Abstract
The aim of the research is to identify the changes which occur in lignin from miscanthus and sorghum, one of the main biomass components, as a result of an anaerobic digestion (AD) process. The percentage content and structure of lignin before and after the fermentation process were analysed using biomass harvested in two growing periods—before and after vegetation. It was shown that plants at different developmental stages differ in lignin content. During plant growth, the lignin structure also changes—the syringyl-to-guaiacyl ratio (S/G) increases, whereas the aliphatic and aromatic structure ratio (Al/Ar) decreases. The AD process leads to an increase in percentage lignin content in cell walls, and the increase is higher for plants harvested during vegetation. It has been shown in studies that the methane fermentation of miscanthus and sorghum produces waste containing a large amount of lignin, the structure of which is altered relative to native lignin. The quantity and the new, simplified structure of lignin create new possibilities for using this aromatic polymer.
1. Introduction
Lignin is a natural polymer constructed from aromatic compounds. It is the third component, next to cellulose and hemicellulose, building plant cell walls [1,2]. It mainly occurs in vascular and strengthening tissues [3]. Bonded to hemicellulose by covalent bonds, it forms a lignin-carbohydrate complex (LCC) giving stiffness and mechanical endurance to a cell wall [2,4]. Lignin is built from monomers (monolignols), which have a phenyl structure varying in the substitution degree of methoxy groups in the ring [2,5]. In terms of the substitution degree of methoxy groups, there are three types of monolignols: alcohol 4-hydroxyphenol (H), syringyl (S), and guaiacyl (G). H, S, and G units are bonded by ester, ether, and C-C bonds which recur arbitrarily and frequently, providing, on the one hand, a high degree of variation in that parameter among plants, and on the other, considerable resistance of the lignin structure to destruction [1,2,6]. The S/G ratio of lignin composition is important for deconstruction, as S subunits are only able to cross-link with two monolignols (creating a less branched structure) and their bonds are generally more reactive and can be broken down more easily by different industrial processes [7,8,9], including methane fermentation [10].
Raw materials used in second-generation biofuel production which do not compete with food include miscanthus and sorghum. The energy value of miscanthus is approximately 18.01–19.20 MJ kg−1 d.m. [11,12], while that of sorghum is 17.75 MJ kg−1 d.m. [13]. Research results on the chemical composition and structure of the main components of these plants in terms of their application for energy purposes have been published widely [14,15,16,17,18]. Research has also been conducted into the possibility of employing these products in biogas production during anaerobic digestion (AD) [19].
It has been shown that the availability of cellulose to microorganisms, as well as the degree of its degradation to glucose, plays a key role in the methane fermentation process [20]. In lignocellulosic raw materials this availability is impaired because cellulose is surrounded by both hemicelluloses and lignin. Hemicelluloses are compounds more susceptible to hydrolysis than cellulose. However, lignin significantly hampers the decomposition of lignocellulosic raw materials [21,22,23,24,25]. Whittaker et al. [26] proved that the efficiency of biogas obtained from miscanthus (Miscanthus ×giganteus and Miscanthus sacchariflorus) depended on the time of harvest, among other factors. The significance of chemical composition in the fermentation process exemplified by Miscanthus ×giganteus and Sorghum bicolor has been investigated by Godin et al. [27]. However, the research conducted to date fails to indicate precisely which chemical changes lignocellulose biomass undergoes during AD. Changes in the chemical structure of cellulose occurring under the influence of the fermentation process have been widely described in the literature [28,29,30,31]. Results on changes involving carbohydrates during that process for miscanthus and sorghum varieties have been presented by Waliszewska et al. [31]. However, there is very little information about lignin. The changes that lignin from the wood of Betula pubescens undergoes due to methane fermentation have been described by Mulat et al. [25], and the changes of lignin from waste corncob were investigated by Stachowiak-Wencek et al. [32]. Their results indicate that the lignin that is the residue after the lignocellulosic biomass methane fermentation has a different structure compared to the lignin present in the lignocellulosic biomass before fermentation or to lignin, which is a waste of cellulose pulp production. Given the need to replace polymers produced from crude oil and the growing interest in lignin as a natural aromatic polymer, lignin from post-fermentation residues seems to be an interesting alternative.
The aim of the present work was to determine the quantitative and qualitative changes of lignin of selected energy plant varieties, such as miscanthus (Miscanthus ×giganteus, Miscanthus sacchariflorus, and Miscanthus sinensis) and sorghum (Sorghum saccharatum and Sorghum bicolor) as a result of the AD process.
2. Materials and Methods
2.1. Plant Material and Sampling
The research was conducted using plants of three varieties of miscanthus: Miscanthus ×giganteus, Miscanthus sacchariflorus, and Miscanthus sinensis, as well as two varieties of sorghum: Sorghum bicolor and Sorghum saccharatum. The varieties of miscanthus were verified from experimental fields of the Institute of Plant Genetics at the Polish Academy of Sciences in Poznan, and the varieties of sorghum were verified from experimental fields of Kazimierz Wielki University in Bydgoszcz. Plants were harvested in two growing phases: during vegetation (in spring) and after vegetation (in autumn). Plants which were harvested during vegetation were labelled as DV (during vegetation), and plants which were harvested after vegetation were labelled as AV (after vegetation). Water content for miscanthus and sorghum ranged from 11.9% to 13% and from 83.7% to 87.7%, respectively. One part of the DV and AV materials was used for the determination of the percentage of lignin, and the second part for the AD process. The plant materials for the determination of lignin were ground in a laboratory mill (Fritsch type 15) using a sieve with 1.0 mm square screens, and then passed through brass sieves to separate the 0.5–1.0 mm fraction. The plant materials for fermentation were only cut into smaller pieces of 100–150 mm using scissors.
2.2. Chemical Analysis
2.2.1. Determination of Lignin Percentage
The percentage lignin content in DV and AV materials was determined before fermentation and in the residue after fermentation of those materials. Determination of the percentage was performed according to the TAPPI standard [33], using 72% sulphuric acid. Lignin obtained from the above determination was used in the identification of its structure. The lignin obtained from DV and AV materials before fermentation was labelled as “native lignin” (NL), while the lignin obtained from DV and AV materials after fermentation was labelled as “residue lignin” (RL).
2.2.2. Determination of Functional Groups
Methoxy groups in NL and RL were determined according to the modified Zeisel‒Vieböcka‒Schwappacha method [34]. Hydroxyl groups were determined using acetylation [35].
2.2.3. Fourier Transform-Infrared Spectroscopy of Lignin
Fourier transform-infrared (FT-IR) spectra of NL and RL were obtained using an Alfa FT-IR spectrometer produced by Bruker Optics GmbH (Ettlingen, Germany) and the software OPUS 6.5 was used to process the data. Powder samples of lignin (2 mg) were dispersed in a matrix of KBr (200 mg), followed by compression to form pellets. The samples were collected using 32 scans, in the range from 4000 to 400 cm−1, at a resolution of 4 cm−1. The syringyl-to-guaiacyl (S/G) ratio in lignin was calculated as the ratio of the FT-IR band intensities at 1325 cm–1 (S units) and 1267 cm–1 (G units), according to Fan et al. [36]. On the basis of the absorbance value ratio for the wavenumbers 2930 cm–1 and 1510 cm–1, a quantitative ratio of aliphatic to aromatic rings (Al/Ar) was also determined [37]. Three different measurements for each cellulose sample were evaluated, and the average value was considered.
2.3. Statistical Analysis
The experimental data were analysed using Dell™ Statistica™ 13.1 software (Dell, Inc., Palo Alto, CA, USA). For the lignin percentage, comparisons were subjected to analysis of variance (ANOVA) and significant differences between the mean values of control and treated samples were determined using Tukey’s HSD test for α = 0.05. Different superscripts (a, b, c…) denote significant difference between mean values of the percentage content of NL and RL (i.e., lignin in plants before fermentation and lignin in post-fermentation residue).
Anaerobic Digestion
Methane fermentation was conducted according to the DIN 38 414-S8 standard [38]. Approximately 60 g of miscanthus and 200 g of sorghum were analysed with the use of an inoculant (rich in methanogenic bacteria with dry matter content 2.7–2.9% and ash content 28–30%) in a mass of 1000 g. The fermentation process was performed in glass bioreactors of 2 dm3 volume. The experiment was carried out in a set of multi-chamber biofermenters [39]. The investigated material (substrate) was placed in the reactor and then covered with a portion of the inoculant. The reactors purged with nitrogen (to create anaerobic conditions), were placed in a water bath with a temperature of 39 ± 1 °C (mesophilic fermentation) to provide optimal conditions for the process. Biogas produced in each separate chamber was transferred to cylindrical store-equalizing reservoirs, filled with liquid resistant to gas solubility. The samples were tested in three replications.
3. Results and Discussion
3.1. Percentage of Lignin
Table 1 presents the percentage lignin content in the investigated plants harvested during two growing periods, before and after AD (i.e., content of NL and RL, respectively). The mean NL percentage of the miscanthus varieties was 18.2% and 21.1% for plants harvested during and after vegetation, respectively. Almost the same percentage content of lignin in varieties of miscanthus harvested at maturity were reported by Broose et al. and by Lee and Kuan [17]. For the sorghum varieties, a lower percentage content of NL was recorded (approximately 16%). A previous study of lignin content with respect to energy applications of sorghum was performed by Anami et al. [18]. Their results were significantly lower, amounting to 11%. Stefaniak et al. also investigated the chemical composition of sorghum, and obtained results for percentage lignin content ranging from 9% to 20%. It was found that for all of the studied miscanthus and sorghum varieties, the NL content in mature plants was higher. This confirms the tendency for lignification to take place at the end of a growing phase, as observed generally in the plant world [40].

Table 1.
Percentage lignin content before (NL) and after (RL) the anaerobic digestion (AD) process of miscanthus and sorghum.
Following the AD process, a higher level of RL was determined in all investigated materials than prior to the process and the determined differences were statistically significant. For all the varieties, the lignin increase was higher in the plants harvested during vegetation. For miscanthus it amounted to more than 50% and for sorghum to more than 100%. The results confirmed the limited potential of lignin fermentation and the carbohydrate’s susceptibility to decomposition due to microorganisms, also under anaerobic conditions. Similar observations have been reported by Sannigrahi and Ragauskas [41] and Mulat et al. [25].
3.2. Structure of Lignin
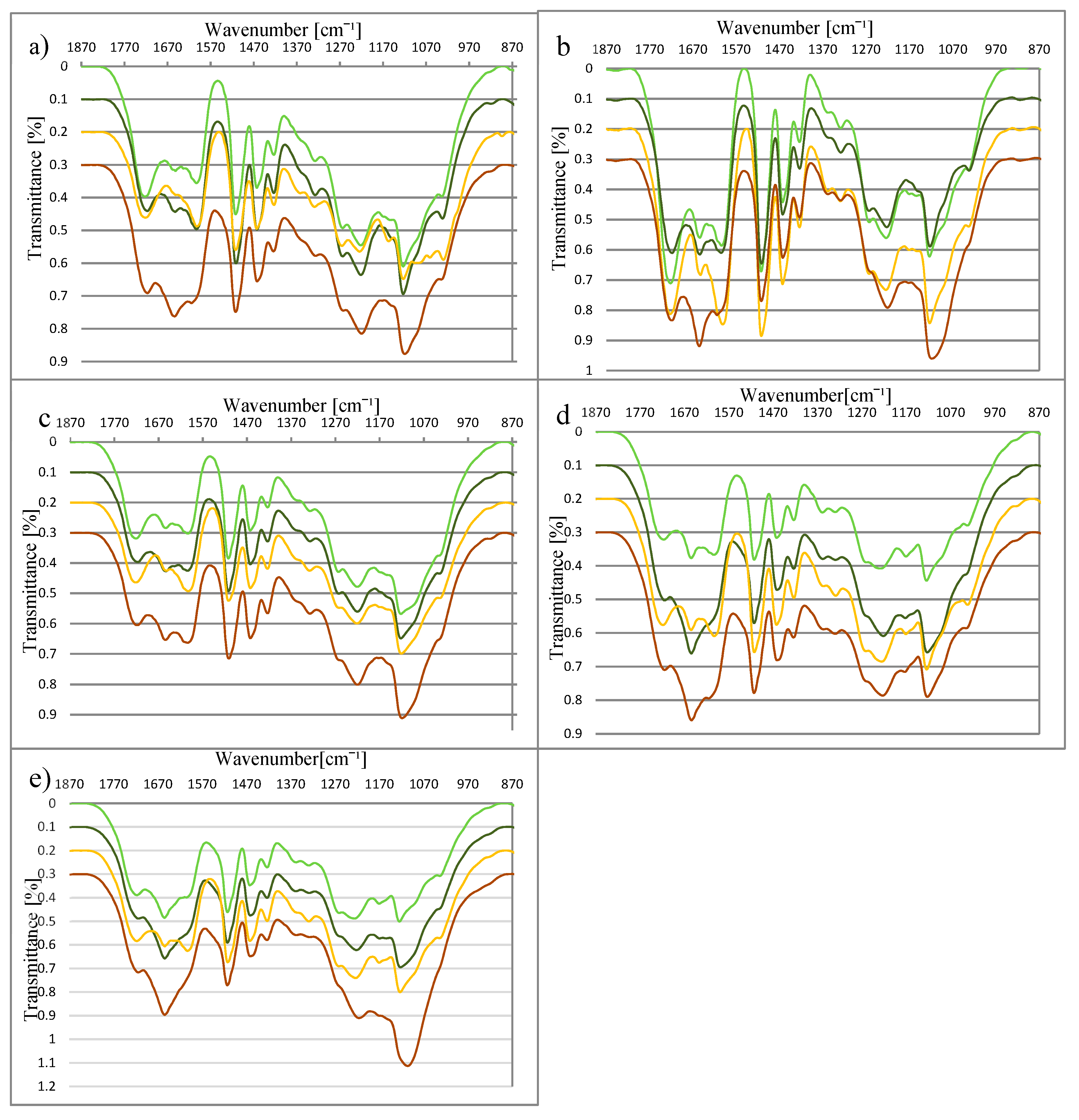
Spectra of lignin (the most interesting range from 870 cm−1 to 1870 cm−1) from plants harvested during (DV) and after (AV) vegetation obtained from material before (NL) and after (RL) fermentation, are shown in Figure 1a–e. All spectra include bands typical for the structure of lignin (Table 2).
Figure 1.
Fourier transform-infrared (FT-IR) of lignin from (a) Miscanthus ×giganteus, (b) M. sacchariflorus, (c) M. sinensis, (d) S. bicolor, and (e) S. saccharatum (bright green line—during vegetation before the AD; dark green line—during vegetation after the AD; yellow line—after vegetation before the AD; brown line—after vegetation after the AD).

Table 2.
FT-IR bands characteristic for lignin obtained from DV and AV materials, before and after the AD process [42,43].
The spectra of DV and AV from miscanthus and sorghum species differ at the wavenumbers 1327 cm−1 and 1600 cm−1. In the AV spectrum, the absorption at these wavenumbers is higher. Their presence in the spectrum corresponds to the existence of syringyl rings and the aromatic structure of lignin respectively. These differences indicate changes in the lignin structure during the vegetation process, and reflect the development of the syringyl type of lignin in the analysed species. Additionally, in the case of miscanthus varieties, signals at 1029 cm−1, 1061 cm−1, and 1156 cm−1, respectively assigned to C–H, C–C, and C–O–C, are stronger for the lignin from plants after vegetation. According to the literature, the regulation of the lignin biosynthesis is controlled very early by the different activities of enzymes under varying conditions, with regard to factors such as light and hormone supply [42,44]. The results obtained here suggest the formation of syringyl rings in the investigated species at the end of the vegetation process.
There were also differences in the structure of NL (lignin before fermentation) and RL (lignin after fermentation). For both miscanthus and sorghum, a decrease in absorption at 1327 cm−1 (assigned to syringyl rings) was observed. The syringyl type of lignin has less crosslinking structure thatn the guaiacyl type, and therefore it is more easierly undergoes decomposition [45,46,47,48]. It is possible that the decrease in syringyl rings content is connected with the fragmentation of the lignin net during the AD process. Additionally, on the spectra of NL and RL, changes at 1653 cm−1 were observed. This band is connected with the presence of C=O groups in the lignin structure. The increase in absorption at 1653 cm−1 may result from the breaking of C–O–C links between monolignols [49], and the release of the corresponding aromatic aldehydes (vanillin in the case of guaiacyl units and syringaldehyde in the case of syringyl units) [50].
Functional group content in lignin is presented in Table 3. The methoxy group content in NL from the investigated plant varieties harvested during and after vegetation ranged from 8.4% to 12.8% and is typical for lignocellulose biomass [51]. In most cases the content of these groups is higher in AV, which may be connected with the increase in syringyl unit content during vegetation. For miscanthus varieties, there was no unambiguous trend in methoxy group content resulting from AD. However, for sorghum their content in RL was lower after AD than in NL. Hydroxy group content in NL from both investigated varieties harvested during and after vegetation ranged from 0.4% to 5.0% and decreased in all the investigated plants during their growth period. For NL from miscanthus varieties, the mean content of OH groups was 3.4% during vegetation and 1.2% after vegetation. In NL from sorghum varieties, mean values of OH groups for the plants harvested during and after vegetation were close to those recorded in the case of miscanthus, amounting to 3.3% and 0.9%, respectively. This is probably connected with the cross-linking of lignin during its biosynthesis, resulting in hydroxyl groups forming ether or ester bonds [52]. The AD process led to a decline in the hydroxyl group content in lignin by approximately one half in all investigated plants. The changes occurring may be related to the utilisation of the oxygen of OH groups by microorganisms for biogas production in the AD process.

Table 3.
Functional group content of lignin before (NL) and after (RL) AD of miscanthus and sorghum.
Table 4 shows characteristics of monolignols of lignin from the miscanthus and sorghum varieties harvested during and after vegetation (the ratio of syringyl (S) and guaiacyl (G) units and the ratio of aromatic (Ar) and aliphatic (Al) structures), obtained by means of FT-IR. The S/G ratio obtained for NL from miscanthus varieties ranged from 0.34 to 0.66, while for sorghum it ranged from 0.57 to 0.64. These values are typical for lignin in non-wooden plants [53]. Lupoli and Smith [54] obtained an S/G ratio of 0.6 for Miscanthus ×giganteus, and Sattler et al. [55] obtained values for sorghum ranging from 0.03 to 0.625. In the case of the investigated plants it was found that for both varieties the S/G ratio was higher after vegetation. An increase in the S/G ratio of non-woody fibres with plant maturity has also been reported previously [56]. After the AD process, the S/G ratio of lignin from miscanthus varieties was altered, but the change did not follow a stable trend. However, for sorghum lignin the change in the S/G ratio was constant, and the ratio was lower after the AD process. Mulat et al. reported a similar pattern after the fermentation of birch wood. The decline of the S/G ratio in the investigated plants is related to the decrease in the content of methoxy groups (Table 2).

Table 4.
Characteristics of lignin structure before (NL) and after (RL) AD of miscanthus and sorghum.
The Al/Ar ratio in lignin from the investigated plants ranged from 0.47 to 0.99 and was significantly higher for the plants harvested during vegetation than after vegetation. The values obtained are characteristic for monocotyledons [37,57]. Similar values of the Al/Ar ratio for lignin of sorghum were obtained by Xiao et al. [58]. The fermentation process resulted in changes in the Al/Ar ratio values, although no clear trend was identified.
4. Conclusions
The results discussed above indicate that the investigated plants harvested in different growing seasons vary in terms of percentage lignin content and lignin structure. Plants during vegetation are characterised by lower lignin content, which indicates the better biogas potential of that raw material. During their growth, functional group content also alters: hydroxy group content decreases during vegetation, which may be related to cross-linking during cell lignification. The process may also additionally impede lignin fermentation. The methane fermentation process contributes to an increase in percentage lignin content in the material. The structure of that component also changes: a decline in hydroxy group content was observed for two varieties, as well as a decrease in a methoxy group content and in the S/G ratio for sorghum.
The studies indicate that despite its limitations, lignin is a biomass component that undergoes chemical changes during fermentation, and its structure becomes simpler, containing fewer functional groups than native lignin. This creates new possibilities for using this waste AD process, which results in a product very rich in lignin with new, specific chemical properties.
Author Contributions
Conceptualization, H.W., M.Z., A.S.-W., and B.W.; formal analysis: H.W.; data curation, H.W., M.Z., A.S.-W., and W.C.; funding acquisition, M.Z. and B.W.; methodology, H.W., M.Z., and W.C.; project administration, M.Z.; supervision, M.Z.; writing—original draft, H.W.
Funding
The work was financially supported by the Ministry of Science and Higher Education grant BIOSTRATEG 298241/10/NCBR/2016 Intelligent systems for breeding and cultivation of wheat, maize and poplar for optimized biomass production, biofuels and modified wood. Research was also granted by the Ministry of Science and Higher Education as the project (No. 506.423.02.0) with subsidies for maintaining the research capacity. The paper was partially financed within the framework of Ministry of Science and Higher Education programme ‘Regional Initiative of Excellence’ in years 2019-2022, Project No. 005/RID/2018/19.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Derkacheva, O.; Sukhov, D. Investigation of lignins by FTIR spectroscopy. Macromol. Symp. 2008, 265, 61–68. [Google Scholar] [CrossRef]
- Banoub, J.; Delmas, G.H.; Mackenzie, G.; Cachet, N.; Benjelloun Mlayah, B.; Delmas, M. A critique on the structural analysis of lignins and application of novel tandem mass spectrometric strategies to determine lignin sequencing. J. Mass Spectr. 2014, 50, 5–48. [Google Scholar] [CrossRef] [PubMed]
- Martone, P.T.; Estevez, J.M.; Lu, F.; Ruel, K.; Denny, M.W.; Somerville, C.; Ralph, J. Discovery of lignin in seaweed reveals convergent evolution of cell-wall architecture. Curr. Biol. 2009, 19, 169–175. [Google Scholar] [CrossRef]
- Doherty, W.; Mousavioun, P.; Fellows, C. Value-adding to cellulosic ethanol: Lignin polymers. Ind. Crop. Prod. 2011, 33, 259–276. [Google Scholar] [CrossRef]
- Lupoi, J.S.; Singh, S.; Parthasarathi, R.; Simmons, B.A.; Henry, R.J. Recent innovations in analytical methods for the qualitative and quantitative assessment of lignin. Renew. Sustain. Energy Rev. 2015, 49, 871–906. [Google Scholar] [CrossRef]
- Freudenberg, K. Biosynthesis and constitution of lignin. Nature 1959, 183, 1152. [Google Scholar] [CrossRef]
- Davison, B.H.; Drescher, S.R.; Tuskan, G.A.; Davis, M.F.; Nghiem, N.P. Variation of S/G ratio and lignin content in a Populus family influences the release of xylose by dilute acid hydrolysis. Appl. Biochem. Biotechnol. 2006, 129–132, 427–435. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef]
- Plomion, C.; Leprovost, G.; Stokes, A. Wood formation in trees. Plant Physiol. 2001, 127, 1513–1523. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tsukahara, K.; Yagishita, T.; Sawayama, S. Performance of a fixed-bed reactor packed with carbon felt during anaerobic digestion of cellulose. Bioresour. Technol. 2004, 94, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Brosse, N.; Dufour, F.A.; Meng, X.; Sun, Q.; Ragauskas, A. Miscanthus: A fast-growing crop for biofuels and chemicals production. Biofuels Bioprod. Bioref. 2012, 6, 580–598. [Google Scholar] [CrossRef]
- Dukiewicz, H.; Waliszewska, B.; Zborowska, M. Higher and lower heating values of selected lignocellulose materials. Ann. Wars. Univ. Life Sci-SGGW For. Wood Technol. 2014, 87, 60–63. [Google Scholar]
- Kozłowski, S.; Zielewicz, W.; Lutyński, A. Określanie wartości energetycznej Sorghum saccharatum (L.) Moench, Zea mays L. i Malva verticillata L. Łąkarstwo W Polsce 2007, 10, 131–140. [Google Scholar]
- She, D.; Xu, F.; Geng, Z.C.; Sun, R.C.; Jones, G.L.; Baird, M.S. Physicochemical characterization of extracted lignin from sweet sorghum stem. Ind. Crop. Prod. 2010, 32, 21–28. [Google Scholar] [CrossRef]
- Stefaniak, T.R.; Dahlberg, J.A.; Bean, B.W.; Dighe, N.; Wolfrum, E.J.; Rooney, W.L. Variation in biomass composition components among forage, biomass, sorghum-sudangrass, and sweet sorghum types. Crop Sci. 2012, 52, 1949–1954. [Google Scholar] [CrossRef]
- Tolbert, A.; Akinosho, H.; Khunsupat, R.; Naskar, A.K.; Ragauskas, A.J. Characterization and analysis of the molecular weight of lignin for biorefining studies. Biofuels. Bioprod. Biorefining 2014, 8, 836–856. [Google Scholar] [CrossRef]
- Lee, W.-C.; Kuan, W.-C. Miscanthus as cellulosic biomass for bioethanol production. Biotechnol. J. 2015, 10, 840–854. [Google Scholar] [CrossRef] [PubMed]
- Anami, S.E.; Zhang, L.-M.; Xia, Y.; Zhang, Y.-M.; Liu, Z.-Q.; Jing, H.-C. Sweet sorghum ideotypes: Genetic improvement of the biofuel syndrome. Food Energy Secur. 2015, 4, 159–177. [Google Scholar] [CrossRef]
- Mayer, F.; Gerin, P.A.; Noo, A.; Lemaigre, S.; Stilmant, D.; Schmit, T.; Leclech, N.; Ruelle, L.; Gennen, J.; von Francken-Welz, H.; et al. Assessment of energy crops alternative to maize for biogas production in the Greater Region. Bioresour. Technol. 2014, 166, 358–367. [Google Scholar] [CrossRef]
- Michalska, K.; Ledakowicz, S. Degradacja struktur lignocelulozowych oraz produktów ich hydrolizy. Inżynieria I Aparatura Chemiczna [Degradation of lignocellulosic structures and products of their hydrolysis. Eng. Chem. Appar. 2012, 51, 157. [Google Scholar]
- Castro, F.B.; Hotten, P.M.; Ørskov, E.R.; Rebeller, M. Inhibition of Rumen microbes by compounds formed in the steam treatment of wheat straw. Bioresour. Technol. 1994, 50, 25–30. [Google Scholar] [CrossRef]
- Pu, Y.; Hu, F.; Huang, F.; Davison, B.H.; Ragauskas, A.J. Assessing the molecular structure basis for biomass recalcitrance during dilute acid and hydrothermal pretreatments. Biotechnol. Biofuels 2013, 6, 1–13. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Theuretzbacher, F.; Lizasoain, J.; Lefever, C.; Saylor, M.K.; Enguidanos, R.; Weran, N.; Gronauer, A.; Bauer, A. Steam explosion pretreatment of wheat straw to improve methane yields: Investigation of the degradation kinetics of structural compounds during anaerobic digestion. Bioresour. Technol. 2015, 179, 299–305. [Google Scholar] [CrossRef]
- Mulat, D.G.; Dibdiakova, J.; Horn, S.J. Microbial biogas production from hydrolysis lignin: Insight into lignin structural changes. Biotechnol. Biofuels 2018, 11, 61–77. [Google Scholar] [CrossRef]
- Whittaker, C.; Hunt, J.; Misselbrook, T.; Shiel, I. How well does Miscanthus ensile for use in an anaerobic digestion plant? Biomass Bioenergy 2016, 88, 24–34. [Google Scholar] [CrossRef]
- Godin, B.; Agneessens, R.; Schmit, T.; Lamaudiere, S.; Goffart, J.P.; Gerin, P.A. Evolution of Sorghum and Corn Composition with the Harvest Period, with Focus on the Hemicelluloses Monosaccharidic Composition; Journée Annuelle de l’EDT Geproc and Envitam: Gembloux, Belgium, 2013. [Google Scholar]
- Galbe, M.; Zacchi, G. Pretreatment: The key to efficient utilization of lignocellulosic materials. Biomass Bioenergy 2012, 46, 70–78. [Google Scholar] [CrossRef]
- Mao, J.D.; Holtman, K.M.; Franqui-Villanueva, D. Chemical structures of corn stover and its residue after dilute acid prehydrolysis and enzymatic hydrolysis: Insight into factors limiting enzymatic hydrolysis. J. Agric. Food Chem. 2010, 58, 11680–11687. [Google Scholar] [CrossRef]
- Jung, S.; Foston, M.; Sullards, M.C.; Ragauskas, A.J. Surface characterization of dilute acid pretreated Populus deltoides by ToF-SIMS. Energy Fuels 2010, 24, 1347–1357. [Google Scholar] [CrossRef]
- Waliszewska, H.; Zborowska, M.; Waliszewska, B.; Borysiak, S.; Antczak, A.; Czekała, W. Transformation of miscanthus and sorghum cellulose during methane fermentation. Cellulose 2018, 25, 1207–1216. [Google Scholar] [CrossRef]
- Stachowiak-Wencek, A.; Zborowska, M.; Waliszewska, H. Waliszewska B., Zmiany struktury ligniny osadków kukurydzy pod wpływem fermentacji metanowej. Przemysł Chem. 2018, 97, 2162–2165. [Google Scholar]
- TAPPI method T 222 om-83. Acid-insoluble lignin in wood and pulp. In Test Methods, 1998–1999; TAPPI Press: Atlanta, GA, USA, 1999.
- Kačík, F.; Kačíková, D.; Bubenikova, T.; Veľková, V. Determination of methoxy groups in lignocellulosic materials. Drewno 2004, 47, 113–119. [Google Scholar]
- Lin, S.Y.; Dence, C.W. Methods in Lignin Chemistry, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 1992. [Google Scholar] [CrossRef]
- Fan, M.; Dai, D.; Huang, B. Fourier Transform Infrared Spectroscopy for Natural Fibres, Fourier Transform Salih Mohammed Salih, IntechOpen. Available online: https://www.intechopen.com/books/fourier-transform-materials-analysis/fourier-transform-infrared-spectroscopy-for-natural-fibres (accessed on 23 May 2012). [CrossRef]
- Ungureanu, E.; Ungureanu, O.; Căpraru, A.-M.; Popa, V.I. Chemical modification and characterization of straw lignin. Cellul. Chem. Technol. 2009, 43, 263–269. [Google Scholar]
- DIN 38 414-S8. Bestimmung des Faulverhaltens “Schlamm und Sedimente”; BeuthVerlag GmbH: Berlin, Germany, 1985.
- Lewicki, A.; Pilarski, K.; Janczak, D.; Czekała, W.; Rodríguez Carmona, P.C.; Cieślik, M.; Witaszek, K. The biogas production from herbs and waste from herbal industry. J. Res. Appl. Agric. Eng. 2013, 58, 114–117. [Google Scholar]
- Shugang, Z.; Jing, W.; Hongxia, W.; Zhihua, Z.; Xibo, L. Changes in lignin content and activity of related enzymes in the endocarp during the walnut shell development period. Hortic. Plant J. 2016, 2, 141–146. [Google Scholar]
- Sannigrahi, P.; Ragauskas, A.J. Characterization of fermentation residues from the production of bio-ethanol from lignocellulosic feedstocks. J. Biobased Mater. Bioenergy 2011, 5, 514–519. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood—Chemistry, Ultrastructure, Reactions, 2nd ed.; Walter de Gruyter: Berlin, Germany, 1989. [Google Scholar]
- Popescu, C.M.; Popescu, M.C.; Singurel, G.; Vasile, C.; Argyropoulos, D.S.; Willfor, S. Spectral characterization of eucalyptus wood. Appl. Spectrosc. 2007, 61, 1168–1177. [Google Scholar] [CrossRef]
- Hatfield, R.; Ralph, J.; Grabber, J.H. A potential role for sinapyl p-coumarate as a radical transfer mechanism in grass lignin formation. Planta 2008, 228, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Faix, O.; Mozuch, M.D.; Kent Kirk, T. Degradation of gymnosperm (guaiacyl) vs. angiosperm (syringyl/guaiacyl) lignins by phanerochaete chrysosporium. Holzforschung 1985, 39, 203–208. [Google Scholar] [CrossRef]
- Lewis, N.; Yamamoto, E. Lignin: Occurrence, biogenesis and biodegradation. Annu. Rev. Plant Biol. 1990, 41, 455–496. [Google Scholar] [CrossRef] [PubMed]
- Skyba, O.; Douglas, C.J.; Mansfield, S.D. Syringyl-rich lignin renders poplars more resistant to degradation by wood decay fungi. Appl. Environ. Microbiol. 2013, 79, 2560–2571. [Google Scholar] [CrossRef]
- Obst, J.R.; Highley, T.L.; Miller, R.B. Influence of Lignin Type on the Decay of Woody Angiosperms by Trametes Versicolor. In Mycotoxins, Wood Decay, Plant Stress: Biocorrosion, and General Biodeterioration; Llewellyn, G.C., Dashek, W.V., O’Rear, C.E., Eds.; Biodeterioration Research; Springer: Boston, MA, USA, 1994; Volume 4. [Google Scholar]
- Chua, M.; Chen, C.; Chang, H. 13C NMR Spectroscopic study of spruce lignin degraded Phanerochaete Chrysosporium. Holzforsch.—Int. J. Biol. Chem. Phys. Technol. Wood 2009, 36, 165–172. [Google Scholar]
- Ruiz-Dueñas, F.J.; Martínez, A.T. Microbial degradation of lignin: How a bulky recalcitrant polymer is efficiently recycled in nature and how we can take advantage of this. Microb. Biotechnol. 2009, 2, 164–177. [Google Scholar] [CrossRef]
- Brauns, F.E.; Brauns, D.A. The Chemistry of Lignin: Covering the Literature for the Years 1949–1958; Academic Press: New York, NY, USA; London, UK, 1960. [Google Scholar]
- Zhao, Q. Lignification: Flexibility, Biosynthesis and Regulation. Trends Plant Sci. 2016, 21, 713–721. [Google Scholar] [CrossRef]
- Marques, G.; Rencoret, J.; Gutiérrez, A.; del Río, J.C. Evaluation of the chemical composition of different non-woody plant fibers used for pulp and paper manufacturing. Open Agric. J. 2010, 3, 93–101. [Google Scholar] [CrossRef]
- Lupoi, J.S.; Smith, E.A. Characterization of woody and herbaceous biomasses lignin composition with 1064 nm dispersive multichannel Raman spectroscopy. Appl. Spectrosc. 2012, 66, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Sattler, S.E.; Palmer, N.A.; Saballos, A.; Greene, A.M.; Xin, Z.; Sarath, G.; Vermerris, W.; Pedersen, J.F. “Identification and Characterization of Four Missense Mutations in Brown midrib12 (Bmr12), the Caffeic O-Methyltranferase (COMT) of Sorghum”. Bioenerg. Res. 2012, 5, 855–865. [Google Scholar] [CrossRef]
- Mazumder, B. Fortunate Sons: New Estimates of Intergenerational Mobility in the United States Using Social Security Earnings Data. Rev. Econ. Stat. 2005, 87, 235–255. [Google Scholar] [CrossRef]
- Todorciuc, T.; Căpraru, A.-M.; Kratochvílová, I.; Popa, V.I. Characterization of non-wood lignin and its hydoxymethylated derivatives by spectroscopy and self-assembling investigations. Cellul. Chem. Technol. 2009, 43, 399–408. [Google Scholar]
- Xiao, Z.; Li, Y.; Wu, X.; Qi, G.; Li, N.; Zhang, K.; Wang, D.; Sun, X.S. Utilization of sorghum lignin to improve adhesion strength of soy protein adhesives on wood veneer. Ind. Crop. Prod. 2013, 50, 501–509. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).