Effect of Resin and Blocked/Unblocked Hardener Mixture on the Production of Epoxy Foams with CO2 Blocked Hardener in Batch Foaming Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
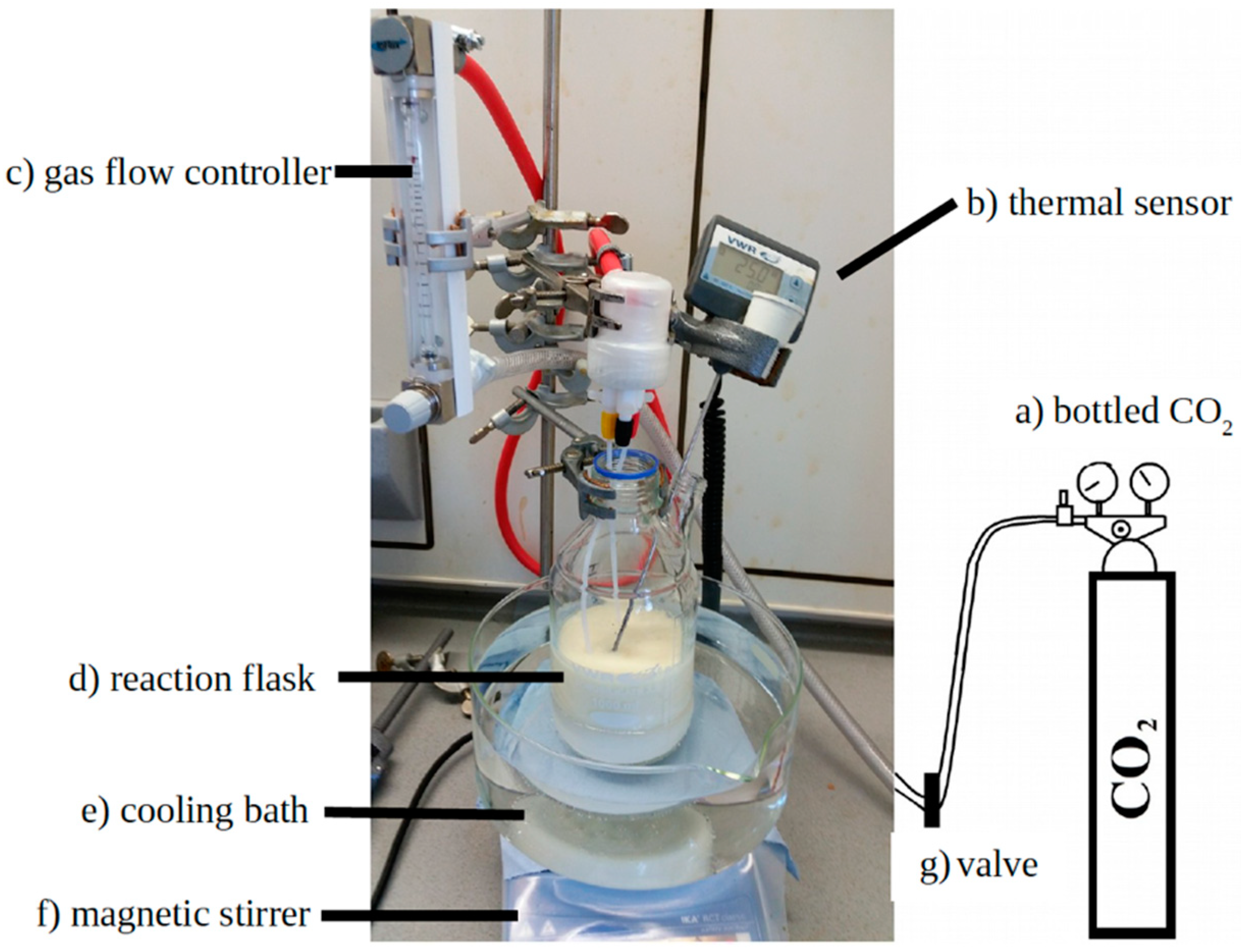
2.2. Procedures
2.2.1. Blending of Resins and Hardeners
2.2.2. Foaming
2.2.3. Characterization
3. Results and Discussion
3.1. Pre-Foaming Experiments
3.1.1. Unblocking Behavior of B-AEP and Decomposition Behavior of B-AEP and TSH
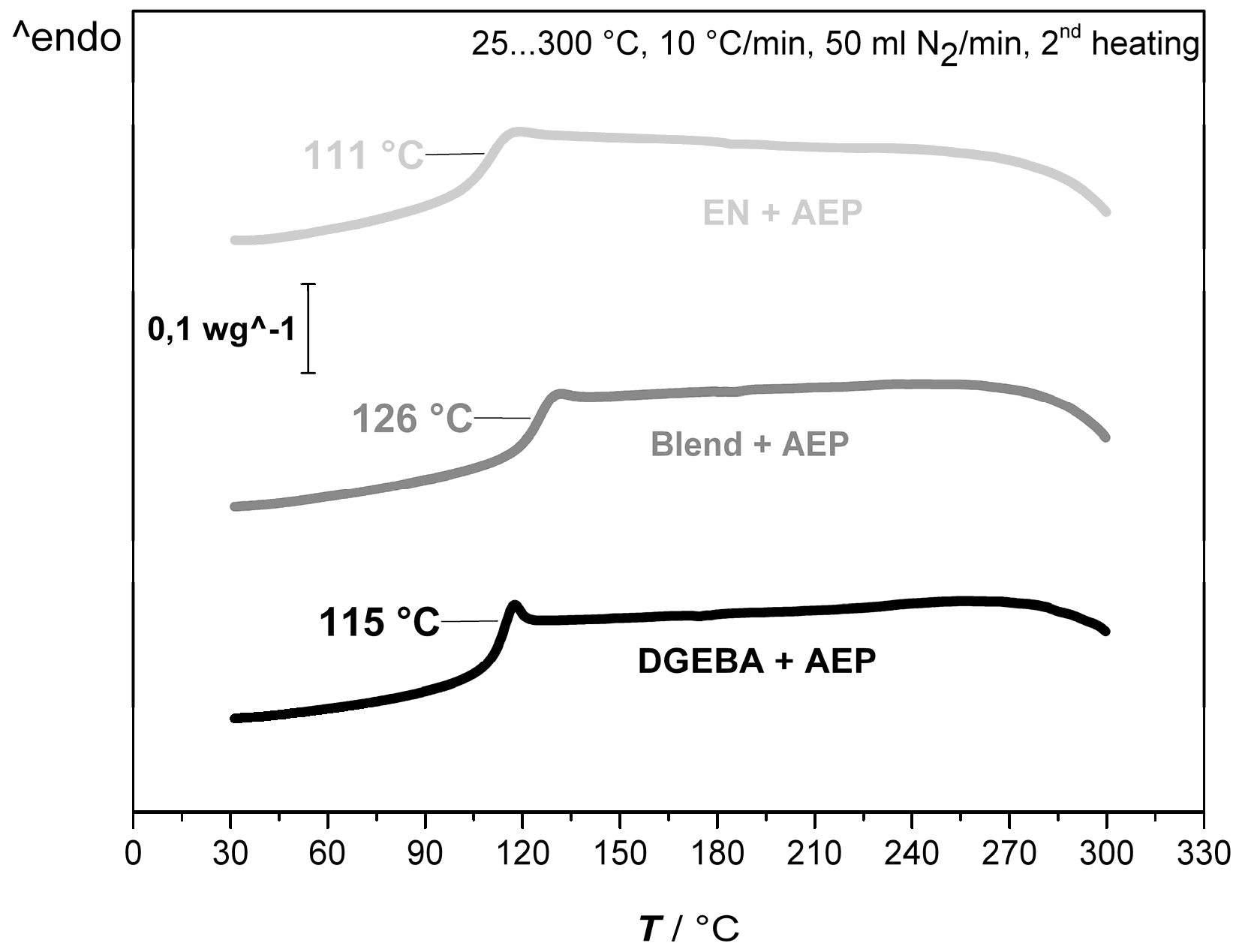
3.1.2. Effect of Blocked and Unblocked Blowing Agent on the Curing Enthalpy and Thermal Properties Relevant for the Foaming Process
3.1.3. Rheological Behavior of Blending of DGEBA and EN and their Blend
3.1.4. Hardener Mixture
3.2. Foaming Experiments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
B-AEP Synthesis and Characterization

References
- Kurek, K.; Bledzki, A. Fatigue Behavior of Composites with Foamed Matrix. J. Reinf. Plast. Compos. 1994, 13, 1116–1134. [Google Scholar] [CrossRef]
- Pascalut, J.-P.; Williams, R.J.J. Epoxy Polymers, New Materials and Innovations; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; ISBN 978-3-527-32480-4. [Google Scholar]
- Takiguchi, O.; Ishikawa, D.; Sugimoto, M. Effect of Rheological Behavior of Epoxy during Precuring on Foaming. J. Appl. Polym. Sci. 2008, 110, 657–662. [Google Scholar] [CrossRef]
- Ren, Q.; Zhu, S. One-Pack Epoxy Foaming with CO2 as Latent Blowing Agent. ACS Macro Lett. 2015, 4, 693–697. [Google Scholar] [CrossRef]
- Kurek, K.; Bledzki, A. Mechanical Behavior of Polyurethane—And Epoxy Foams and Their Glass Fiber Composites. Mech. Compos. Mater. 1994, 30, 105–109. [Google Scholar] [CrossRef]
- Hajimichael, M.; Lewis, A.; Scholey, D. Investigation and Development of Epoxy Foams. Br. Polym. J. 1986, 18, 307–311. [Google Scholar] [CrossRef]
- Kuhlkamp, A.; Mauz, O.; Göwecke, S. Process for Making Epoxy Resin Foam Plastics. U.S. Patent US3406131, 15 October 1968. [Google Scholar]
- Fauzi, M.S.; Lan, D.N.U.; Osman, H.; Ghani, S.A. Effect of Sodium Bicarbonate as Blowing Agent on Production of Epoxy Shape Memory Foam using Aqueous Processing Method. Sains Malays. 2015, 44, 869–874. [Google Scholar] [CrossRef]
- Lyu, J.; Liu, T.; Xi, Z. Effect of Pre-Curing Process on Epoxy Resin Foaming Using Carbon Dioxide as Blowing Agent. J. Cell. Plast. 2017, 53, 181–197. [Google Scholar] [CrossRef]
- Alonso, M.V.; Auad, M.L.; Nutt, S. Short-Fiber-Reinforced Epoxy Foams. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1952–1960. [Google Scholar] [CrossRef]
- Gazzani, S.E.; Nassiet, V.; Habas, J. High Temperature Epoxy Foam: Optimization of Process Parameters. Polymers 2016, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N. A Functionally Graded Syntactic Foam Material for High Energy Absorption under Compression. Mater. Lett. 2007, 61, 979–982. [Google Scholar] [CrossRef]
- Caeti, R.; Gupta, N.; Porfiri, M. Processing and Compressive Response of Functionally Graded Composites. Mater. Lett. 2009, 63, 1964–1967. [Google Scholar] [CrossRef]
- Doddamani, M.; Kishore; Shunmugasamy, V.C.; Gupta, N.; Vijayakumar, H.B. Compressive and Flexural Properties of Functionally Graded Fly Ash Cenosphere—Epoxy Resin Syntactic Foams. Polym. Compos. 2015, 36, 685–693. [Google Scholar] [CrossRef]
- Klempner, D.; Frisch, K.C. Handbook of Polymeric Foams and Foam Technology; Carl Hanser Verlag Munich Vienna: New York, NY, USA; Barcelona, Spain, 1991; ISBN 3-446-15097-8. [Google Scholar]
- Dong, Y.; Fu, Y.; Ni, Q.-Q. In-Situ Grown Silica/Water-Borne Epoxy Shape Memory Composite Foams Prepared without Blowing Agent Addition. J. Appl. Polym. Sci. 2015, 132, 1–11. [Google Scholar] [CrossRef]
- Bakar, A.A.; Lan, D.N.U.; Azahari, B.; Ariff, Z.M. Production of Novel Epoxy Micro-Balloons. Mater. Lett. 2009, 63, 827–829. [Google Scholar] [CrossRef]
- Significant New Alternatives Policy (SNAP). Available online: https://www.epa.gov/snap/foam-blowing-agents (accessed on 4 April 2019).
- Ren, Q.; Zhu, S. Development of Epoxy Foaming with CO2 as Latent Blowing Agent and Principle in Selection of Amine Curing Agent. Ind. Eng. Chem. Res. 2015, 54, 11056–11064. [Google Scholar] [CrossRef]
- Barrisu, E.; Bankmann, D.; Lammerschop, O.; Renkel, M.; Wucherpfennig, S.; Braun, K. Use of Low-Temperature Foamable Epoxide Resins in Hollow Chamber Structures. E.U. Patent EP2473559A1, 11 July 2012. [Google Scholar]
- Weller, E.E.; Plagains, J. Adhesive Composition Comprising an Epoxy Resin, an Amine Carbamate and Water. U.S. Patent US3275587, 27 July 1966. [Google Scholar]
- Information Taken from Safety Data Sheet, BASF SE. Available online: https://www.basf.com (accessed on 15 December 2016).
- Information Taken from Product Information Sheet, Dow Chemical Company. Available online: https://de.dow.com/de-de (accessed on 19 October 2015).
- Oberbach, K.; Baur, E.; Brinkmann, S.; Schmachtenberg, E. Saechtling Kunststoff Taschenbuch, 29th ed.; Carl Hanser Verlag: München, Germary, 2004; ISBN 3-446-22670-2. [Google Scholar]
- Information Taken from Safety Data Sheet, Sigma-Aldrich. Available online: www.sigmaaldrich.com (accessed on 22 March 2018).
- Brostow, W.; Chiu, R.; Kalogeras, L.M.; Vassilikou-Dova, A. Prediction of Glass Transition Temperatures: Binary Blends and Copolymers. Mater. Lett. 2008, 62, 3152–3155. [Google Scholar] [CrossRef]
- Costeux, S.; Khan, I.; Bunker, S.P. Experimental Study and Modeling of Nanofoams Formation from Single Phase Acrylic Copolymers. J. Cell. Plast. 2014, 51, 197–221. [Google Scholar] [CrossRef]









| Formulation | Resin | Hardener | Blocked Hardener | Blowing Agent | Amount [g] | Density [kg/m3] |
|---|---|---|---|---|---|---|
| b-DGEBA | DGEBA | B-AEP | 3.2 | 215 ± 13 | ||
| m1-DGEBA | DGEBA | AEP (20%) | B-AEP (80%) | 4.0 | 357 ± 22 | |
| m2-DGEBA | DGEBA | AEP (50%) | B-AEP (50%) | 5.1 | 496 ± 49 | |
| DGEBA-TSH | DGEBA | AEP (100%) | - | TSH (10 wt.%) | 3.5 | 331 ± 40 |
| b-Blend | DGEBA:EN 50:50 | B-AEP | 3.2 | 273 ± 16 | ||
| m1-Blend | DGEBA:EN 50:50 | AEP (20%) | B-AEP (80%) | 4.0 | 403 ± 23 | |
| Blend-TSH | DGEBA:EN 50:50 | AEP (100%) | - | TSH (10 wt.%) | 3.6 | 335 ± 19 |
| b-EN | EN | B-AEP | 3.5 | 357 ± 43 | ||
| m3-EN | EN | AEP (15%) | B-AEP (85%) | 4.0 | 394 ± 13 | |
| EN-TSH | EN | AEP (100%) | - | TSH (10 wt.%) | 4.4 | 459 ± 46 |
| Formulation | Peak [°C] | ∆H (J/g) | Tg [°C] |
|---|---|---|---|
| AEP + DGEBA | 93 | −501 | 115 |
| b-DGEBA | 133/155 | −208 | 104 |
| m1-DGEBA | 128/160 | −238 | 99 |
| m2-DGEBA | 100/160 | −282 | 106 |
| DGEBA-TSH | 84 | −375 | 98 |
| AEP + Blend 50:50 | 90 | −482 | 126 |
| b-Blend | 132/160 | −127 | 117 |
| m1-Blend | 124/158 | −168 | 106 |
| Blend-TSH | 82 | −403 | 107 |
| AEP + EN | 86 | −417 | 111 |
| b-EN | 130/164 | −200 | 121 |
| m3-EN | 118/155 | −201 | 121 |
| EN-TSH | 77 | −334 | 121 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bethke, C.; Sanchez-Vazquez, S.A.; Raps, D.; Bakis, G.; Bard, S.; Du Ngoc, U.L.; Volker, A. Effect of Resin and Blocked/Unblocked Hardener Mixture on the Production of Epoxy Foams with CO2 Blocked Hardener in Batch Foaming Process. Polymers 2019, 11, 793. https://doi.org/10.3390/polym11050793
Bethke C, Sanchez-Vazquez SA, Raps D, Bakis G, Bard S, Du Ngoc UL, Volker A. Effect of Resin and Blocked/Unblocked Hardener Mixture on the Production of Epoxy Foams with CO2 Blocked Hardener in Batch Foaming Process. Polymers. 2019; 11(5):793. https://doi.org/10.3390/polym11050793
Chicago/Turabian StyleBethke, Christian, Sandra A. Sanchez-Vazquez, Daniel Raps, Gökhan Bakis, Simon Bard, Uy Lan Du Ngoc, and Altstädt Volker. 2019. "Effect of Resin and Blocked/Unblocked Hardener Mixture on the Production of Epoxy Foams with CO2 Blocked Hardener in Batch Foaming Process" Polymers 11, no. 5: 793. https://doi.org/10.3390/polym11050793
APA StyleBethke, C., Sanchez-Vazquez, S. A., Raps, D., Bakis, G., Bard, S., Du Ngoc, U. L., & Volker, A. (2019). Effect of Resin and Blocked/Unblocked Hardener Mixture on the Production of Epoxy Foams with CO2 Blocked Hardener in Batch Foaming Process. Polymers, 11(5), 793. https://doi.org/10.3390/polym11050793








