Electrochemical Sensor of Double-Thiol Linked PProDOT@Si Composite for Simultaneous Detection of Cd(II), Pb(II), and Hg(II)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of Monomer
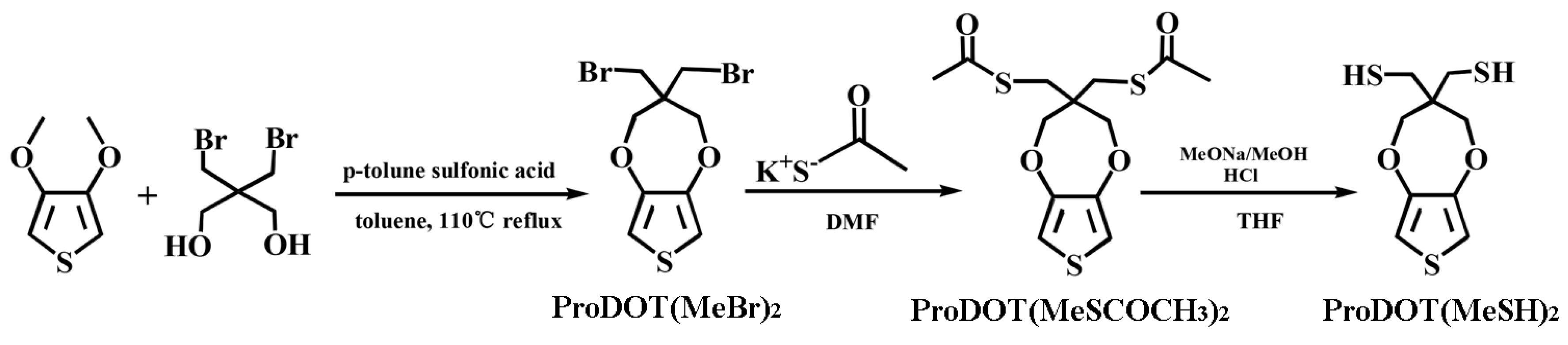
2.2.1. Synthesis of ProDOT(MeBr)2
2.2.2. Synthesis of ProDOT(MeSCOMe)2
2.2.3. Synthesis of ProDOT(MeSH)2
2.3. Preparation of Composite
2.3.1. Synthesis of SiO2 Spheres
2.3.2. Synthesis of Porous Silicon Spheres (Si)
2.3.3. Fabrication of PProDOT(MeSH)2@Si Composites
2.4. Structure Characterization
2.5. Measurement of Electrocatalytic Activity
3. Results and Discussion
3.1. Structure Characterization
3.2. Morphology Analysis
3.3. Electrochemical Determination of Cd(II), Pb(II), and Hg(II)
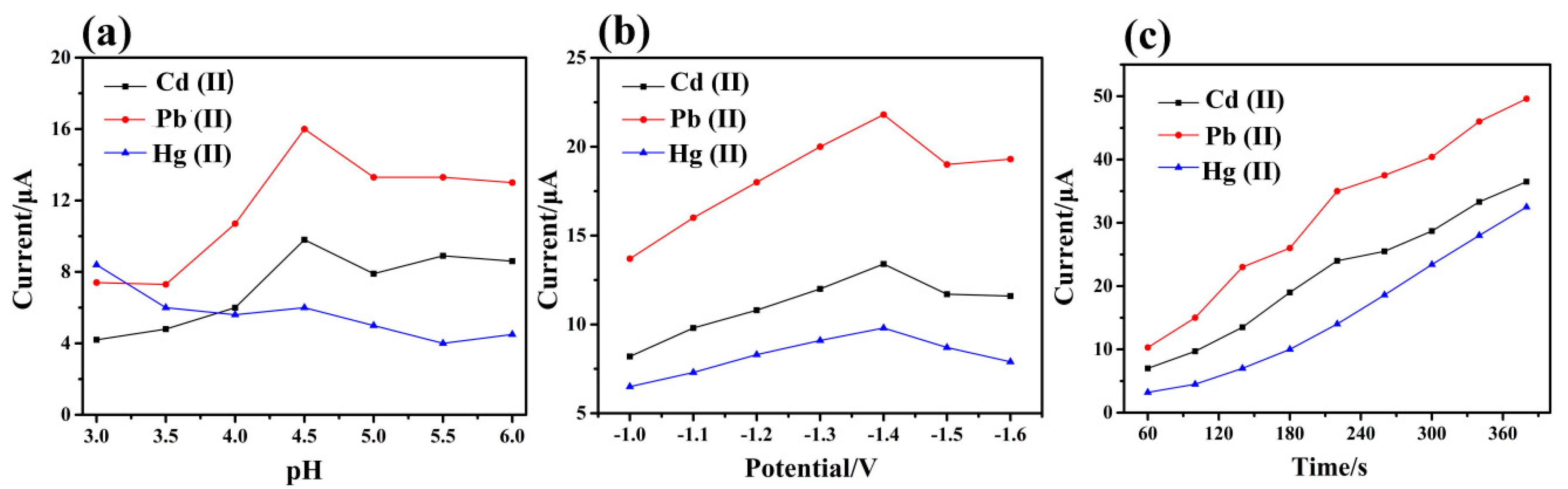
3.4. Optimization of Experimental Condition
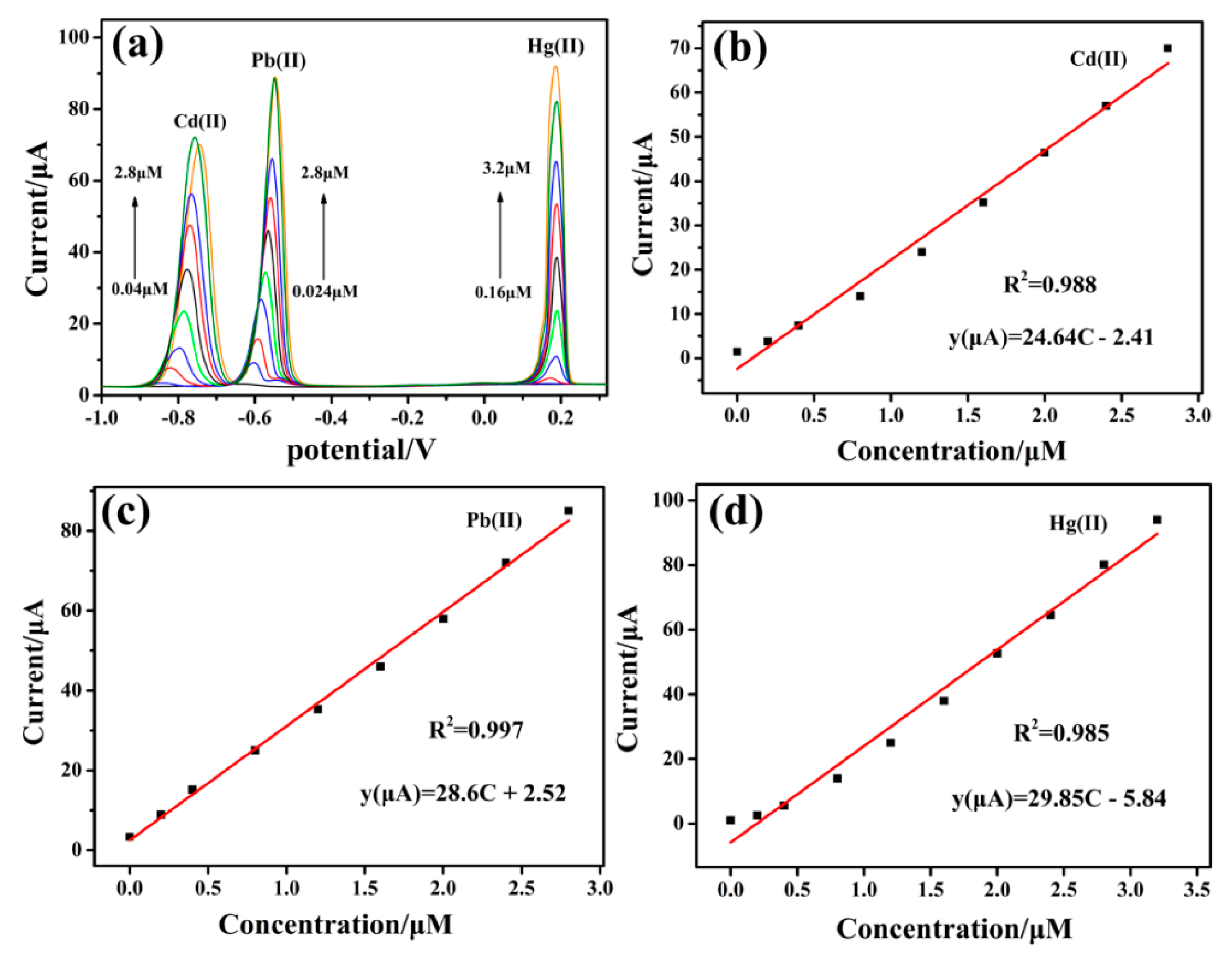
3.5. Individual Determination of Cd(II), Pb(II) and Hg(II)
3.6. Evaluation of Mutual Interferences
3.7. Simultaneous Determination of Cd(II), Pb(II), and Hg(II)
3.8. Reproducibility and Stability of Modified Electrode Study
3.9. Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wan, N.W.; Hanafiah, M.A. Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: A review. Bioresour. Technol. 2008, 99, 3935–3948. [Google Scholar]
- Wen, L.; Nan, X.; Wang, T.; Lin, X.; Ni, J. Influence of ph, ionic strength and humic acid on competitive adsorption of pb(ii), cd(ii) and cr(iii) onto titanate nanotubes. Chem. Eng. J. 2013, 215, 366–374. [Google Scholar]
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef]
- Lu, Y.; Liang, X.; Niyungeko, C.; Zhou, J.; Xu, J.; Tian, G. A review of the identification and detection of heavy metal ions in the environment by voltammetry. Talanta 2018, 178, 324–338. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, P.; Ouyang, Z.; Zhang, M.; Lin, Z.; Li, J.; Su, Z.; Gang, W. Nanoscale graphene doped with highly dispersed silver nanoparticles: Quick synthesis, facile fabrication of 3D membrane-modified electrode, and super performance for electrochemical sensing. Adv. Funct. Mater. 2016, 26, 2122–2134. [Google Scholar]
- Lee, S.; Oh, J.; Kim, D.; Piao, Y. A sensitive electrochemical sensor using an iron oxide/graphene composite for the simultaneous detection of heavy metal ions. Talanta 2016, 160, 528–536. [Google Scholar] [CrossRef]
- Li, D.; Liu, X.; Ran, Y.; Zhang, J.; Su, Z.; Gang, W. Electrochemical sensor based on novel two-dimensional nanohybrids: MoS2 nanosheets conjugated with organic copper nanowires for simultaneous detection of hydrogen peroxide and ascorbic acid. Inorg. Chem. Front. 2018, 5, 112–119. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, W.; Zhang, P.; Su, Z. Fabrication technologies and sensing applications of graphene-based composite films: Advances and challenges. Biosens. Bioelectron. 2017, 89, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Liu, W.; Deng, X.; Yan, S.; Su, Z. Gold nanocluster embedded bovine serum albumin nanofibers-graphene hybrid membranes for the efficient detection and separation of mercury ion. Chem. Eng. J. 2018, 335, 176–184. [Google Scholar] [CrossRef]
- Zhu, G.; Ge, Y.; Yong, D.; Shang, X.; Yang, J.; Liu, J. Size-tunable polyaniline nanotube-modified electrode for simultaneous determination of pb(ii) and cd(ii). Electrochim. Acta 2018, 268, 202–210. [Google Scholar] [CrossRef]
- Liu, T.; Guo, Y.; Zhang, Z.; Miao, Z.; Zhang, X.; Su, Z. Fabrication of hollow cuo/pani hybrid nanofibers for non-enzymatic electrochemical detection of H2O2 and glucose. Sens. Actuators B Chem. 2019, 286, 370–376. [Google Scholar] [CrossRef]
- Sundramoorthy, A.K.; Premkumar, B.S.; Gunasekaran, S. Reduced graphene oxide-poly (3,4-ethylenedioxythiophene) polystyrenesulfonate based dual-selective sensor for iron in different oxidation states. ACS Sens. 2015, 1, 151–157. [Google Scholar] [CrossRef]
- Serra, A.; Valles, E. Advanced electrochemical synthesis of multicomponent metallic nanorods and nanowires: Fundamentals and applications. Appl. Mater. Today 2018, 12, 207–234. [Google Scholar] [CrossRef]
- Abdiryim, T.; Ubul, A.; Jamal, R.; Feng, X.; Rahman, A. Electrochemical properties of the poly (3,4-ethylenedioxythiophene)/single-walled carbon nanotubes composite synthesized by solid-state heating method. Synth. Met. 2012, 162, 1604–1608. [Google Scholar] [CrossRef]
- Zhang, L.; Jamal, R.; Zhao, Q.; Wang, M.; Abdiryim, T. Preparation of pedot/go, pedot/MnO2, and pedot/go/MnO2 nanocomposites and their application in catalytic degradation of methylene blue. Nanoscale Res. Lett. 2015, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Abdiryim, T.; Huang, X.; Jamal, R.; Simayi, R. Hollow, spherical, poly (3,4-ethylenedioxythiophene)-bearing methanethiol as a gold stabilizer for high-efficiency electrochemical sensors. J. Electrochem. Soc. 2018, 165, B335–B343. [Google Scholar] [CrossRef]
- Lamy, M.; Darmanin, T.; Guittard, F. Highly hydrophobic films with high water adhesion by electrodeposition of poly (3, 4-propylenedioxythiophene) containing two alkoxy groups. Colloid Polym. Sci. 2015, 293, 933–940. [Google Scholar] [CrossRef]
- Welsh, D.M.; Kloeppner, L.J.; Madrigal, L.; Pinto, M.R.; Thompson, B.C.; Schanze, K.S.; Abboud, K.A.; Powell, D.; Reynolds, J.R. Regiosymmetric dibutyl-substituted poly (3,4-propylenedioxythiophene) s as highly electron-rich electroactive and luminescent polymers. Macromolecules 2002, 35, 6517–6525. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Liu, J.; Qiang, X.; Xiao, H.; Wang, X.; Hui, X.; Jing, Z. Thiol modified Fe3O4 @ SiO2 as a robust, high effective, and recycling magnetic sorbent for mercury removal. Chem. Eng. J. 2013, 226, 30–38. [Google Scholar] [CrossRef]
- Zhong, Z.; Ali, A.; Jamal, R.; Simayi, R.; Xiang, L.; Ding, S.; Abdiryim, T. Poly (edot-pyridine-edot) and poly (edot-pyridazine-edot) hollow nanosphere materials for the electrochemical detection of pb2+ and cu2+. J. Electroanal. Chem. 2018, 822, 112–122. [Google Scholar] [CrossRef]
- Ding, S.; Ali, A.; Jamal, R.; Xiang, L.; Zhong, Z.; Abdiryim, T. An electrochemical sensor of poly (edot-pyridine-edot)/graphitic carbon nitride composite for simultaneous detection of cd2+ and pb2+. Materials 2018, 11, 702. [Google Scholar] [CrossRef]
- Zuo, X.; Xia, Y.; Ji, Q.; Gao, X.; Yin, S.; Wang, M.; Wang, X.; Qiu, B.; Wei, A.; Sun, Z. Self-templating construction of 3d hierarchical macro-/mesoporous silicon from 0d silica nanoparticles. ACS Nano 2017, 11, 889. [Google Scholar] [CrossRef]
- Khdary, N.H.; Howard, A.G. New solid-phase-nanoscavenger for the analytical enrichment of mercury from water. Analyst 2011, 136, 3004–3009. [Google Scholar] [CrossRef]
- Akhavan, B.; Jarvis, K.; Majewski, P. Plasma polymer-functionalized silica particles for heavy metals removal. ACS Appl. Mater. Interfaces 2015, 7, 4265. [Google Scholar] [CrossRef]
- Guo, Z.; Seol, M.L.; Gao, C.; Kim, M.S.; Ahn, J.H.; Choi, Y.K.; Huang, X.J. Functionalized porous si nanowires for selective and simultaneous electrochemical detection of cd(ii) and pb(ii) ions. Electrochim. Acta 2016, 211, 998–1005. [Google Scholar] [CrossRef]
- Yu, X.; Sun, S.; Zhou, L.; Miao, Z.; Zhang, X.; Su, Z.; Wei, G. Removing metal ions from water with graphene–bovine serum albumin hybrid membrane. Nanomaterials 2019, 9, 276. [Google Scholar] [CrossRef]
- Reeves, B.D.; Grenier, C.R.G.; Argun, A.A.; Cirpan, A.; Mccarley, T.D.; Reynolds, J.R. Spray coatable electrochromic dioxythiophene polymers with high coloration efficiencies. Macromolecules 2004, 37, 7559–7569. [Google Scholar] [CrossRef]
- Reeves, B.D.; Unur, E.; Ananthakrishnan, N.; Reynolds, J.R. Defunctionalization of ester-substituted electrochromic dioxythiophene polymers. Macromolecules 2007, 40, 5344–5352. [Google Scholar] [CrossRef]
- Goda, T.; Toya, M.; Matsumoto, A.; Miyahara, Y. Poly (3,4-ethylenedioxythiophene) bearing phosphorylcholine groups for metal-free, antibody-free, and low-impedance biosensors specific for c-reactive protein. ACS Appl. Mater. Interfaces 2015, 7, 27440–27448. [Google Scholar] [CrossRef]
- Zhang, T.; Qiao, Z.; Ge, J.; Goebl, J.; Sun, M.; Yan, Y.; Liu, Y.S.; Chang, C.; Guo, J.; Yin, Y. A self-templated route to hollow silica microspheres. J. Phys. Chem. C 2009, 113, 3168–3175. [Google Scholar] [CrossRef]
- Ping, N.; Liu, X.; Fu, R.; Wu, Y.; Jiang, J.; Hui, D.; Zhang, X. Mesoporous silicon anodes by using polybenzimidazole derived pyrrolic n-enriched carbon toward high-energy li-ion batteries. ACS Energy Lett. 2017, 2. [Google Scholar]
- Hao, Z.; Shan, F.; Tong, Z.; Hui, D.; Zhang, X. Porous silicon@polythiophene core-shell nanospheres for lithium-ion batteries. Part. Part. Syst. Charact. 2016, 33, 75–81. [Google Scholar]
- Osman, Y.; Jamal, R.; Rahman, A.; Xu, F.; Ali, A.; Abdiryim, T. Comparative study on poly (3,4-propylenedioxythiophene)/TiO2 nanocomposites synthesized by mechanochemical and chemical solution methods. Synth. Met. 2013, 179, 54–59. [Google Scholar] [CrossRef]
- Liu, S.M.; Yang, S.Y.; Sato, K. Enhanced photoluminescence from si nano-organosols by functionalization with alkenes and their size evolution. Chem. Mater. 2006, 18, 637–642. [Google Scholar] [CrossRef]
- Mora, M.B.D.L.; Bornacelli, J.; Nava, R.; Zanella, R.; Reyes-Esqueda, J.A. Porous silicon photoluminescence modification by colloidal gold nanoparticles: Plasmonic, surface and porosity roles. J. Lumin. 2014, 146, 247–255. [Google Scholar] [CrossRef]
- Jamal, R.; Li, Z.; Wang, M.; Qin, Z.; Abdiryim, T. Synthesis of poly (3,4-propylenedioxythiophene)/MnO2 composites and their applications in the adsorptive removal of methylene blue. Prog. Nat. Sci. Mater. Int. 2016, 26, 32–40. [Google Scholar] [CrossRef]
- Li, Z.; Jamal, R.; Qin, Z.; Yu, Z.; Wang, M.; Abdiryim, T. The structure and electrochemical properties of poly (3,4-propylenedioxythiophene)/SnO2 nanocomposites synthesized by mechanochemical route. Polym. Compos. 2016, 37, 2884–2896. [Google Scholar]
- Liu, Z.; Chang, X.; Wang, T.; Li, W.; Ju, H.; Zheng, X.; Wu, X.; Wang, C.; Zheng, J.; Li, X. Silica derived hydrophobic colloidal nano-si for lithium ion batteries. ACS Nano 2017, 11, 6065. [Google Scholar] [CrossRef]
- Wei, Y.; Gao, C.; Meng, F.-L.; Li, H.-H.; Wang, L.; Liu, J.-H.; Huang, X.-J. SnO2/reduced graphene oxide nanocomposite for the simultaneous electrochemical detection of cadmium(ii), lead(ii), copper(ii), and mercury(ii): An interesting favorable mutual interference. J. Phys. Chem. C 2011, 116, 1034–1041. [Google Scholar] [CrossRef]
- Xie, Y.L.; Zhao, S.Q.; Ye, H.L.; Jing, Y.; Ping, S.; Hu, S.Q. Graphene/CeO2 hybrid materials for the simultaneous electrochemical detection of cadmium(ii), lead(ii), copper(ii), and mercury(ii). J. Electroanal. Chem. 2015, 757, 235–242. [Google Scholar] [CrossRef]
- Xiong, S.; Yang, B.; Cai, D.; Qiu, G.; Wu, Z. Individual and simultaneous stripping voltammetric and mutual interference analysis of cd2+, pb2+ and hg2+ with reduced graphene oxide-Fe3O4 nanocomposites. Electrochim. Acta 2015, 185, 52–61. [Google Scholar] [CrossRef]
- Guo, X.; Du, B.; Qin, W.; Jian, Y.; Hu, L.; Yan, L.; Xu, W. Synthesis of amino functionalized magnetic graphenes composite material and its application to remove cr(vi), pb(ii), hg(ii), cd(ii) and ni(ii) from contaminated water. J. Hazard. Mater. 2014, 278, 211–220. [Google Scholar] [CrossRef]
- Lv, G.; Zhu, B.; Li, X.; Chen, C.; Li, J.; Jin, Y.; Hu, X.; Zhu, J. Simultaneous perforation and doping of si nanoparticles for lithium-ion battery anode. ACS Appl. Mater. Interfaces 2017, 9, 44452–44457. [Google Scholar] [CrossRef]
- Feng, G.; Gao, N.; Nishitani, A.; Tanaka, H. Rod-like hydroxyapatite and nafion nanocomposite as an electrochemical matrix for simultaneous and sensitive detection of hg2+, cu2+, pb2+ and cd2+. J. Electroanal. Chem. 2016, 775, 212–218. [Google Scholar]
- Gumpu, M.B.; Veerapandian, M.; Krishnan, U.M.; Rayappan, J.B.B. Simultaneous electrochemical detection of cd(ii), pb(ii), as(iii) and hg(ii) ions using ruthenium(ii)-textured graphene oxide nanocomposite. Talanta 2017, 162, 574–582. [Google Scholar] [CrossRef]
- Yue, M.; Wang, Y.; Xie, D.; Yue, G.; Zhu, X.; Zhang, H.; Wang, G.; Zhang, Y.; Zhao, H. Hierarchical mgfe-layered double hydroxide microsphere/graphene composite for simultaneous electrochemical determination of trace pb(ii) and cd(ii). Chem. Eng. J. 2018, 347, 953–962. [Google Scholar]
- Xing, H.; Xu, J.; Zhu, X.; Duan, X.; Lu, L.; Wang, W.; Zhang, Y.; Yang, T. Highly sensitive simultaneous determination of cadmium(ii), lead(ii), copper(ii), and mercury(ii) ions on n-doped graphene modified electrode. J. Electroanal. Chem. 2016, 760, 52–58. [Google Scholar] [CrossRef]
- Mei, L.; Guan, Q.; Liu, S. Nitrogen-doped hollow carbon spheres for electrochemical detection of heavy metal ions. Ionics 2018, 24, 2783–2793. [Google Scholar]










| Analyties | Cd(Ⅱ) | Pb(Ⅱ) | Hg(Ⅱ) | ||||
|---|---|---|---|---|---|---|---|
| N.D | (ìM) | (ìA) | (ìM) | (ìA) | (ìM) | (ìA) | |
| 1 | 0.04 | 2.4 | 0.024 | 3 | 0.07 | 3 | |
| 2 | 0.2 | 5 | 0.3 | 10 | 0.4 | 6 | |
| 3 | 0.4 | 7.3 | 0.8 | 20 | 0.8 | 10 | |
| 4 | 0.8 | 12 | 1.2 | 29 | 1.6 | 17 | |
| 5 | 1.2 | 23.5 | 2 | 44 | 2.8 | 24.5 | |
| 6 | 1.6 | 29.2 | 2.4 | 50 | 3.6 | 29 | |
| 7 | 1.8 | 34 | 2.8 | 55 | 4.8 | 36 | |
| 8 | 2.2 | 44.5 | 3.2 | 60 | 5.6 | 40.2 | |
| 9 | 2.6 | 48.5 | 3.6 | 65 | 6.4 | 44 | |
| 10 | 3 | 53 | 4 | 70 | 7.6 | 50 | |
| Liner range | 0.04–3.0 | 0.024–4.0 | 0.07–7.6 | ||||
| Regression Equation | i(μA) = −0.457 + 17.08C(μM) | i(μA) = −0.457 + 17.08C(μM) | i(μA) = −0.457 + 17.08C(μM) | ||||
| R2 | 0.983 | 0.983 | 0.98 | ||||
| LOD(nm) | 8.3 | 4.7 | 8.0 | ||||
| Electrode | Methods | Analytes | Linear Range (µM) | LOD (nM) | Ref. |
|---|---|---|---|---|---|
| Nafion-HAP | DPASV | Cd2+ Pb2+ Hg2+ | 0.1–10 0.1–10 0.1–1.0, 3.0–10.0 | 35 49 30 | [44] |
| [Ru(bpy)3]2+-GO/Au | DPSV | Cd2+ Pb2+ Hg2+ | 0.02–0.9 0.05–1.5 0.1–0.9 | 2.8 1.14 1.6 | [45] |
| rGO-Fe3O4 | SWASV | Cd2+ Pb2+ Hg2+ | 0.1–1.7 0.1–1.7 0.1–1.7 | 28 8 17 | [41] |
| poly(BPE)HNs | DPV | Pb2+ | 0.012–4 | 5 | [20] |
| MgFe-LDH/graphene | SWASV | Cd2+ Pb2+ | 0.1–1.0 0.1–1.0 | 5.9 2.7 | [46] |
| N-doped graphene | DPSV | Cd2+ Pb2+ Hg2+ | 0.05–9.0 0.01–9.0 0.2–9.0 | 50 5 50 | [47] |
| poly(BPE)/g-C3N4 | DPV | Cd2+ Pb2+ | 0.12–7.2 0.08–7.2 | 18 3.34 | [21] |
| Grapheme-CeO2 | DPASV | Cd2+ Pb2+ Hg2+ | 0.2–2.5 0.2–2.5 0.2–2.5 | 0.1994 0.1057 0.2771 | [40] |
| N-HCS/CS/gold | SWASW | Pb2+ Hg2+ | 0.05–0.7 0.01–0.9 | 15 2.35 | [48] |
| PProDOT(MeSH)2@ Si | DPV | Cd(Ⅱ) Pb(Ⅱ) Hg(Ⅱ) | 0.04–2.8 0.024–2.8 0.16–3.2 | 5.75 2.7 1.7 | this work |
| Original (µM) | Added (µM) | Found (µM) | Recovery (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cd(II) | Pb(II) | Hg(II) | Cd(II) | Pb(II) | Hg(II) | Cd(II) | Pb(II) | Hg(II) | |
| N.D | 0.5 | 0.5 | 0.5 | 0.52 | 0.53 | 0.49 | 104 | 105 | 97.2 |
| N.D | 1.0 | 1.0 | 1.0 | 0.92 | 1.01 | 0.97 | 92.0 | 101 | 97.0 |
| N.D | 1.5 | 1.5 | 1.5 | 1.45 | 1.4 | 1.59 | 96.6 | 93.3 | 106 |
| N.D | 2.0 | 2.0 | 2.0 | 2.06 | 2.06 | 1.94 | 103 | 103 | 97.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulla, M.; Ali, A.; Jamal, R.; Bakri, T.; Wu, W.; Abdiryim, T. Electrochemical Sensor of Double-Thiol Linked PProDOT@Si Composite for Simultaneous Detection of Cd(II), Pb(II), and Hg(II). Polymers 2019, 11, 815. https://doi.org/10.3390/polym11050815
Abdulla M, Ali A, Jamal R, Bakri T, Wu W, Abdiryim T. Electrochemical Sensor of Double-Thiol Linked PProDOT@Si Composite for Simultaneous Detection of Cd(II), Pb(II), and Hg(II). Polymers. 2019; 11(5):815. https://doi.org/10.3390/polym11050815
Chicago/Turabian StyleAbdulla, Mihray, Ahmat Ali, Ruxangul Jamal, Tursunnisahan Bakri, Wei Wu, and Tursun Abdiryim. 2019. "Electrochemical Sensor of Double-Thiol Linked PProDOT@Si Composite for Simultaneous Detection of Cd(II), Pb(II), and Hg(II)" Polymers 11, no. 5: 815. https://doi.org/10.3390/polym11050815





