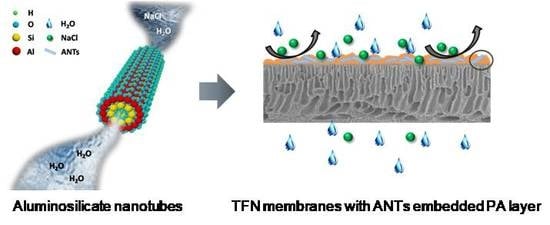
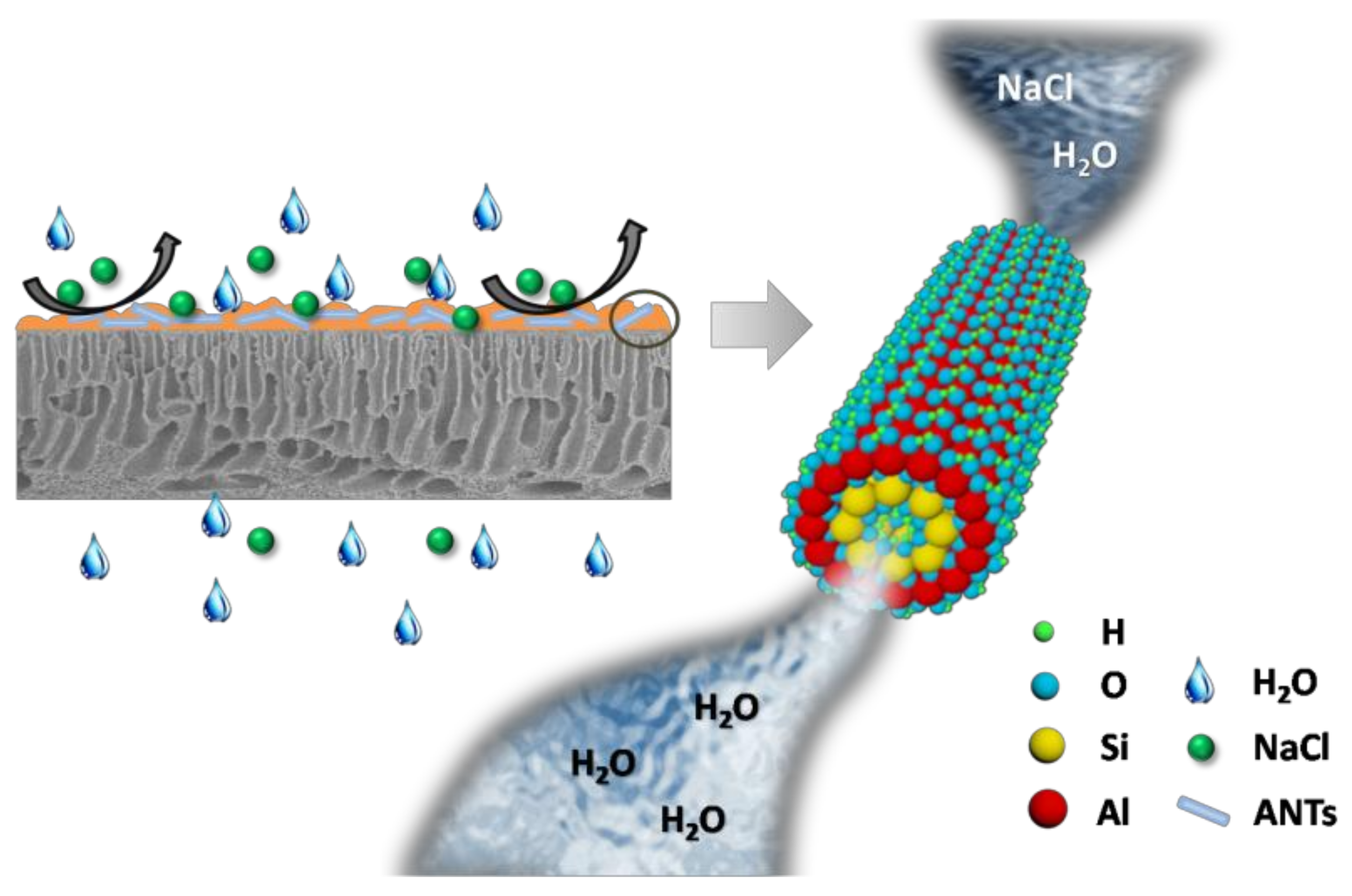
Aluminosilicate Nanotubes Embedded Polyamide Thin Film Nanocomposite Forward Osmosis Membranes with Simultaneous Enhancement of Water Permeability and Selectivity
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis and Characterization of ANTs
2.3. Fabrication and Characterization of Forward Osmosis (FO) Membranes
2.3.1. Preparation of PSf Substrates
2.3.2. Preparation of Thin Film Nanocomposite (TFN) Membranes
2.3.3. Membrane Characterization
2.4. Evaluation of Membrane Performance
2.5. Molecular Dynamics Simulation
3. Results and Discussion
3.1. Structure and Chemical Composition of Synthesized ANTs
3.2. Surface Composition and Morphology of TFN Membranes
3.3. Effect of ANTs Loading on Intrinsic Transport Performance of TFN Membranes
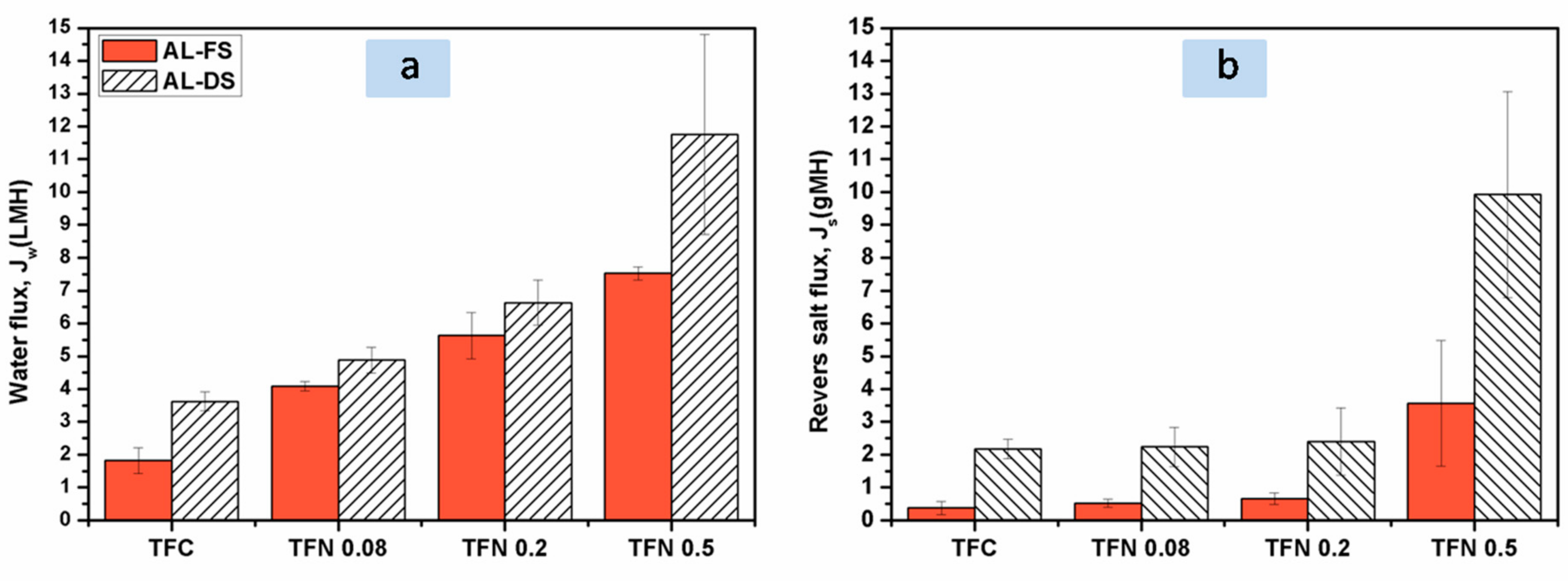
3.4. Effect of ANTs Loading on FO Performance of TFN Membranes
3.5. Molecular Dynamic Simulation of Water Diffusion through ANTs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Montgomery, M.A.; Elimelech, M. Water and sanitation in developing countries: Including health in the equation. Environ. Sci. Technol. 2007, 41, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef]
- Faria, A.F.; Liu, C.; Xie, M.; Perreault, F.; Nghiem, L.D.; Ma, J.; Elimelech, M. Thin-film composite forward osmosismembranes functionalized with graphene oxide–silver nanocomposites for biofouling control. J. Membr. Sci. 2017, 525, 146–156. [Google Scholar] [CrossRef]
- Xiong, S.; Zuo, J.; Ma, Y.G.; Liu, L.; Wu, H.; Wang, Y. Novel thin film composite forward osmosis membrane of enhanced water flux and anti-fouling property with N-[3-(trimethoxysilyl) propyl] ethylenediamine incorporated. J. Membr. Sci. 2016, 520, 400–414. [Google Scholar] [CrossRef]
- Cheng, Z.L.; Li, X.; Chung, T.-S. The forward osmosis-pressure retarded osmosis (FO-PRO) hybrid system: A new process to mitigate membrane fouling for sustainable osmotic power generation. J. Membr. Sci. 2018, 559, 63–74. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Xing, X.Y.; Yu, Y.; Gu, L.; Xu, Z.K. Novel thin film composite membranes supported by cellulose triacetate porous substrates for high-performance forward osmosis. Polymer. 2018, 153, 150–160. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Pan, Y.H.; Jin, W.Z.; Xu, J.M.; Lao, K.K.; Gu, L. Preparation of sodium lignin sulfonate modified polysulfone membranes and their use as supports for forward osmosis membrane. Acta Polym. Sin. 2017, 5, 851–857. [Google Scholar]
- Liang, H.Q.; Hung, W.S.; Yu, H.H.; Hu, C.C.; Lee, K.R.; Lai, J.Y.; Xu, Z.K. Forwar dosmosis membranes with unprecedented water flux. J. Membr. Sci. 2017, 529, 47–54. [Google Scholar] [CrossRef]
- Shen, Y.-X.; Saboe, P.O.; Sines, I.T.; Erbakan, M.; Kumar, M. Biomimetic membranes: Areview. J. Membr. Sci. 2014, 454, 359–381. [Google Scholar] [CrossRef]
- Song, W.; Lang, C.; Shen, Y.-x.; Kumar, M. Design considerations for artificial water channel–based membranes. Annu. Rev. Mater. Res. 2018, 48, 57–82. [Google Scholar] [CrossRef]
- Xia, L.; Andersen, M.F.; Hélix-Nielsen, C.; McCutcheon, J.R. Novel commercial aquaporin flat-sheet membrane for forward osmosis. Ind. Eng. Chem. Res. 2017, 56, 11919–11925. [Google Scholar] [CrossRef]
- Shen, Y.-X.; Song, W.C.; Barden, D.R.; Ren, T.; Lang, C.; Feroz, H.; Henderson, C.B.; Saboe, P.O.; Tsai, D.; Yan, H. Achieving high permeability and enhanced selectivity for angstrom-scale separations using artificial water channel membranes. Nat. Commun. 2018, 9, 2294–2305. [Google Scholar] [CrossRef]
- Shen, Y.-X.; Si, W.; Erbakan, M.; Decker, K.; DeZorzi, R.; Saboe, P.O.; Kang, Y.J.; Majd, S.; Butler, P.J.; Walz, T. Highly permeable artificial water channels that can self-assemble into two-dimensional arrays. Proc. Natl. Acad. Sci. USA 2015, 112, 9810–9815. [Google Scholar] [CrossRef] [Green Version]
- Ghaseminezhad, S.M.; Barikani, M.; Salehirad, M. Development of graphene oxide-cellulose acetate nanocomposite reverse osmosis membrane for seawater desalination. Compos. Part B Eng. 2019, 161, 320–327. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zhao, S.; Fang, Z.; Ng, D.; Xie, C.; Wang, H.; Xie, Z. Thin-film composite membrane with interlayer decorated metal–organic framework Uio-66 toward enhanced forward osmosis performance. Ind. Eng. Chem. Res. 2019, 58, 195–206. [Google Scholar] [CrossRef]
- Tian, E.; Wang, X.; Wang, X.; Ren, Y.; Zhao, Y.; An, X. Preparation and characterization of thin-film nanocomposite membrane with high flux and antibacterial performance for forward osmosis. Ind. Eng. Chem. Res. 2019, 58, 897–907. [Google Scholar] [CrossRef]
- Emadzadeh, D.; Lau, W.J.; Rahbari-Sisakht, M.; Ilbeygi, H.; Rana, D.; Matsuura, T.; Ismail, A.F. Synthesis, modification and optimization of titanate nanotubes-polyamide thin film nanocomposite (TFN) membrane for forward osmosis (FO) application. Chem. Eng. J. 2015, 281, 243–251. [Google Scholar] [CrossRef]
- Ghanbari, M.; Emadzadeh, D.; Lau, W.J.; Lai, S.O.; Matsuura, T.; Ismail, A.F. Synthesis and characterization of novel thin film nanocomposite (TFN) membranes embedded with halloysite nanotubes (HNTs) for water desalination. Desalination 2015, 358, 33–41. [Google Scholar] [CrossRef]
- Tunuguntla, R.H.; Henley, R.Y.; Yao, Y.-C.; Pham, T.A.; Wanunu, M.; Noy, A. Enhanced water permeability and tunable ion selectivity in subnanometer carbon nanotube porins. Science 2017, 357, 792–796. [Google Scholar] [CrossRef]
- Holt, J.K.; Park, H.G.; Wang, Y.; Stadermann, M.; Artyukhin, A.B.; Grigoropoulos, C.P.; Noy, A.; Bakajin, O. Fast mass transport through sub-2-nanometer carbon nanotubes. Science 2006, 312, 1034–1037. [Google Scholar] [CrossRef]
- Hummer, G.; Rasaiah, J.C.; Noworyta, J.P. Water conduction through the hydrophobic channel of a carbon nanotube. Nature 2001, 414, 188–190. [Google Scholar] [CrossRef]
- Gethard, K.; Sae-Khow, O.; Mitra, S. Water desalination using carbon-nanotube-enhanced membrane distillation. Acs Appl. Mater. Interfaces 2011, 3, 110–114. [Google Scholar] [CrossRef]
- Son, M.; Choi, H.-G.; Liu, L.; Celik, E.; Park, H.; Choi, H. Efficacy of carbon nanotube positioning in the polyethersulfone support layer on the performance of thin-film composite membrane for desalination. Chem. Eng. J. 2015, 266, 376–384. [Google Scholar] [CrossRef]
- Song, X.; Wang, L.; Tang, C.Y.; Wang, Z.; Gao, C. Fabrication of carbon nanotubes incorporated double-skinned thin film nanocomposite membranes for enhanced separation performance and antifouling capability in forward osmosis process. Desalination 2015, 369, 1–9. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Qiu, S.; Wu, L.G.; Zhang, L.; Chen, H.L.; Gao, C.J. Improving the performance of polyamide reverse osmosis membrane by incorporation of modified multi-walled carbon nanotubes. J. Membr. Sci. 2014, 450, 249–256. [Google Scholar] [CrossRef]
- Chan, W.-F.; Chen, H.-Y.; Surapathi, A.; Taylor, M.G.; Shao, X.; Marand, E.; Johnson, J.K. Zwitterion functionalized carbon nanotube/polyamide nanocomposite membranes for water desalination. Acs Nano 2013, 7, 5308–5319. [Google Scholar] [CrossRef]
- Azelee, I.W.; Goh, P.S.; Lau, W.J.; Ismail, A.F.; Rezaei-DashtArzhandi, M.; Wong, K.C.; Subramaniam, M.N. Enhanced desalination of polyamide thin film nanocomposite incorporated with acid treated multiwalled carbon nanotube-titania nanotube hybrid. Desalination 2017, 409, 163–170. [Google Scholar] [CrossRef]
- Wang, L.; Song, X.; Wang, T.; Wang, S.; Wang, Z.; Gao, C. Fabrication and characterization of polyethersulfone/carbon nanotubes (PES/CNTs) based mixed matrix membranes (MMMs) for nanofiltration application. Appl. Surf. Sci. 2015, 330, 118–125. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Y.; Tian, E.; Ren, Y. Preparation and characterization of novel forward osmosis membrane incorporated with sulfonated carbon nanotubes. Rsc Adv. 2018, 8, 41032–41039. [Google Scholar] [CrossRef]
- Creton, B.; Bougeard, D.; Smirnov, K.S.; Guilment, J.; Poncelet, O. Molecular dynamics study of hydrated imogolite 2. Structure and dynamics of confined water. Phys. Chem. Chem. Phys. 2008, 10, 4879–4888. [Google Scholar] [CrossRef]
- Liou, K.H.; Kang, D.Y.; Lin, L.C. Investigating the potential of single-walled aluminosilicate nanotubes in water desalination. ChemPhysChem 2017, 18, 179–183. [Google Scholar] [CrossRef]
- Zang, J.; Konduri, S.; Nair, S.; Sholl, D.S. Self-diffusion of water and simple alcohols in single-walled aluminosilicate nanotubes. ACS Nano 2009, 3, 1548–1556. [Google Scholar] [CrossRef]
- Liou, K.H.; Tsou, N.T.; Kang, D.Y. Relationships among the structural topology, bond strength, and mechanical properties of single-walled aluminosilicate nanotubes. Nanoscale 2015, 7, 16222–16229. [Google Scholar] [CrossRef]
- Farmer, V.C.; Fraser, A.R.; Tait, J.M. Synthesis of imogolite: A tubular aluminium silicate polymer. J. Chem. Soc. Chem. Commun. 1977, 13, 462–463. [Google Scholar] [CrossRef]
- Kang, D.-Y.; Brunelli, N.A.; Yucelen, G.I.; Venkatasubramanian, A.; Zang, J.; Leisen, J.; Hesketh, P.J.; Jones, C.W.; Nair, S. Direct synthesis of single-walled aminoaluminosilicate nanotubes with enhanced molecular adsorption selectivity. Nat. Commun. 2014, 5, 3342–3351. [Google Scholar] [CrossRef]
- Baroña, G.N.B.; Lim, J.; Choi, M.; Jung, B. Interfacial polymerization of polyamide-aluminosilicate SWNT nanocomposite membranes for reverse osmosis. Desalination 2013, 325, 138–147. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.-H.; Zhao, Q.-Y.; Gu, L.; Wu, Q.-Y. Thin film nanocomposite membranes based on imologite nanotubes blended substrates for forward osmosis desalination. Desalination 2017, 421, 160–168. [Google Scholar] [CrossRef]
- Kang, D.-Y.; Tong, H.M.; Zang, J.; Choudhury, R.P.; Sholl, D.S.; Beckham, H.W.; Jones, C.W.; Nair, S. Single-walled aluminosilicate nanotube/poly(vinylalcohol) nanocomposite membranes. Acs Appl. Mater. Interfaces 2012, 4, 965–976. [Google Scholar] [CrossRef]
- Li, M.; Brant, J.A. Synthesis of polyamide thin-film nanocomposite membranes using surface modified imogolite nanotubes. J. Membr. Sci. 2018, 563, 664–675. [Google Scholar] [CrossRef]
- Scalfi, L.; Fraux, G.; Boutin, A.; Coudert, F.-X. Structure and dynamics of water confined in imogolite nanotubes. Langmuir 2018, 34, 6748–6756. [Google Scholar] [CrossRef]
- Arancibia-Miranda, N.; Escudey, M.; Molina, M.; García-González, M.T. Use of isoelectric point and pH to evaluate the synthesis of a nanotubular aluminosilicate. J. Non-Cryst. Solids 2011, 357, 1750–1756. [Google Scholar] [CrossRef] [Green Version]
- Horvath, G.; Kawazoe, K. Method for the calculation of effective pore-size distribution in molecular-sieve carbon. J. Chem. Eng. Jpn. 1983, 16, 470–475. [Google Scholar] [CrossRef]
- Albo, J.; Wang, J.; Tsuru, T. Gas transport properties of interfacially polymerized polyamide composite membranes under different pre-treatments and temperatures. J. Membr. Sci. 2014, 449, 109–118. [Google Scholar] [CrossRef]
- Albo, J.; Wang, J.; Tsuru, T. Application of interfacially polymerized polyamide composite membranes to isopropanol dehydration: Effect of membrane pre-treatment and temperature. J. Membr. Sci. 2014, 453, 384–393. [Google Scholar] [CrossRef]
- Albo, J.; Hagiwara, H.; Yanagishita, H.; Ito, K.; Tsuru, T. Structural characterization of thin-film polyamide reverse osmosis membranes. Ind. Eng. Chem. Res. 2014, 53, 1442–1451. [Google Scholar] [CrossRef]
- Elber, R.; Roitberg, A.; Simmerling, C.; Goldstein, R.; Li, H.; Verkhivker, G.; Chen, K.; Zhang, J.; Ulitsky, A. MOIL: A program for simulations of macromolecules. Comput. Phys. Commun. 1995, 91, 159–189. [Google Scholar] [CrossRef]
- Tucker, S.C.; Maddox, M.W. The effect of solvent density in homogeneities on solute dynamics in super critical fluids: A theoretical perspective. J. Phys. Chem. B 1998, 102, 2437–2453. [Google Scholar] [CrossRef]
- Guimarães, L.; Enyashin, A.N.; Frenzel, J.; Heine, T.; Duarte, H.A.; Seifert, G. Imogolite nanotubes: Stability, electronic, and mechanical properties. ACS Nano 2007, 1, 362–368. [Google Scholar] [CrossRef]
- Skyner, R.; McDonagh, J.; Groom, C.; VanMourik, T.; Mitchell, J. A review of methods for the calculation of solution free energies and the modelling of systems insolution. Phys. Chem. Chem. Phys. 2015, 17, 6174–6191. [Google Scholar] [CrossRef]
- Kolesnikov, A.I.; Zanotti, J.M.; Loong, C.K.; Thiyagarajan, P.; Moravsky, A.P.; Loutfy, R.O.; Burnham, C.J. Anomalously soft dynamics of water in a nanotube: A revelation of nanoscale confinement. Phys. Rev. Lett. 2004, 93, 035503. [Google Scholar] [CrossRef]
- Tani, M.; Liu, C.; Huang, P.M. Atomic force microscopy of synthetic imogolite. Geoderma 2004, 118, 209–220. [Google Scholar] [CrossRef]
- Yamamoto, K.; Otsuka, H.; Takahara, A. Preparation of novel polymer hybrids from imogolite nanofiber. Polym. J. 2006, 39, 1–15. [Google Scholar] [CrossRef]
- Koenderink, G.H.; Kluijtmans, S.; Philipse, A.P. On the synthesis of colloidal imogolite fibers. J. Colloid Interface Sci. 1999, 216, 429–431. [Google Scholar] [CrossRef]
- Kang, D.-Y.; Zang, J.; Wright, E.R.; McCanna, A.L.; Jones, C.W.; Nair, S. Dehydration, dehydroxylation, and rehydroxylation of single-walled aluminosilicate nanotubes. ACS Nano 2010, 4, 4897–4907. [Google Scholar] [CrossRef]
- AbuTarboush, B.J.; Rana, D.; Matsuura, T.; Arafat, H.; Narbaitz, R. Preparation of thin-film-composite polyamide membranes for desalination using novel hydrophilic surface modifying macromolecules. J. Membr. Sci. 2008, 325, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.Y.; Kwon, Y.-N.; Leckie, J.O. Effect of membrane chemistry and coating layer on physiochemical properties of thin film composite polyamide RO and NF membranes. Desalination 2009, 242, 149–167. [Google Scholar] [CrossRef]
- Lind, M.L.; Ghosh, A.K.; Jawor, A.; Huang, X.; Hou, W.; Yang, Y.; Hoek, E.M. Influence of zeolite crystal size on zeolite-polyamide thin film nanocomposite membranes. Langmuir 2009, 25, 10139–10145. [Google Scholar] [CrossRef]
- Ghanbari, M.; Emadzadeh, D.; Lau, W.J.; Matsuura, T.; Davoody, M.; Ismail, A.F. Super hydrophilic TiO2/HNT nanocomposites as a new approach for fabrication of high performance thin film nanocomposite membranes for FO application. Desalination 2015, 371, 104–114. [Google Scholar] [CrossRef]
- Karan, S.; Jiang, Z.; Livingston, A.G. Membrane filtration. Sub-10 nm polyamide nanofilms with ultrafast solvent transport for molecular separation. Science 2015, 348, 1347. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, Y.; Yang, H.C.; Du, Y.; Xu, Z.K. Polyphenol coating as an interlayer for thin-film composite membranes with enhanced nanofiltration performance. ACS Appl. Mater. Interfaces 2016, 8, 32512–32519. [Google Scholar] [CrossRef]
- Park, M.J.; Phuntsho, S.; He, T.; Nisola, G.M.; Tijing, L.D.; Li, X.-M.; Chen, G.; Chung, W.-J.; Shon, H.K. Graphene oxide incorporated polysulfone substrate for the fabrication of flat-sheet thin-film composite forward osmosis membranes. J. Membr. Sci. 2015, 493, 496–507. [Google Scholar] [CrossRef]
- Amini, M.; Jahanshahi, M.; Rahimpour, A. Synthesis of novel thin film nanocomposite (TFN) forward osmosis membranes using functionalized multi-walled carbon nanotubes. J. Membr. Sci. 2013, 435, 233–241. [Google Scholar] [CrossRef]
- Tang, C.Y.; She, Q.; Lay, W.C.L.; Wang, R.; Field, R.; Fane, A.G. Modeling double-skinned FO membranes. Desalination 2011, 283, 178–186. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Zhou, Z.; Xie, J.; Lee, J.Y. Hydrophilic mineral coating of membrane substrate for reducing internal concentration polarization (ICP) in forward osmosis. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Tian, M.; Qiu, C.; Liao, Y.; Chou, S.; Wang, R. Preparation of polyamide thin film composite forward osmosis membranes using electrospun polyvinylidene fluoride (PVDF) nanofibers as substrates. Sep. Purif. Technol. 2013, 118, 727–736. [Google Scholar] [CrossRef]
- Huang, L.; McCutcheon, J.R. Hydrophilic nylon 6,6 nanofibers supported thin film composite membranes for engineered osmosis. J. Membr. Sci. 2014, 457, 162–169. [Google Scholar] [CrossRef]
- Liu, X.; Ng, H.Y. Fabrication of layered silica–polysulfone mixed matrix substrate membrane for enhancing performance of thin-film composite forward osmosis membrane. J. Membr. Sci. 2015, 481, 148–163. [Google Scholar] [CrossRef]
- Ma, N.; Wei, J.; Liao, R.; Tang, C.Y. Zeolite-polyamide thin film nanocomposite membranes: Towards enhanced performance for forward osmosis. J. Membr. Sci. 2012, 405–406, 149–157. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef] [Green Version]






| Membrane | C (%) | O (%) | N (%) | Al (%) | Si (%) | C/N |
|---|---|---|---|---|---|---|
| TFC | 73.19 | 12.51 | 11.49 | 0 | 2.82 | 6.37 |
| TFN 0.08 | 71.23 | 17.13 | 9.27 | 0.52 | 1.85 | 7.68 |
| TFN 0.2 | 71.89 | 14.43 | 10.28 | 0.99 | 2.4 | 6.99 |
| TFN 0.5 | 72.6 | 14.31 | 9.79 | 1.11 | 2.19 | 7.28 |
| FO membranes | Aa (L/m2 h bar) | Bb (L/m2 h) | Rs (%) | B/A (bar) | S (mm) |
|---|---|---|---|---|---|
| TFC | 0.26 ± 0.03 | 0.58 ± 0.05 | 67.17 ± 8.28 | 2.22 | 5.09 |
| TFN 0.08 | 0.59 ± 0.20 | 0.79 ± 0.12 | 75.51 ± 2.34 | 1.34 | 2.37 |
| TFN 0.2 | 0.66 ± 0.17 | 0.44 ± 0.08 | 86.67 ± 6.13 | 0.67 | 1.61 |
| TFN 0.5 | 2.15 ± 0.53 | 9.60 ± 0.37 | 48.43 ± 11.66 | 4.47 | 1.36 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, S.-J.; Pan, Y.-H.; Wang, S.-F.; Dai, Z.-W.; Gu, L.; Wu, Q.-Y. Aluminosilicate Nanotubes Embedded Polyamide Thin Film Nanocomposite Forward Osmosis Membranes with Simultaneous Enhancement of Water Permeability and Selectivity. Polymers 2019, 11, 879. https://doi.org/10.3390/polym11050879
Shi S-J, Pan Y-H, Wang S-F, Dai Z-W, Gu L, Wu Q-Y. Aluminosilicate Nanotubes Embedded Polyamide Thin Film Nanocomposite Forward Osmosis Membranes with Simultaneous Enhancement of Water Permeability and Selectivity. Polymers. 2019; 11(5):879. https://doi.org/10.3390/polym11050879
Chicago/Turabian StyleShi, She-Ji, Ye-Han Pan, Shao-Fei Wang, Zheng-Wei Dai, Lin Gu, and Qing-Yun Wu. 2019. "Aluminosilicate Nanotubes Embedded Polyamide Thin Film Nanocomposite Forward Osmosis Membranes with Simultaneous Enhancement of Water Permeability and Selectivity" Polymers 11, no. 5: 879. https://doi.org/10.3390/polym11050879
APA StyleShi, S.-J., Pan, Y.-H., Wang, S.-F., Dai, Z.-W., Gu, L., & Wu, Q.-Y. (2019). Aluminosilicate Nanotubes Embedded Polyamide Thin Film Nanocomposite Forward Osmosis Membranes with Simultaneous Enhancement of Water Permeability and Selectivity. Polymers, 11(5), 879. https://doi.org/10.3390/polym11050879