chem. Rev. 2008, 108, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Faridbod, F.; Norouzi, P.; Dinarvand, R.; Ganjali, M.R. Developments in the Field of Conducting and Non-conducting Polymer Based Potentiometric Membrane Sensors for Ions Over the Past Decade. Sensors 2008, 8, 2331–2412. [Google Scholar] [CrossRef] [PubMed]
- Hangarter, C.M.; Bangar, M.; Mulchandani, A.; Myung, N.V. Conducting polymer nanowires for chemiresistive and FET-based bio/chemical sensors. J. Mater. Chem. 2010, 20, 3131–3140. [Google Scholar] [CrossRef]
- Marsella, M.J.; Newland, R.J.; Carroll, P.J.; Swager, T.M. Ionoresistivity as a highly sensitive sensory probe: investigations of polythiophenes functionalized with calix[4]arene-based ion receptors. J. Am. Chem. Soc. 1995, 117, 9842–9848. [Google Scholar] [CrossRef]
- Swager, T.M.; Marsella, M.J. Molecular recognition and chemoresistive materials. Adv. Mater. 1994, 6, 595–597. [Google Scholar] [CrossRef]
- Groenendaal, L.; Zotti, G.; Aubert, P.-H.; Waybright, S.M.; Reynolds, J.R. Electrochemistry of Poly(3,4-alkylenedioxythiophene) Derivatives. Adv. Mater. 2003, 15, 855–879. [Google Scholar] [CrossRef]
- Jang, J.; Chang, M.; Yoon, H. Chemical Sensors Based on Highly Conductive Poly(3,4-ethylenedioxythiophene) Nanorods. Adv. Mater. 2005, 17, 1616–1620. [Google Scholar] [CrossRef]
- Louwet, F.; Groenendaal, L.; D’Haen, J.; Manca, J.; Van Luppen, J.; Verdonck, E.; Leenders, L. PEDOT/PSS: synthesis, characterization, properties and applications. Synth. Met. 2003, 135, 115–117. [Google Scholar] [CrossRef]
- Borges-González, J.; Kousseff, C.J.; Nielsen, C.B. Organic semiconductors for biological sensing. J. Mater. Chem. C 2019, 7, 1111–1130. [Google Scholar] [CrossRef]
- Gualandi, I.; Marzocchi, M.; Scavetta, E.; Calienni, M.; Bonfiglio, A.; Fraboni, B. A simple all-PEDOT:PSS electrochemical transistor for ascorbic acid sensing. J. Mater. Chem. B 2015, 3, 6753–6762. [Google Scholar] [CrossRef]
- Zhang, L.; Wen, Y.; Yao, Y.; Xu, J.; Duan, X.; Zhang, G. Synthesis and Characterization of PEDOT Derivative with Carboxyl Group and Its Chemo/Bio Sensing Application as Nanocomposite, Immobilized Biological and Enhanced Optical Materials. Electrochimica Acta 2014, 116, 343–354. [Google Scholar] [CrossRef]
- Xie, H.; Luo, S.-C.; Yu, H.-h. Electric-Field-Assisted Growth of Functionalized Poly(3,4-ethylenedioxythiophene) Nanowires for Label-Free Protein Detection. Small 2009, 5, 2611–2617. [Google Scholar] [CrossRef]
- Luo, S.-C.; Xie, H.; Chen, N.; Yu, H.-h. Trinity DNA Detection Platform by Ultrasmooth and Functionalized PEDOT Biointerfaces. ACS Appl. Mater. Interfaces 2009, 1, 1414–1419. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.-C.; Ali, E.M.; Tansil, N.C.; Yu, H.-h.; Gao, S.; Kantchev, E.A.B.; Ying, J.Y. Poly(3,4-ethylenedioxythiophene) (PEDOT) Nanobiointerfaces: Thin, Ultrasmooth, and Functionalized PEDOT Films with in Vitro and in Vivo Biocompatibility. Langmuir 2008, 24, 8071–8077. [Google Scholar] [CrossRef] [PubMed]
- Povlich, L.K.; Cho, J.C.; Leach, M.K.; Corey, J.M.; Kim, J.; Martin, D.C. Synthesis, copolymerization and peptide-modification of carboxylic acid-functionalized 3,4-ethylenedioxythiophene (EDOTacid) for neural electrode interfaces. Biochim. et Biophys. (BBA) Gen. Subj. 2013, 1830, 4288–4293. [Google Scholar] [CrossRef]
- Penner, R.M. Chemical Sensing with Nanowires. Annu. Rev. Anal. Chem. 2012, 5, 461–485. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H. Current Trends in Sensors Based on Conducting Polymer Nanomaterials. Nanomaterials 2013, 3, 524–549. [Google Scholar] [CrossRef] [PubMed]
- Le, T.-H.; Kim, Y.; Yoon, H. Electrical and Electrochemical Properties of Conducting Polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef]
- Chandran, G.T.; Li, X.; Ogata, A.; Penner, R.M. Electrically Transduced Sensors Based on Nanomaterials (2012–2016). Anal. Chem. 2016, 89, 249–275. [Google Scholar] [CrossRef]
- Kwon, O.S.; Park, S.J.; Lee, J.S.; Park, E.; Kim, T.; Park, H.-W.; You, S.A.; Yoon, H.; Jang, J. Multidimensional Conducting Polymer Nanotubes for Ultrasensitive Chemical Nerve Agent Sensing. Nano Lett. 2012, 12, 2797–2802. [Google Scholar] [CrossRef]
- Chen, C.-H.; Luo, S.-C. Tuning Surface Charge and Morphology for the Efficient Detection of Dopamine under the Interferences of Uric Acid, Ascorbic Acid, and Protein Adsorption. ACS Appl. Mater. Interfaces 2015, 7, 21931–21938. [Google Scholar] [CrossRef]
- Sekine, J.; Luo, S.-C.; Wang, S.; Zhu, B.; Tseng, H.-R.; Yu, H.-h. Functionalized Conducting Polymer Nanodots for Enhanced Cell Capturing: The Synergistic Effect of Capture Agents and Nanostructures. Adv. Mater. 2011, 23, 4788–4792. [Google Scholar] [CrossRef]
- Lin, H.-A.; Luo, S.-C.; Zhu, B.; Chen, C.; Yamashita, Y.; Yu, H.-h. Molecular or Nanoscale Structures? The Deciding Factor of Surface Properties on Functionalized Poly(3,4-ethylenedioxythiophene) Nanorod Arrays. Adv. Funct. Mater. 2013, 23, 3212–3219. [Google Scholar] [CrossRef]
- Ali, E.M.; Kantchev, E.A.B.; Yu, H.-h.; Ying, J.Y. Conductivity Shift of Polyethylenedioxythiophenes in Aqueous Solutions from Side-Chain Charge Perturbation. Macromolecules 2007, 40, 6025–6027. [Google Scholar] [CrossRef]
- Ayalew, H.; Wang, T.-L.; Wang, T.-H.; Hsu, H.-F.; Yu, H.-h. Direct C–H Arylation Polymerization to form Anionic Water-Soluble Poly(3,4-ethylenedioxythiophenes) with Higher Yields and Molecular Weights. Synlett 2018, 29, 2660–2668. [Google Scholar]
- Lima, A.; Schottland, P.; Sadki, S.; Chevrot, C. Electropolymerization of 3,4-ethylenedioxythiophene and 3,4-ethylenedioxythiophene methanol in the presence of dodecylbenzenesulfonate. Synth. Met. 1998, 93, 33–41. [Google Scholar] [CrossRef]
- Lee, D.; Swager, T.M. Defining Space around Conducting Polymers: Reversible Protonic Doping of a Canopied Polypyrrole. J. Am. Chem. Soc. 2003, 125, 6870–6871. [Google Scholar] [CrossRef]
- Lee, D.; Swager, T.M. Toward Isolated Molecular Wires: A pH-Responsive Canopied Polypyrrole. Chem. Mater. 2005, 17, 4622–4629. [Google Scholar] [CrossRef]
- MacDiarmid, A.G.; Chiang, J.C.; Richter, A.F.; Epstein, A.J. Polyaniline: a new concept in conducting polymers. Synth. Met. 1987, 18, 285–290. [Google Scholar] [CrossRef]
- Yu, H.-h.; Xu, B.; Swager, T.M. A Proton-Doped Calix[4]arene-Based Conducting Polymer. J. Am. Chem. Soc. 2003, 125, 1142–1143. [Google Scholar] [CrossRef]
- Kanicky, J.R.; Poniatowski, A.F.; Mehta, N.R.; Shah, D.O. Cooperativity among Molecules at Interfaces in Relation to Various Technological Processes: Effect of Chain Length on the pKa of Fatty Acid Salt Solutions. Langmuir 2000, 16, 172–177. [Google Scholar] [CrossRef]
- Zotti, G.; Zecchin, S.; Schiavon, G.; Groenendaal, L.B. Electrochemical and chemical synthesis and characterization of sulfonated poly(3,4-ethylenedioxythiophene): A novel water-soluble and highly conductive conjugated oligomer. Macromol. Chem. Physic. 2002, 203, 1958–1964. [Google Scholar] [CrossRef]
- Kim, B.S.; Chen, L.; Gong, J.; Osada, Y. Titration Behavior and Spectral Transitions of Water-Soluble Polythiophene Carboxylic Acids. Macromolecules 1999, 32, 3964–3969. [Google Scholar] [CrossRef]
- Leclerc, M.; Guay, J.; Dao, L.H. Synthesis and characterization of poly(alkylanilines). Macromolecules 1989, 22, 649–653. [Google Scholar] [CrossRef]
- Öztemiz, S.; Beaucage, G.; Ceylan, O.; Mark, H.B.J. Synthesis, characterization and molecular weight studies of certain soluble poly(3-alkylthiophene) conducting polymers. J. Electrochem. 2004, 8, 928–931. [Google Scholar] [CrossRef]
- Tekbaşoğlu, T.Y.; Soganci, T.; Ak, M.; Koca, A.; Şener, M.K. Enhancing biosensor properties of conducting polymers via copolymerization: Synthesis of EDOT-substituted bis(2-pyridylimino)isoindolato-palladium complex and electrochemical sensing of glucose by its copolymerized film. Biosens. Bioelectron. 2017, 87, 81–88. [Google Scholar] [CrossRef]
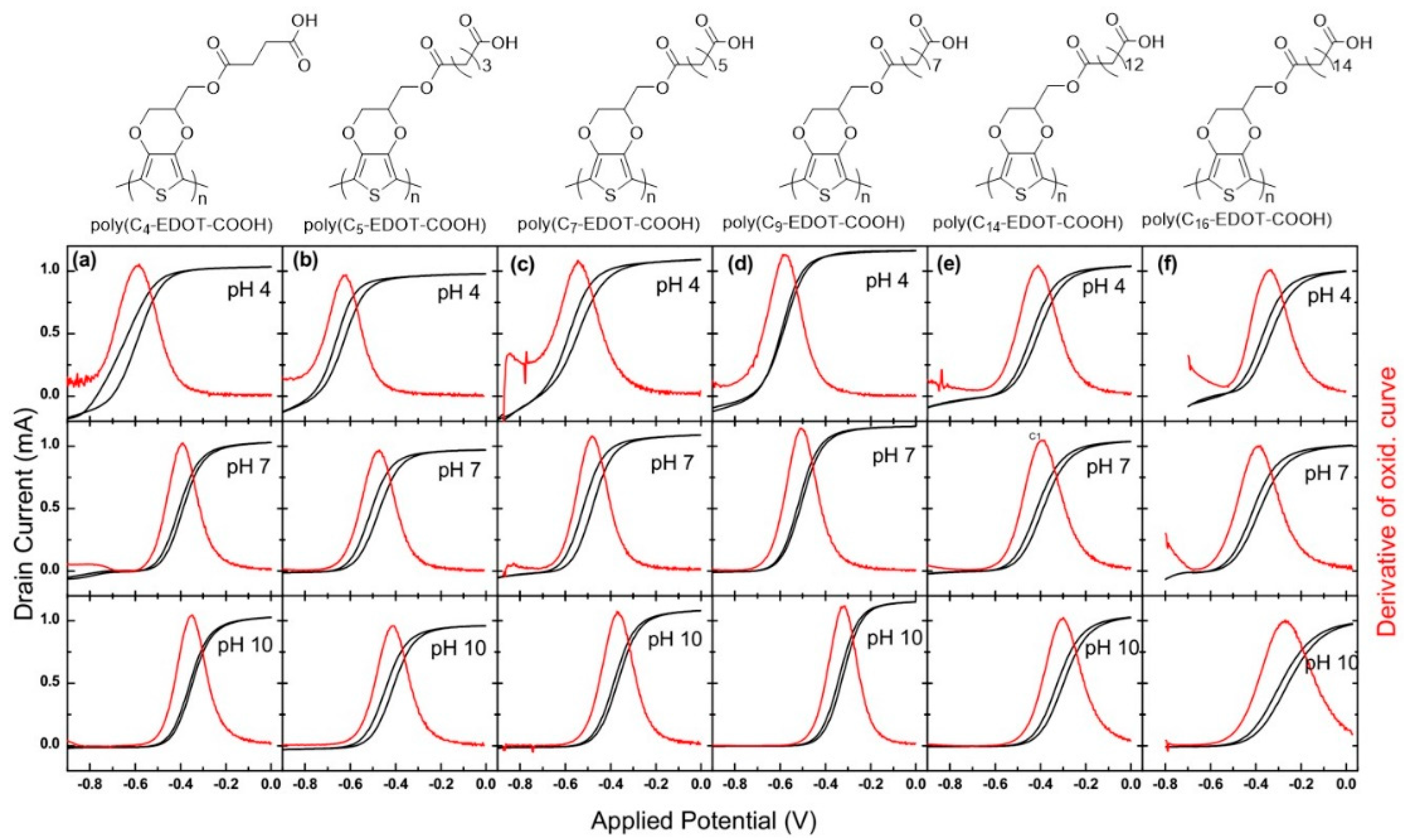

| Poly(Cn-EDOT-COOH) | Eonset (V) a | ΔV (V) | ||||
|---|---|---|---|---|---|---|
| pH 4 | pH 7 | pH 10 | ΔV4→7 | ΔV7→10 | ΔV4→10 | |
| poly(C4-EDOT-COOH) | −0.606 | −0.399 | −0.356 | 0.207 | 0.043 | 0.250 |
| poly(C5-EDOT-COOH) | −0.635 | −0.491 | −0.428 | 0.144 | 0.063 | 0.207 |
| poly(C7-EDOT-COOH) | −0.557 | −0.503 | −0.374 | 0.054 | 0.129 | 0.183 |
| poly(C9-EDOT-COOH) | −0.594 | −0.518 | −0.325 | 0.076 | 0.193 | 0.269 |
| poly(C14-EDOT-COOH) | −0.425 | −0.403 | −0.312 | 0.022 | 0.091 | 0.113 |
| poly(C16-EDOT-COOH) | −0.331 | −0.420 | −0.313 | −0.089 | 0.107 | 0.018 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayalew, H.; Wang, T.-l.; Yu, H.-h. Deprotonation-Induced Conductivity Shift of Polyethylenedioxythiophenes in Aqueous Solutions: The Effects of Side-Chain Length and Polymer Composition. Polymers 2019, 11, 659. https://doi.org/10.3390/polym11040659
Ayalew H, Wang T-l, Yu H-h. Deprotonation-Induced Conductivity Shift of Polyethylenedioxythiophenes in Aqueous Solutions: The Effects of Side-Chain Length and Polymer Composition. Polymers. 2019; 11(4):659. https://doi.org/10.3390/polym11040659
Chicago/Turabian StyleAyalew, Hailemichael, Tian-lin Wang, and Hsiao-hua Yu. 2019. "Deprotonation-Induced Conductivity Shift of Polyethylenedioxythiophenes in Aqueous Solutions: The Effects of Side-Chain Length and Polymer Composition" Polymers 11, no. 4: 659. https://doi.org/10.3390/polym11040659