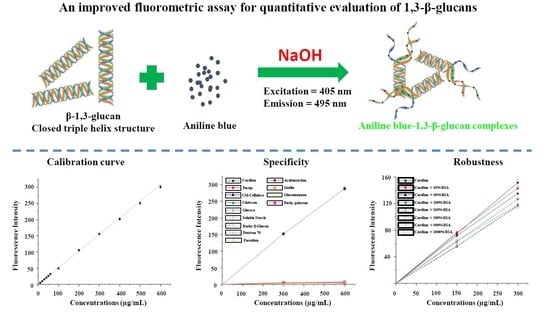
Quantitative Evaluation of Ultrasound-Assisted Extraction of 1,3-β-glucans from Dictyophora indusiata Using an Improved Fluorometric Assay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Chemicals
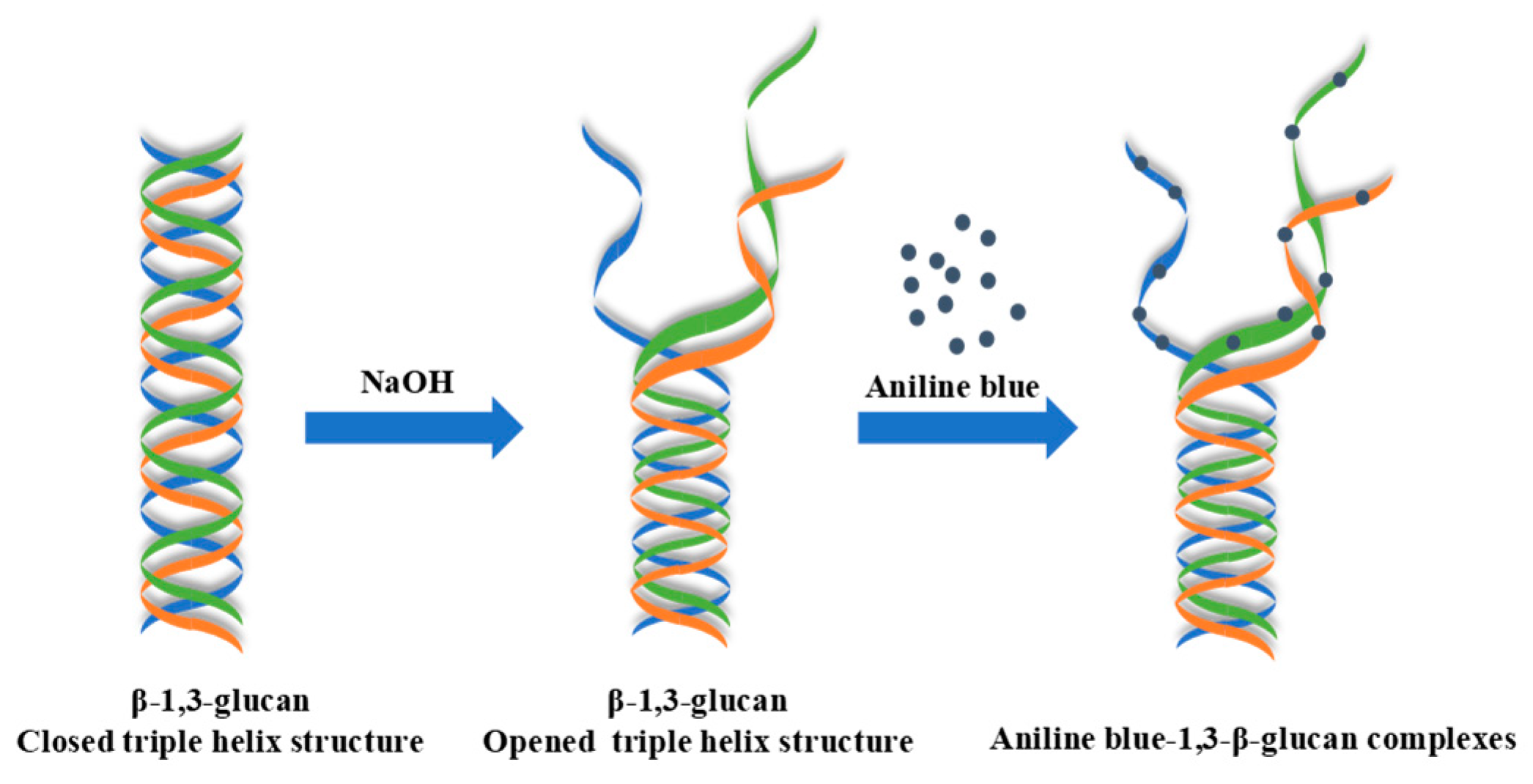
2.2. Fluorometric Assay for 1,3-β-glucans Quantification
2.2.1. Optimization, Calibration Curve, and Specificity of the Fluorometric Assay
2.2.2. Precision, Repeatability, Stability, Accuracy, Limit of Determination (LOD), and Limit of Quantification (LOQ) of the Improved Fluorometric Assay
2.3. Ultrasound-Assisted Extraction of Water Soluble 1,3-β-glucans from D. indusiata
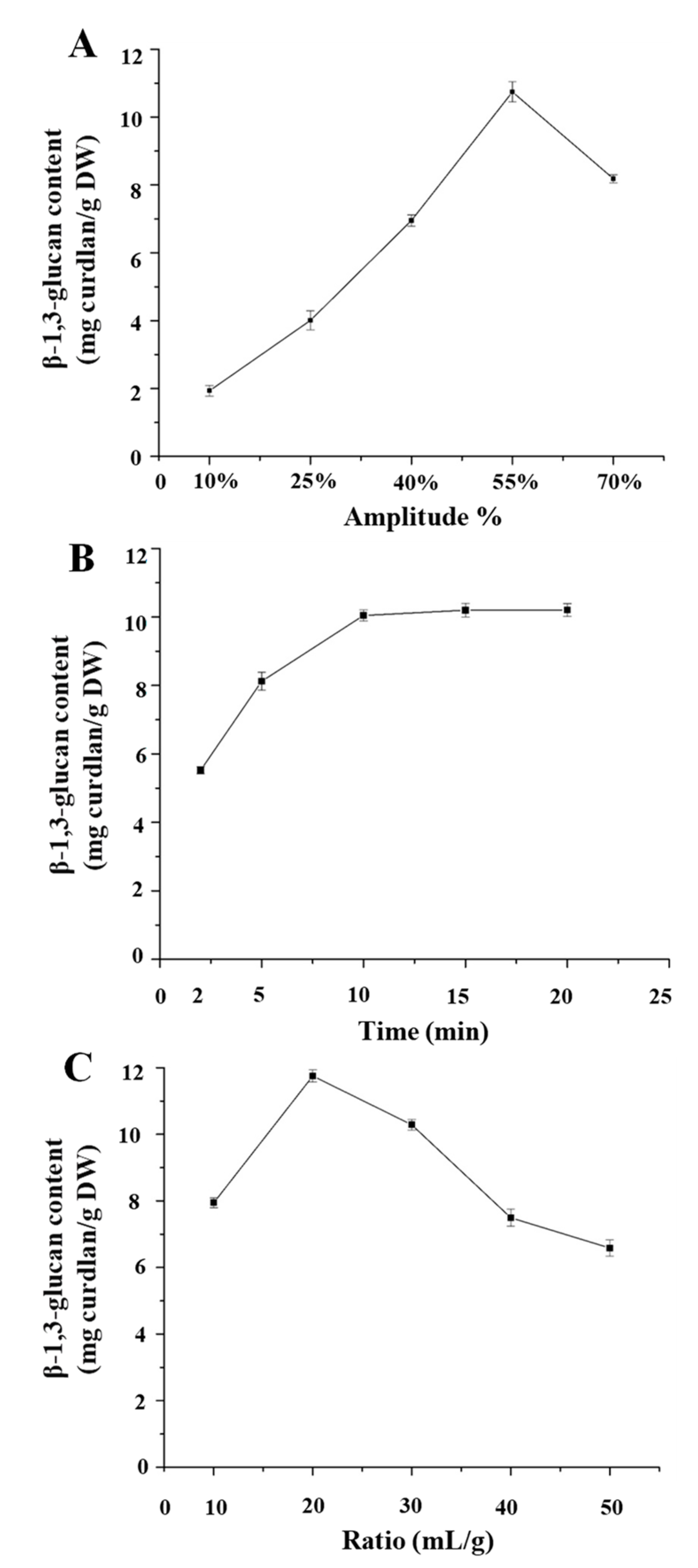
2.3.1. Single Factor Experimental Design
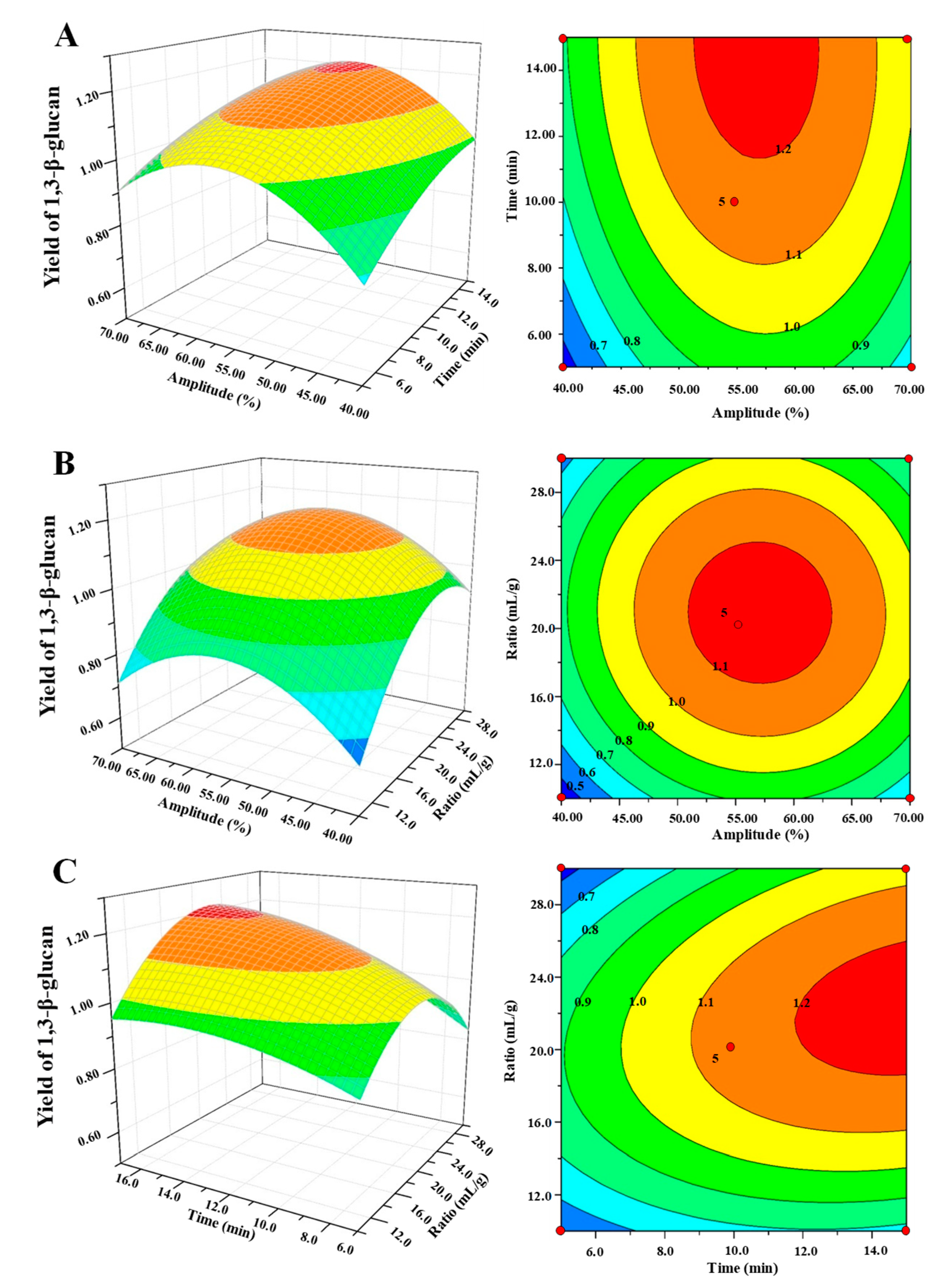
2.3.2. Box–Behnken Experimental Design
2.3.3. Hot Water Extraction of Water Soluble 1,3-β-glucans from D. indusiata
2.4. Statistical Analysis
3. Results and Discussions
3.1. Optimal Conditions, Calibration Curve, and Specificity of the Fluorometric Assay
3.2. Method Validation of the Improved Fluorometric Assay
3.3. Single Factor Experimental Analysis
3.4. Model Fitting and Statistical Analysis
3.5. Analysis of Response Surface Plot and Contour Plot
3.6. Verification of the Predictive Model and Comparison of UAE and HWE
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Deng, C.; Shang, J.; Fu, H.; Chen, J.; Liu, H.; Chen, J. Mechanism of the immunostimulatory activity by a polysaccharide from Dictyophora indusiata. Int. J. Biol. Macromol. 2016, 91, 752–759. [Google Scholar] [CrossRef]
- Liao, W.; Luo, Z.; Liu, D.; Ning, Z.; Yang, J.; Ren, J. Structure characterization of a novel polysaccharide from Dictyophora indusiata and its macrophage immunomodulatory activities. J. Agric. Food Chem. 2015, 63, 535–544. [Google Scholar] [CrossRef]
- Yaw-Bee, K.; Chen, K.C.; Peng, C.C.; Chiu-Lan, H.; Peng, R.Y. Structural Characteristics and Antioxidative Capability of the Soluble Polysaccharides Present in Dictyophora indusiata (Vent. Ex Pers.) Fish Phallaceae. J. Evid.-Based Complement. Altern. Med. 2011, 2011, 396013. [Google Scholar]
- Wang, Y.; Shi, X.; Yin, J.; Nie, S. Bioactive polysaccharide from edible Dictyophora spp.: Extraction, purification, structural features and bioactivities. Bioact. Carbohydr. Diet. Fibre 2018, 14, 25–32. [Google Scholar] [CrossRef]
- Deng, C.; Hu, Z.; Fu, H.; Hu, M.; Xu, X.; Chen, J. Chemical analysis and antioxidant activity in vitro of a β-D-glucan isolated from Dictyophora indusiata. Int. J. Biol. Macromol. 2012, 51, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Fu, H.; Teng, L.; Hu, Z.; Xu, X.; Chen, J.; Ren, T. Anti-tumor activity of the regenerated triple-helical polysaccharide from Dictyophora indusiata. Int. J. Biol. Macromol. 2013, 61, 453–458. [Google Scholar] [CrossRef]
- Wang, J.; Xu, X.; Zheng, H.; Li, J.; Deng, C.; Xu, Z.; Chen, J. Structural characterization, chain conformation, and morphology of a β-(1→3)-d-glucan isolated from the fruiting body of Dictyophora indusiata. J. Agric. Food Chem. 2009, 57, 5918–5924. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Deng, C.; Teng, L.; Yu, L.; Su, T.; Xu, X.; Chen, J.; Yang, C. Immunomodulatory Activities on RAW 264.7 Macrophages of a Polysaccharide from Veiled Lady Mushroom, Dictyophora indusiata (Higher Basidiomycetes). Int. J. Med. Mushrooms 2015, 17, 151–160. [Google Scholar]
- Liu, X.; Chen, Y.; Wu, L.; Wu, X.; Huang, Y.; Liu, B. Optimization of polysaccharides extraction from Dictyophora indusiata and determination of its antioxidant activity. Int. J. Biol. Macromol. 2017, 103, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Ren, D.; Yang, N.; Yang, X. Optimization for ultrasound-assisted extraction of polysaccharides with chemical composition and antioxidant activity from the Artemisia sphaerocephala Krasch seeds. Int. J. Biol. Macromol. 2016, 91, 856–866. [Google Scholar] [CrossRef]
- Jiang, C.; Li, X.; Jiao, Y.; Jiang, D.; Zhang, L.; Fan, B.; Zhang, Q. Optimization for ultrasound-assisted extraction of polysaccharides with antioxidant activity in vitro from the aerial root of Ficus microcarpa. Carbohydr. Polym. 2014, 110, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Li, F.; Xu, X.; Tang, J. Optimization of ultrasonic-assisted extraction of antioxidant polysaccharides from the stem of Trapa quadrispinosa using response surface methodology. Int. J. Biol. Macromol. 2017, 94, 335–344. [Google Scholar] [CrossRef]
- Aguiló-Aguayo, I.; Walton, J.; Viñas, I.; Tiwari, B.K. Ultrasound assisted extraction of polysaccharides from mushroom by-products. LWT-Food Sci. Technol. 2017, 77, 92–99. [Google Scholar] [CrossRef]
- Guo, X.; Shang, X.; Zhou, X.; Zhao, B.; Zhang, J. Ultrasound-assisted extraction of polysaccharides from Rhododendron aganniphum: Antioxidant activity and rheological properties. Ultrason. Sonochem. 2017, 38, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Maran, J.P.; Mekala, V.; Manikandan, S. Modeling and optimization of ultrasound-assisted extraction of polysaccharide from Cucurbita moschata. Carbohydr. Polym. 2013, 92, 2018–2026. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, W.P.; Zhang, H.S.; Huang, Q. Optimization of ultrasonic-assisted extraction of water-soluble polysaccharides from Boletus edulis mycelia using response surface methodology. Carbohydr. Polym. 2012, 87, 614–619. [Google Scholar] [CrossRef]
- Ko, Y.T.; Lin, Y.L. 1,3-β-glucan Quantification by a Fluorescence Microassay and Analysis of Its Distribution in Foods. J. Agric. Food Chem. 2004, 52, 3313–3318. [Google Scholar] [CrossRef]
- Nitschke, J.; Modick, H.; Busch, E.; von Rekowski, R.W.; Altenbach, H.J.; Mölleken, H. A new colorimetric method to quantify β-1,3-1,6-glucans in comparison with total β-1,3-glucans in edible mushrooms. Food Chem. 2011, 127, 791–796. [Google Scholar] [CrossRef]
- Smiderle, F.R.; Morales, D.; Gil-Ramírez, A.; Jesus, L.I.D.; Gilbert-López, B.; Iacomini, M.; Soler-Rivas, C. Evaluation of microwave-assisted and pressurized liquid extractions to obtain β-d-glucans from mushrooms. Carbohydr. Polym. 2017, 156, 165–174. [Google Scholar] [CrossRef]
- Koenig, S.; Rühmann, B.; Sieber, V.; Schmid, J. Quantitative assay of β-(1,3)–β-(1,6)–glucans from fermentation broth using aniline blue. Carbohydr. Polym. 2017, 174, 57–64. [Google Scholar] [CrossRef]
- Long, G.L.; Winefordner, J.D. Limit of Detection A Closer Look at the IUPAC Definition. Anal. Chem. 1983, 55, 712A–724A. [Google Scholar]
- Young, S.H.; Jacobs, R.R. Sodium hydroxide-induced conformational change in schizophyllan detected by the fluorescence dye, aniline blue. Carbohydr. Res. 1998, 310, 91–99. [Google Scholar] [CrossRef]
- Wood, P.J.; Fulcher, R.G. Specific interaction of aniline blue with (13)-β-D-glucan. Carbohydr. Polym. 1984, 4, 49–72. [Google Scholar] [CrossRef]
- Young, S.H. Observation of a Partially Opened Triplehelix Conformation in 1right-arrow3-beta Glucan by Fluorescence Resonance Energy Transfer Spectroscopy. J. Biol. Chem. 2000, 275, 11874–11879. [Google Scholar] [CrossRef] [PubMed]
- Knill, C.J.; Kennedy, J.F. Degradation of cellulose under alkaline conditions. Carbohydr. Polym. 2003, 51, 281–300. [Google Scholar] [CrossRef]
- Jin, W.; Mei, T.; Wang, Y.; Xu, W.; Li, J.; Zhou, B.; Li, B. Synergistic degradation of konjac glucomannan by alkaline and thermal method. Carbohydr. Polym. 2014, 99, 270–277. [Google Scholar] [CrossRef]
- Zeng, H.; Zhang, Y.; Lin, S.; Jian, Y.; Miao, S.; Zheng, B. Ultrasonic–microwave synergistic extraction (UMSE) and molecular weight distribution of polysaccharides from Fortunella margarita (Lour.) Swingle. Sep. Purif. Technol. 2015, 144, 97–106. [Google Scholar] [CrossRef]
- Ren, B.; Chen, C.; Li, C.; Fu, X.; You, L.; Liu, R.H. Optimization of microwave-assisted extraction of Sargassum thunbergii polysaccharides and its antioxidant and hypoglycemic activities. Carbohydr. Polym. 2017, 173, 192–201. [Google Scholar] [CrossRef]
- Wu, J.; Yu, D.; Sun, H.; Zhang, Y.; Zhang, W.; Meng, F.; Du, X. Optimizing the extraction of anti-tumor alkaloids from the stem of Berberis amurensis by response surface methodology. Ind. Crop. Prod. 2015, 69, 68–75. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, Q.; Li, Y.; Li, L.; Lan, W.; He, J.; Li, H.; Xiong, Y.; Qin, W. Extraction, characterization and antioxidant activities of polysaccharides of Chuanminshen violaceum. Int. J. Biol. Macromol. 2016, 86, 224–232. [Google Scholar] [CrossRef]

| Runs | Levels of Independent Factors a | Extraction Yield % | |||
|---|---|---|---|---|---|
| X1 (%) | X2 (min) | X3 (mL/g) | Observed b | Predicted | |
| 1 | 1 (70.00) | 1 (15.00) | 0 (20.00) | 1.055 | 1.069 |
| 2 | 0 (55.00) | 0 (10.00) | 0 (20.00) | 1.138 | 1.163 |
| 3 | 0 (55.00) | 0 (10.00) | 0 (20.00) | 1.158 | 1.163 |
| 4 | 0 (55.00) | 0 (10.00) | 0 (20.00) | 1.178 | 1.163 |
| 5 | −1 (40.00) | 0 (10.00) | 1 (30.00) | 0.762 | 0.799 |
| 6 | 0 (55.00) | 1 (15.00) | −1 (10.00) | 0.929 | 0.952 |
| 7 | −1 (40.00) | −1 (5.00) | 0 (20.00) | 0.795 | 0.781 |
| 8 | 0 (55.00) | 0 (10.00) | 0 (20.00) | 1.199 | 1.163 |
| 9 | 0 (55.00) | 1 (15.00) | 1 (30.00) | 1.138 | 1.114 |
| 10 | 1 (70.00) | 0 (10.00) | −1 (10.00) | 0.869 | 0.832 |
| 11 | 1 (70.00) | 0 (10.00) | 1 (30.00) | 0.875 | 0.886 |
| 12 | 0 (55.00) | −1 (5.00) | 1 (30.00) | 0.855 | 0.832 |
| 13 | 0 (55.00) | 0 (10.00) | 0 (20.00) | 1.142 | 1.163 |
| 14 | 1 (70.00) | −1 (5.00) | 0 (20.00) | 0.892 | 0.905 |
| 15 | 0 (55.00) | −1 (5.00) | −1 (10.00) | 0.835 | 0.860 |
| 16 | −1 (40.00) | 1 (15.00) | 0 (20.00) | 1.003 | 0.991 |
| 17 | −1(40.00) | 0 (10.00) | −1 (10.00) | 0.875 | 0.886 |
| Source a | Sum of Squares | df b | Mean Square | F-Value | p-Value c |
|---|---|---|---|---|---|
| Model | 40.75 | 9 | 4.53 | 37.20 | <0.0001** |
| X1 | 2.03 | 1 | 2.03 | 16.68 | 0.0047** |
| X2 | 7.00 | 1 | 7.00 | 57.52 | 0.0001** |
| X3 | 0.89 | 1 | 0.89 | 7.35 | 0.0302* |
| X1X2 | 0.051 | 1 | 0.051 | 0.42 | 0.5385 |
| X1X3 | 0.018 | 1 | 0.018 | 0.15 | 0.7087 |
| X2X3 | 0.90 | 1 | 0.90 | 7.37 | 0.0300* |
| X12 | 13.43 | 1 | 13.43 | 110.38 | <0.0001** |
| X22 | 0.97 | 1 | 0.97 | 8.01 | 0.0254* |
| X32 | 13.01 | 1 | 13.01 | 106.88 | <0.0001** |
| Residual error | 0.85 | 7 | 0.12 | ||
| Lack of fit | 0.59 | 3 | 0.20 | 3.07 | 0.1534 |
| Pure error | 0.26 | 4 | 0.064 | ||
| Correlation total | 41.60 | 16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Lin, S.; Lu, M.; Wei, S.-Y.; Zhou, J.; Zhao, L.; Zhang, Q.; Lin, D.-R.; Liu, Y.-T.; Chen, H.; et al. Quantitative Evaluation of Ultrasound-Assisted Extraction of 1,3-β-glucans from Dictyophora indusiata Using an Improved Fluorometric Assay. Polymers 2019, 11, 864. https://doi.org/10.3390/polym11050864
Fu Y, Lin S, Lu M, Wei S-Y, Zhou J, Zhao L, Zhang Q, Lin D-R, Liu Y-T, Chen H, et al. Quantitative Evaluation of Ultrasound-Assisted Extraction of 1,3-β-glucans from Dictyophora indusiata Using an Improved Fluorometric Assay. Polymers. 2019; 11(5):864. https://doi.org/10.3390/polym11050864
Chicago/Turabian StyleFu, Yuan, Shang Lin, Min Lu, Si-Yu Wei, Jia Zhou, Li Zhao, Qing Zhang, De-Rong Lin, Yun-Tao Liu, Hong Chen, and et al. 2019. "Quantitative Evaluation of Ultrasound-Assisted Extraction of 1,3-β-glucans from Dictyophora indusiata Using an Improved Fluorometric Assay" Polymers 11, no. 5: 864. https://doi.org/10.3390/polym11050864
APA StyleFu, Y., Lin, S., Lu, M., Wei, S.-Y., Zhou, J., Zhao, L., Zhang, Q., Lin, D.-R., Liu, Y.-T., Chen, H., Qin, W., & Wu, D.-T. (2019). Quantitative Evaluation of Ultrasound-Assisted Extraction of 1,3-β-glucans from Dictyophora indusiata Using an Improved Fluorometric Assay. Polymers, 11(5), 864. https://doi.org/10.3390/polym11050864