Room-Temperature Fabrication of a Nickel-Functionalized Copper Metal–Organic Framework (Ni@Cu-MOF) as a New Pseudocapacitive Material for Asymmetric Supercapacitors
Abstract
:1. Introduction
2. Materials and Method
2.1. Synthesis of the Cu-MOF
2.2. Synthesis of the Ni@Cu-MOF
2.3. Electrochemical Measurements
2.4. Materials Characterization
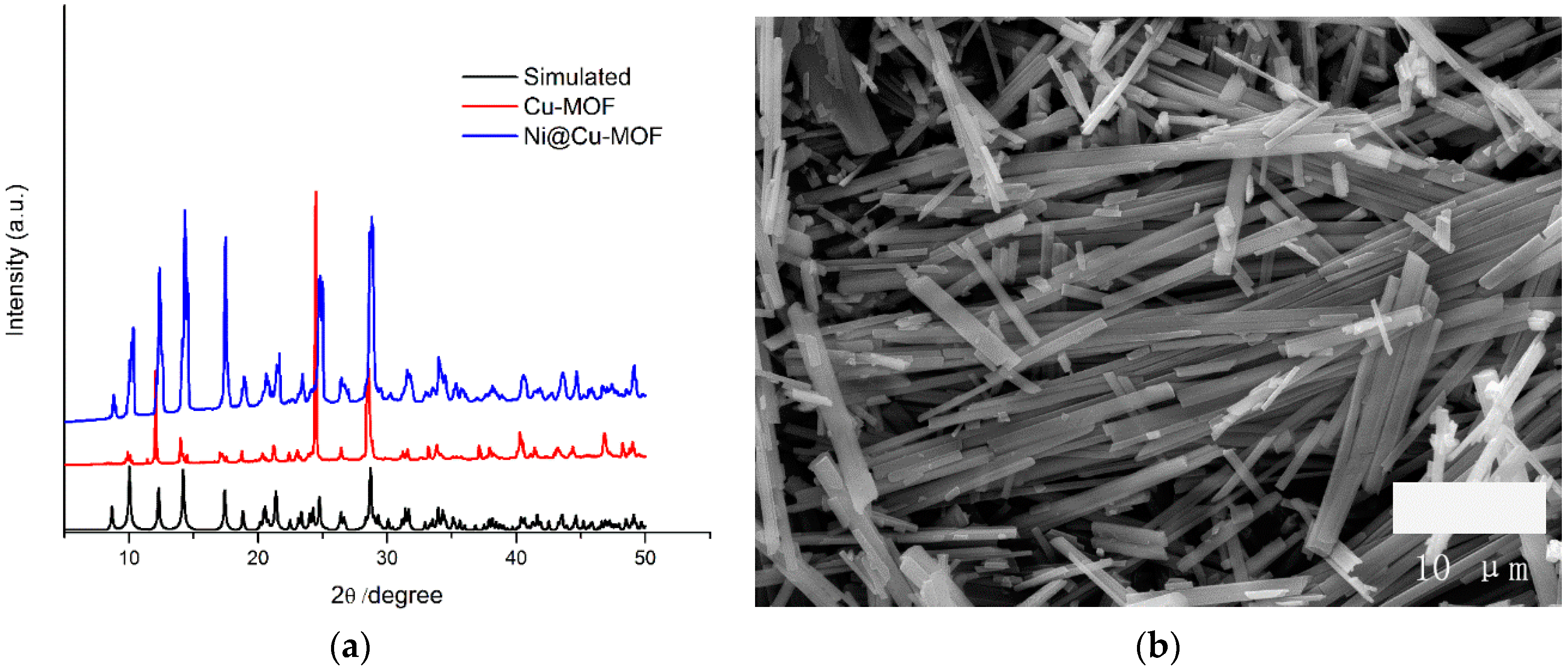
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Cao, X.H.; Tan, C.L.; Sindoro, M.; Zhang, H. Hybrid micro-/nano-structuresderived from metal–organic frameworks: Preparation and applications in energy storage and conversion. Chem. Soc. Rev. 2017, 46, 2660–2677. [Google Scholar] [CrossRef]
- Zhou, J.W.; Wang, B. Emerging crystalline porous materials as a multifunctional platform for electrochemical energy storage. Chem. Soc. Rev. 2017, 46, 6927–6945. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, S.Y.; Liu, Y.N.; Meng, T.; Ma, L.; Zhang, H.; Xu, M.W.; Zhu, J.H.; Li, C.M. Electrode engineering starting from live biomass: A ‘smart’ way to construct smart pregnant hybrids for sustainable charge storage devices. Mater. Chem. Front. 2019, 3, 796–805. [Google Scholar] [CrossRef]
- Haregewoin, A.M.; Wotango, A.S.; Wang, B.-J. Electrolyte additives for lithium ion battery electrodes: Progress and perspectives. Energy Environ. Sci. 2016, 9, 1955–1988. [Google Scholar] [CrossRef]
- Huang, S.Z.; Lim, Y.V.; Zhang, X.M.; Wang, Y.; Zheng, Y.; Kong, D.Z.; Ding, M.; Yang SY, A.; Yang, H.Y. Regulating the polysulfide redox conversion by iron phosphide nanocrystals for high-rate and ultrastable lithium-sulfur battery. Nano Energy 2018, 51, 340–348. [Google Scholar] [CrossRef]
- Liu, L.L.; Niu, Z.Q.; Chen, J. Unconventional supercapacitors from nanocarbon-based electrode materials to device configurations. Chem. Soc. Rev. 2016, 45, 4340–4363. [Google Scholar] [CrossRef] [PubMed]
- Sui, Z.Y.; Zhang, P.Y.; Xu, M.Y.; Liu, Y.W.; Wei, Z.X.; Han, B.H. Metal–Organic Framework-Derived Metal Oxide Embedded in Nitrogen-Doped Graphene Network for High-Performance Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 43171–43178. [Google Scholar] [CrossRef] [PubMed]
- Raza, W.; Ali, F.; Raza, N.; Luo, Y.; Kwon, E.E.; Yang, J.; Kumar, S.; Mehmood, A.; Kim, K.H. Recent advancements in supercapacitor technology. Nano Energy 2018, 52, 441–473. [Google Scholar] [CrossRef]
- Del Pino, Á.P.; Villarroya, A.M.; Chuquitarqui, A.; Logofatu, C.; Tonti, D.; György, E. Reactive laser synthesis of nitrogen-doped hybrid graphene-based electrodes for energy storage. J. Mater. Chem. A 2018, 6, 16074–16086. [Google Scholar] [CrossRef]
- Ali, B.A.; Metwalli, O.I.; Khalil AS, G.; Allam, N.K. Unveiling the Effect of the Structure of Carbon Material on the Charge Storage Mechanism in MoS2 -Based Supercapacitors. ACS Omega 2018, 3, 16301–16308. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Kaneti, Y.V.; Kim, J.H.; Yamauchi, Y. Nanoarchitectures for Metal–Organic Framework-Derived Nanoporous Carbons toward Supercapacitor Applications. Acc. Chem. Res. 2016, 49, 2796–2806. [Google Scholar] [CrossRef] [PubMed]
- Down, M.P.; Rowley-Neale, S.J.; Smith, G.C.; Banks, C.E. Fabrication of Graphene Oxide Supercapacitor Devices. ACS Appl. Energy Mater. 2018, 1, 707–714. [Google Scholar] [CrossRef]
- Liu, D.B.; Ni, K.; Ye, J.L.; Xie, J.; Zhu, Y.W.; Song, L. Tailoring the Structure of Carbon Nanomaterials toward High-End Energy Applications. Adv. Mater. 2018, 30, 1802104. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Zhao, M.; Yu, Y.; Chen, B.; Huang, Y.; Yang, J.; Cao, X.; Lu, Q.; Zhang, X.; Zhang, Z.; et al. Synthesis of Two-Dimensional CoS1.097/Nitrogen-Doped Carbon Nanocomposites Using Metal–Organic Framework Nanosheets as Precursors for Supercapacitor. J. Am. Chem. Soc. 2016, 138, 6924–6927. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.S.; Ye, Z.C.; Hu, S.Z.; Hao, C.; Wang, X.H.; Huang, C.X.; Wu, F.S. Designed formation of Co3O4/ZnCo2O4/CuO hollow polyhedral nanocages derived from zeolitic imidazolate framework-67 for high-performance supercapacitors. Nanoscale 2018, 10, 15771–15781. [Google Scholar] [CrossRef]
- Lin, J.H.; Zheng, X.H.; Wang, Y.H.; Liang, H.Y.; Jia, H.N.; Chen, S.L.; Qi, J.L.; Cao, J.; Fei, W.D.; Feng, J.C. Rational construction of core–shell Ni3S2@Ni(OH)2 nanostructures as battery-like electrodes for supercapacitors. Inorg. Chem. Front. 2018, 5, 1985–1991. [Google Scholar] [CrossRef]
- Qu, C.; Jiao, Y.; Zhao, B.T.; Chen, D.C.; Zou, R.Q.; Walton, K.S.; Liu, M.L. Nickel-based pillared MOFs for high-performance supercapacitors: Design, synthesis and stability study. Nano Energy 2016, 26, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wöll, C. Surface-supported metal–organic framework thin films: Fabrication methods, applications, and challenges. Chem. Soc. Rev. 2017, 46, 5730–5770. [Google Scholar] [CrossRef]
- Wang, L.; Feng, X.; Ren, L.T.; Piao, Q.H.; Zhong, J.Q.; Wang, Y.B.; Li, H.W.; Chen, Y.F.; Wang, B. Flexible Solid-State Supercapacitor Based on a Metal–Organic Framework Interwoven by Electrochemically-Deposited PANI. J. Am. Chem. Soc. 2015, 137, 4920–4923. [Google Scholar] [CrossRef]
- Wang, J.; Luo, X.L.; Young, C.; Kim, J.H.; Kaneti, Y.V.; You, J.; Kang, Y.-M.; Yamauchi, Y.; Wu, K.C.-W. A Glucose-Assisted Hydrothermal Reaction for Directly Transforming Metal–Organic Frameworks into Hollow Carbonaceous Materials. Chem. Mater. 2018, 30, 4401–4408. [Google Scholar] [CrossRef]
- Cao, X.-M.; Sun, Z.-J.; Zhao, S.Y.; Wang, B.; Han, Z.-B. MOF-derived sponge-like hierarchical porous carbon for flexible all-solid-state supercapacitors. Mater. Chem. Front. 2018, 2, 1692–1699. [Google Scholar] [CrossRef]
- Guan, C.; Liu, X.M.; Ren, W.N.; Li, X.; Cheng, C.W.; Wang, J. Rational Design of Metal-Organic Framework Derived Hollow NiCo2O4 Arrays for Flexible Supercapacitor and Electrocatalysis. Adv. Energy Mater. 2017, 7, 1602391. [Google Scholar] [CrossRef]
- Xiao, Z.Y.; Fan, L.L.; Xu, B.; Zhang, S.Q.; Kang, W.P.; Kang, Z.X.; Lin, H.; Liu, X.P.; Zhang, S.Y.; Sun, D.F. Green Fabrication of Ultrathin Co3O4 Nanosheets from Metal–Organic Framework for Robust High-Rate Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 41827–41836. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Tao, K.; Wang, D.; Han, L. Design of a porous cobalt sulfide nanosheet array on Ni foam from zeolitic imidazolate frameworks as an advanced electrode for supercapacitors. Nanoscale 2018, 10, 2735–2741. [Google Scholar] [CrossRef]
- Kulkarni, P.; Nataraj, S.K.; Balakrishna, R.G.; Nagaraju, D.H.; Reddy, M.V. Nanostructured binary and ternary metal sulfides: Synthesis methods and their application in energy conversion and storage devices. J. Mater. Chem. A 2017, 5, 22040–22094. [Google Scholar] [CrossRef]
- Huang, Y.Z.; Quan, L.; Liu, T.Q.; Chen, Q.D.; Cai, D.P.; Zhan, H.B. Construction of MOF-derived hollow Ni–Zn–Co–S nanosword arrays as binder-free electrodes for asymmetric supercapacitors with high energy density. Nanoscale 2018, 10, 14171–14181. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.M.; Chen, D.Z.; Quan, H.Y.; Zou, R.; Wang, W.X.; Luo, X.B.; Guo, L. Fabrication of Hierarchical Porous Metal–Organic Framework Electrode for Aqueous Asymmetric Supercapacitor. ACS Sustain. Chem. Eng. 2017, 5, 4144–4153. [Google Scholar] [CrossRef]
- Xia, W.; Mahmood, A.; Zou, R.Q.; Xu, Q. Metal–organic frameworks and their derived nanostructures for electrochemical energy storage and conversion. Energy Environ. Sci. 2015, 8, 1837–1866. [Google Scholar] [CrossRef]
- HarmeetKaur, S.; KumarBhardwaj, S.; Ki-HyunKimcAkashDeep, S. Metal-organic frameworks and their composites as efficient electrodes for supercapacitor applications. Coord. Chem. Rev. 2018, 369, 15–38. [Google Scholar]
- Liang, Z.; Qu, C.; Guo, W.; Zou, R.; Xu, Q. Pristine Metal–Organic Frameworks and their Composites for Energy Storage and Conversion. Adv. Mater. 2017, 30, 1702891. [Google Scholar] [CrossRef]
- Han, Y.; Qi, P.; Feng, X.; Li, S.; Fu, X.; Li, H.; Chen, Y.; Zhou, J.; Li, X.; Wang, B. In Situ Growth of MOFs on the Surface of Si Nanoparticles for Highly Efficient Lithium Storage: Si@MOF Nanocomposites as Anode Materials for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 2178–2182. [Google Scholar] [CrossRef]
- Gong, T.; Lou, X.B.; Gao, E.Q.; Hu, B.W. Pillared-Layer Metal–Organic Frameworks for Improved Lithium-Ion Storage Performance. ACS Appl. Mater. Interfaces 2017, 9, 21839–21847. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.X.; Sun, W.W.; Lv, L.-P.; Kong, S.F.; Wang, Y. Microwave-Assisted Morphology Evolution of Fe-Based Metal–Organic Frameworks and Their Derived Fe2O3 Nanostructures for Li-Ion Storage. ACS Nano 2017, 11, 4198–4205. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M.; Zhao, Q.; Li, Q.; Pang, H. Facile synthesis of silver nanowire-zeolitic imidazolate framework 67 composites as high-performance bifunctional oxygen catalysts. Nanoscale 2018, 10, 15755–15762. [Google Scholar] [CrossRef]
- Qamar, M.; Adam, A.; Merzougui, B.; Helal, A.; Abdulhamid, O.; Siddiqui, M.N. Metal–organic framework-guided growth of Mo2C embedded in mesoporous carbon as a high-performance and stable electrocatalyst for the hydrogen evolution reaction. J. Mater. Chem. A 2016, 4, 16225–16232. [Google Scholar] [CrossRef]
- Aiyappa, H.B.; Thote, J.; Shinde, D.B.; Banerjee, R.; Kurungot, S. Cobalt-Modified Covalent Organic Framework as a Robust Water Oxidation Electrocatalyst. Chem. Mater. 2016, 28, 4375–4379. [Google Scholar] [CrossRef]
- Zhao, X.; Pachfule, P.; Li, S.; Simke, J.R.J.; Schmidt, J.; Thomas, A. Bifunctional Electrocatalysts for Overall Water Splitting from an Iron/Nickel-Based Bimetallic Metal–Organic Framework/Dicyandiamide Composite. Angew. Chem. Int. Ed. 2018, 57, 8921–8926. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, A.; Antony, R.P.; Wang, R.; Lee, J.M. MOF-Derived Hollow Cage NixCo3−xO4 and Their Synergy with Graphene for Outstanding Supercapacitors. Small 2017, 13, 1603102. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.W.; Hong, J.; An, G.H.; Pak, S.; Lee, J.; Cho, Y.; Lee, S.; Cha, S.; Sohn, J.I.; Kim, J.M. Synergistic effects of engineered spinel hetero-metallic cobaltites on electrochemical pseudo-capacitive behaviors. J. Mater. Chem. A 2018, 6, 15033–15039. [Google Scholar] [CrossRef]
- Niu, S.; Wang, Z.; Zhou, T.; Yu, M.; Yu, M.; Qiu, J. A Polymetallic Metal–Organic Framework-Derived Strategy toward Synergistically Multidoped Metal Oxide Electrodes with Ultralong Cycle Life and High Volumetric Capacity. Adv. Funct. Mater. 2017, 27, 1605332. [Google Scholar] [CrossRef]
- Guan, B.Y.; Kushima, A.; Yu, L.; Li, S.; Li, J.; Lou, X.W. Coordination Polymers Derived General Synthesis of Multishelled Mixed Metal-Oxide Particles for Hybrid Supercapacitors. Adv. Mater. 2017, 29, 1605902. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Zhao, B.T.; Jiao, Y.; Chen, D.C.; Dai, S.G.; Deglee, B.M.; Chen, Y.; Walton, K.S.; Zou, R.Q.; Liu, M.L. Functionalized Bimetallic Hydroxides Derived from Metal–Organic Frameworks for High-Performance Hybrid Supercapacitor with Exceptional Cycling Stability. ACS Energy Lett. 2017, 2, 1263–1269. [Google Scholar] [CrossRef]
- Zhang, S.; Li, D.; Chen, S.; Yang, X.; Zhao, X.; Zhao, Q.; Komarneni, S.; Yang, D. Highly Stable Supercapacitors with MOF-derived Co9S8/Carbon Electrodes for High Rate Electrochemical Energy Storage. J. Mater. Chem. A 2017, 5, 12453–12461. [Google Scholar] [CrossRef]
- Koman, M. Copper(II) Pyridine-2,6-dicarboxylates. Coordination and Distortion Isomers of [Cu(pydca)(H2O)2]. Pol. J. Chem. 2001, 75, 957–964. [Google Scholar]
- Chaudhari, N.K.; Chaudhari, S.; Yu, J.S. Cube-like α-Fe2O3 Supported on Ordered Multimodal Porous Carbon as High Performance Electrode Material for Supercapacitors. ChemSusChem 2014, 7, 3102–3111. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Nie, S.; Liu, Y.; Yan, W.; Lin, S.; Cheng, G.; Yang, H.; Luo, J. Room-Temperature Fabrication of a Nickel-Functionalized Copper Metal–Organic Framework (Ni@Cu-MOF) as a New Pseudocapacitive Material for Asymmetric Supercapacitors. Polymers 2019, 11, 821. https://doi.org/10.3390/polym11050821
Wang Y, Nie S, Liu Y, Yan W, Lin S, Cheng G, Yang H, Luo J. Room-Temperature Fabrication of a Nickel-Functionalized Copper Metal–Organic Framework (Ni@Cu-MOF) as a New Pseudocapacitive Material for Asymmetric Supercapacitors. Polymers. 2019; 11(5):821. https://doi.org/10.3390/polym11050821
Chicago/Turabian StyleWang, Yi, Shengqiang Nie, Yuan Liu, Wei Yan, Shaomin Lin, Gang Cheng, Huan Yang, and Jun Luo. 2019. "Room-Temperature Fabrication of a Nickel-Functionalized Copper Metal–Organic Framework (Ni@Cu-MOF) as a New Pseudocapacitive Material for Asymmetric Supercapacitors" Polymers 11, no. 5: 821. https://doi.org/10.3390/polym11050821
APA StyleWang, Y., Nie, S., Liu, Y., Yan, W., Lin, S., Cheng, G., Yang, H., & Luo, J. (2019). Room-Temperature Fabrication of a Nickel-Functionalized Copper Metal–Organic Framework (Ni@Cu-MOF) as a New Pseudocapacitive Material for Asymmetric Supercapacitors. Polymers, 11(5), 821. https://doi.org/10.3390/polym11050821




