A Way to Increase the Bioaccesibility and Photostability of Roflumilast, a COPD Treatment, by Cyclodextrin Monomers
Abstract
1. Introduction
- (1)
- analyze the encapsulation mechanism of roflumilast by different types of natural and modified CDs.
- (2)
- evaluate the effect of temperature and pH on the encapsulation mechanism of roflumilast.
- (3)
- study the physical interactions between roflumilast and CDs using molecular docking.
- (4)
- determine the stability and bioaccessibility(the quantity of a compound that is released from its matrix in the gastrointestinal tract, becoming available for absorption [20]) of the complex in digestion.
- (5)
- understand the increased photostability of the complex.
2. Materials and Methods
2.1. Materials
2.2. Equipment and Experimental Procedure
2.2.1. Inclusion Complex Characterization
2.2.2. Temperature and pH
2.2.3. Molecular Docking
2.2.4. In Vitro Digestion
2.2.5. Photostability Study
2.2.6. Photodegradation Kinetic Study
2.2.7. Data Analysis
3. Results
3.1. Effect of CDs on the Solubility of Roflumilast
3.2. Effect of Temperature on Roflumilast and β-CD Complexation
3.3. Thermodynamic Parameters for the Roflumilast- β-CD Complexes
3.4. Effect of pH on Complexation Constant of Roflumilast with β-CD
3.5. Molecular Docking Simulations of Roflumilast/β-CD Complex
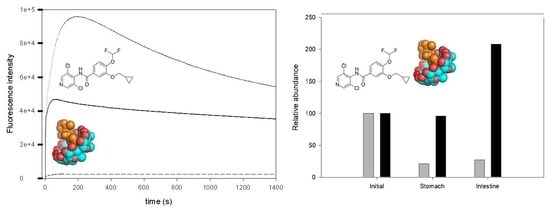
3.6. Effect of CD Addition on the Roflumilast Digestion
3.7. Effect of CD Addition on Roflumilast Photostability
3.8. Kinetics Analysis of the Reaction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hatzelmann, A.; Schudt, C. Anti-Inflammatory and Immunomodulatory Potential of the Novel PDE4 Inhibitor Roflumilast in Vitro. J. Pharmacol. Exp. Ther. 2001, 297, 267–279. [Google Scholar] [PubMed]
- Wedzicha, J.A.; Calverley, P.M.; Rabe, K.F. Roflumilast: a review of its use in the treatment of COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 81–90. [Google Scholar] [CrossRef]
- DailyMed - DALIRESP- Roflumilast Tablet. Available online: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=c9a1d0a8-581f-4f91-a2b8-f419192d0ebf (accessed on 11 October 2018).
- Woods, T.A. Diarrhea. In Clinical Methods: The History, Physical, and Laboratory Examinations; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990; ISBN 978-0-409-90077-4. [Google Scholar]
- Beghè, B.; Rabe, K.F.; Fabbri, L.M. Phosphodiesterase-4 inhibitor therapy for lung diseases. Am. J. Respir. Crit. Care Med. 2013, 188, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Szente, L.; Szejtli, J. Cyclodextrins as food ingredients. Trends Food Sci. Technol. 2004, 15, 137–142. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Fourmentin, S.; Crini, G.; Lichtfouse, E. (Eds.) Cyclodextrin Fundamentals, Reactivity and Analysis; Environmental Chemistry for a Sustainable World; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-76158-9. [Google Scholar]
- López-Nicolás, J.M.; Bru, R.; Sánchez-Ferrer, A.; García-Carmona, F. Use of “soluble lipids” for biochemical processes: Linoleic acid-cyclodextrin inclusion complexes in aqueous solutions. Biochem. J. 1995, 308, 151–154. [Google Scholar] [CrossRef]
- López-Nicolás, J.M.; García-Carmona, F. Rapid, simple and sensitive determination of the apparent formation constants of trans-resveratrol complexes with natural cyclodextrins in aqueous medium using HPLC. Food Chem. 2008, 109, 868–875. [Google Scholar] [CrossRef]
- López-Nicolás, J.M.; Rodríguez-Bonilla, P.; García-Carmona, F. Complexation of pinosylvin, an analogue of resveratrol with high antifungal and antimicrobial activity, by different types of cyclodextrins. J. Agric. Food Chem. 2009, 57, 10175–10180. [Google Scholar] [CrossRef] [PubMed]
- López-Nicolás, J.M.; Rodríguez-Bonilla, P.; García-Carmona, F. Cyclodextrins and Antioxidants. Crit. Rev. Food Sci. Nutr. 2014, 54, 251–276. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Matencio, A.; García-Carmona, F.; López-Nicolás, J.M. Encapsulation of piceatannol, a naturally occurring hydroxylated analogue of resveratrol, by natural and modified cyclodextrins. Food Funct. 2016, 7, 2367–2373. [Google Scholar] [CrossRef]
- Matencio, A.; García-Carmona, F.; López-Nicolás, J.M. The inclusion complex of oxyresveratrol in modified cyclodextrins: A thermodynamic, structural, physicochemical, fluorescent and computational study. Food Chem. 2017, 232, 177–184. [Google Scholar] [CrossRef]
- Matencio, A.; Hernández-García, S.; García-Carmona, F.; Manuel López-Nicolás, J. An integral study of cyclodextrins as solubility enhancers of α-methylstilbene, a resveratrol analogue. Food Funct. 2017, 8, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Matencio, A.; Hernández-Gil, C.J.G.; García-Carmona, F.; López-Nicolás, J.M. Physicochemical, thermal and computational study of the encapsulation of rumenic acid by natural and modified cyclodextrins. Food Chem. 2017, 216, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Matencio, A.; García-Carmona, F.; López-Nicolás, J.M. Aggregation of t10,c12 conjugated linoleic Acid in presence of natural and modified cyclodextrins. A physicochemical, thermal and computational analysis. Chem. Phys. Lipids 2017, 204, 57–64. [Google Scholar] [CrossRef]
- Suzuki, É.Y.; Amaro, M.I.; de Almeida, G.S.; Cabral, L.M.; Healy, A.M.; de Sousa, V.P. Development of a new formulation of roflumilast for pulmonary drug delivery to treat inflammatory lung conditions. Int. J. Pharm. 2018, 550, 89–99. [Google Scholar] [CrossRef]
- Galanakis, C. What is the Difference between Bioavailability Bioaccessibility and Bioactivity of Food Components? SciTech Connect. Available online: http://scitechconnect.elsevier.com/bioavailability-bioaccessibility-bioactivity-food-components/ (accessed on 27 April 2017).
- Connors KA, H.T. Phase solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–210. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Ilyasoglu, H.; El, S.N. Nanoencapsulation of EPA/DHA with sodium caseinate–gum arabic complex and its usage in the enrichment of fruit juice. LWT - Food Sci. Technol. 2014, 56, 461–468. [Google Scholar] [CrossRef]
- Mudie, D.M.; Murray, K.; Hoad, C.L.; Pritchard, S.E.; Garnett, M.C.; Amidon, G.L.; Gowland, P.A.; Spiller, R.C.; Amidon, G.E.; Marciani, L. Quantification of Gastrointestinal Liquid Volumes and Distribution Following a 240 mL Dose of Water in the Fasted State. Mol. Pharm. 2014, 11, 3039–3047. [Google Scholar] [CrossRef]
- Questions and Answers on Cyclodextrins Used as Excipients in Medicinal Products for Human Use. Available online: https://www.ema.europa.eu/en/documents/report/cyclodextrins-used-excipients-report-published-support-questions-answers-cyclodextrins-used_en.pdf (accessed on 9 October 2017).
- Paul, S.K.; Dash, U.N. Identification of Degradation Products in the Phosphodiesterase (PDE-4) Inhibitor Roflumilast Using High Resolution Mass Spectrometry and Density Functional Theory Calculations. Mass Spectrom. Lett. 2015, 6, 38–42. [Google Scholar] [CrossRef]
- Moore, D.E.; Tamat, S.R. Photosensitization by drugs: photolysis of some chlorine-containing drugs. J. Pharm. Pharmacol. 1980, 32, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Hreinsdóttir, D.; Másson, M. Evaluation of cyclodextrin solubilization of drugs. Int. J. Pharm. 2005, 302, 18–28. [Google Scholar] [CrossRef]
- Connors, K.A. Population characteristics of cyclodextrin complex stabilities in aqueous solution. J. Pharm. Sci. 1995, 84, 843–848. [Google Scholar] [CrossRef]
- Rekharsky, M.V.; Inoue, Y. Complexation Thermodynamics of Cyclodextrins. Chem. Rev. 1998, 98, 1875–1918. [Google Scholar] [CrossRef] [PubMed]
- López-Nicolás, J.M.; Rodríguez-Bonilla, P.; Méndez-Cazorla, L.; García-Carmona, F. Physicochemical study of the complexation of pterostilbene by natural and modified cyclodextrins. J. Agric. Food Chem. 2009, 57, 5294–5300. [Google Scholar] [CrossRef]
- Bru, R.; López-Nicolás, J.M.; Núñez-Delicado, E.; Nortes-Ruipérez, D.; Sánchez-Ferrer, A.; Garciá-Carmona, F. Cyclodextrins as hosts for poorly water-soluble compounds in enzyme catalysis. Appl. Biochem. Biotechnol. 1996, 61, 189–198. [Google Scholar] [CrossRef]
- Saenger, W. Cyclodextrin Inclusion Compounds in Research and Industry. Angew. Chem. Int. Ed. Engl. 1980, 19, 344–362. [Google Scholar] [CrossRef]




| B-CD | Mβ-CD | HPβ-CD | γ-CD | α-CD | |
|---|---|---|---|---|---|
| K11 (M−1) | 646 | 418 | 329 | 300 | 143 |
| SD (±) | 32 | 20 | 16 | 15 | 7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matencio, A.; Hernández-García, S.; García-Carmona, F.; López-Nicolás, J.M. A Way to Increase the Bioaccesibility and Photostability of Roflumilast, a COPD Treatment, by Cyclodextrin Monomers. Polymers 2019, 11, 801. https://doi.org/10.3390/polym11050801
Matencio A, Hernández-García S, García-Carmona F, López-Nicolás JM. A Way to Increase the Bioaccesibility and Photostability of Roflumilast, a COPD Treatment, by Cyclodextrin Monomers. Polymers. 2019; 11(5):801. https://doi.org/10.3390/polym11050801
Chicago/Turabian StyleMatencio, Adrián, Samanta Hernández-García, Francisco García-Carmona, and José Manuel López-Nicolás. 2019. "A Way to Increase the Bioaccesibility and Photostability of Roflumilast, a COPD Treatment, by Cyclodextrin Monomers" Polymers 11, no. 5: 801. https://doi.org/10.3390/polym11050801
APA StyleMatencio, A., Hernández-García, S., García-Carmona, F., & López-Nicolás, J. M. (2019). A Way to Increase the Bioaccesibility and Photostability of Roflumilast, a COPD Treatment, by Cyclodextrin Monomers. Polymers, 11(5), 801. https://doi.org/10.3390/polym11050801