Simultaneous Recovery of Matrix and Fiber in Carbon Reinforced Composites through a Diels–Alder Solvolysis Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Furan Precursors
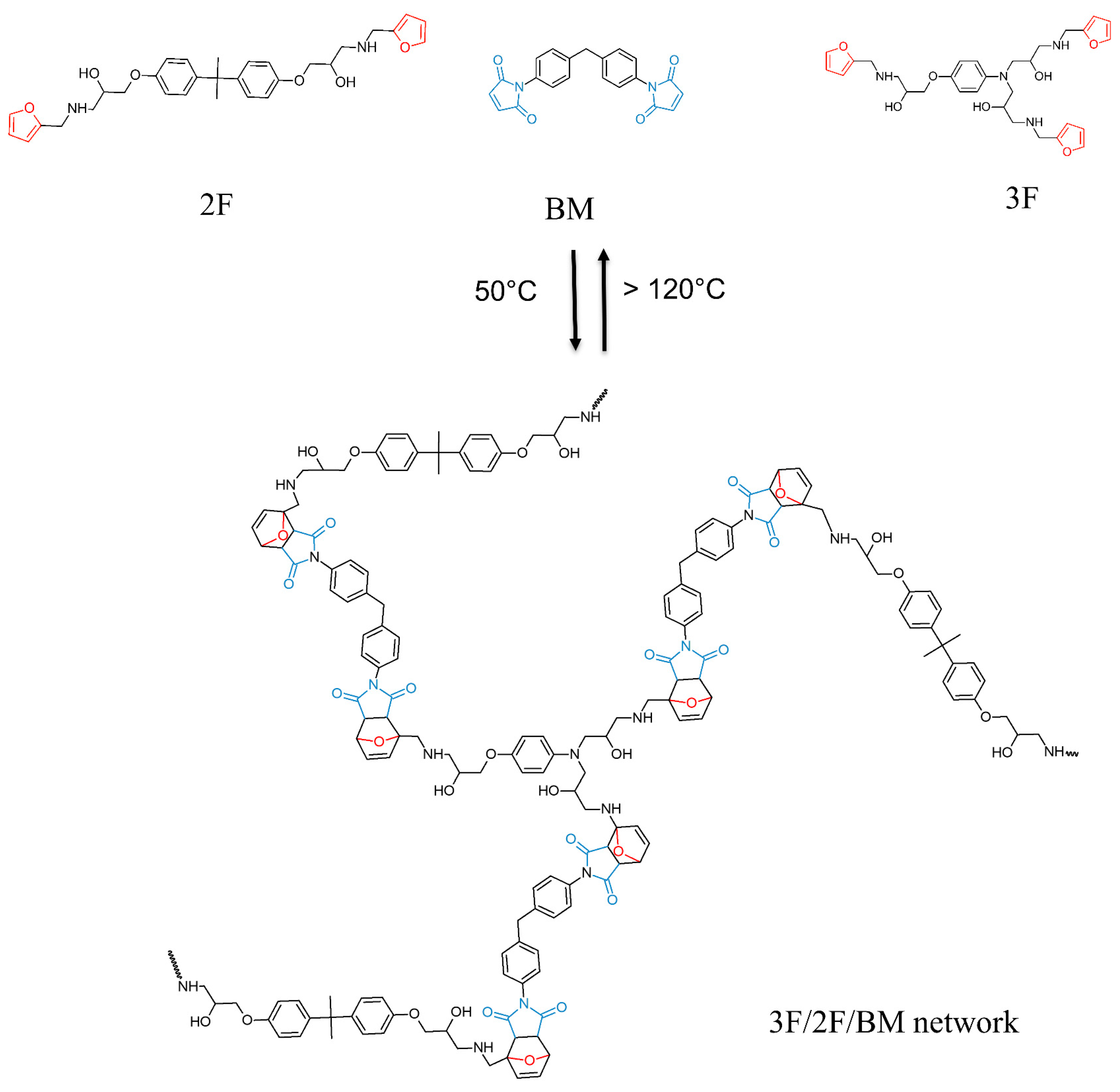
2.3. Synthesis of Diels–Alder Polymer Networks
2.4. Composite Fabrication
2.5. Characterization
3. Results and Discussion
3.1. Synthesis and Characterization of Diels–Alder (DA) Polymer Networks
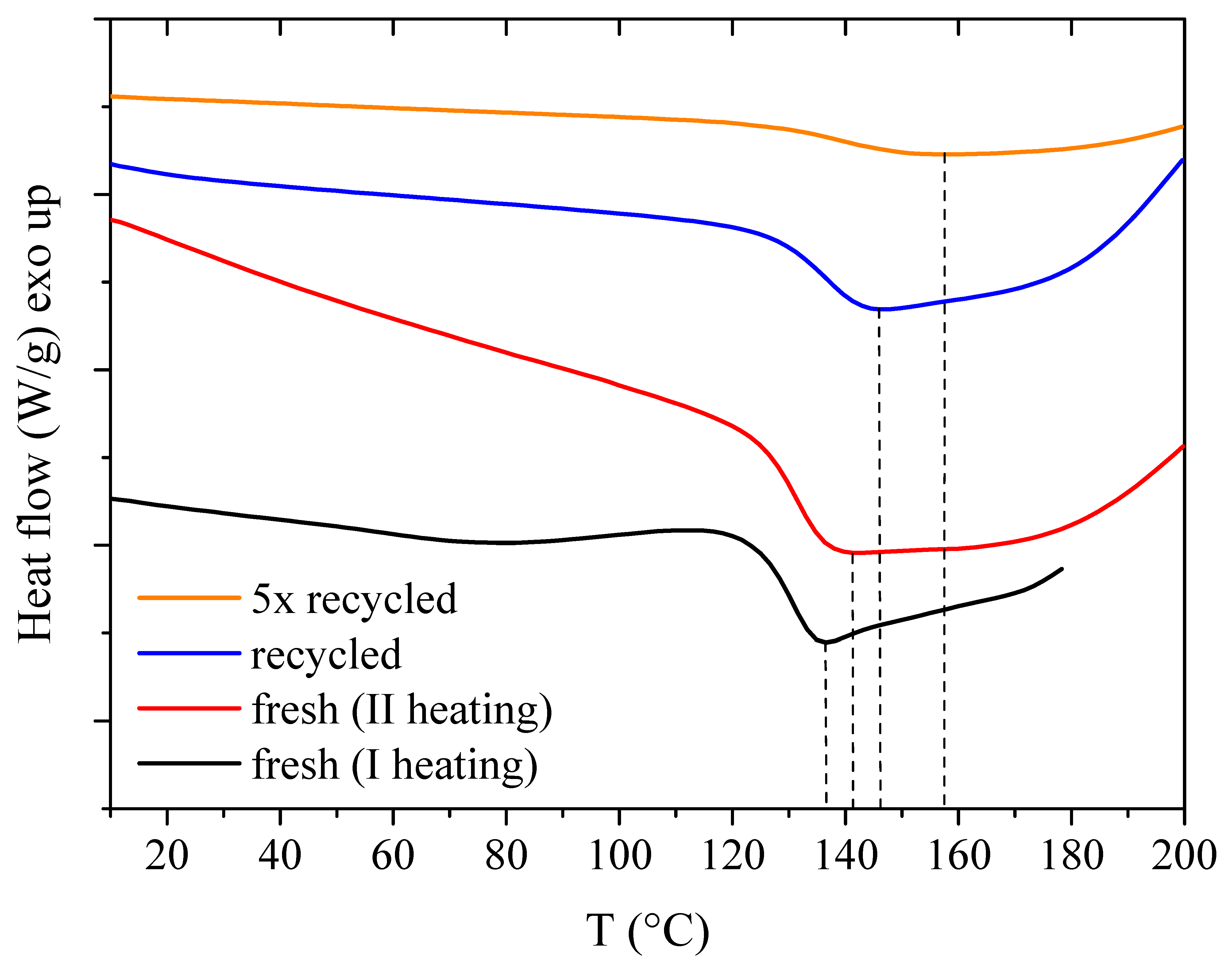
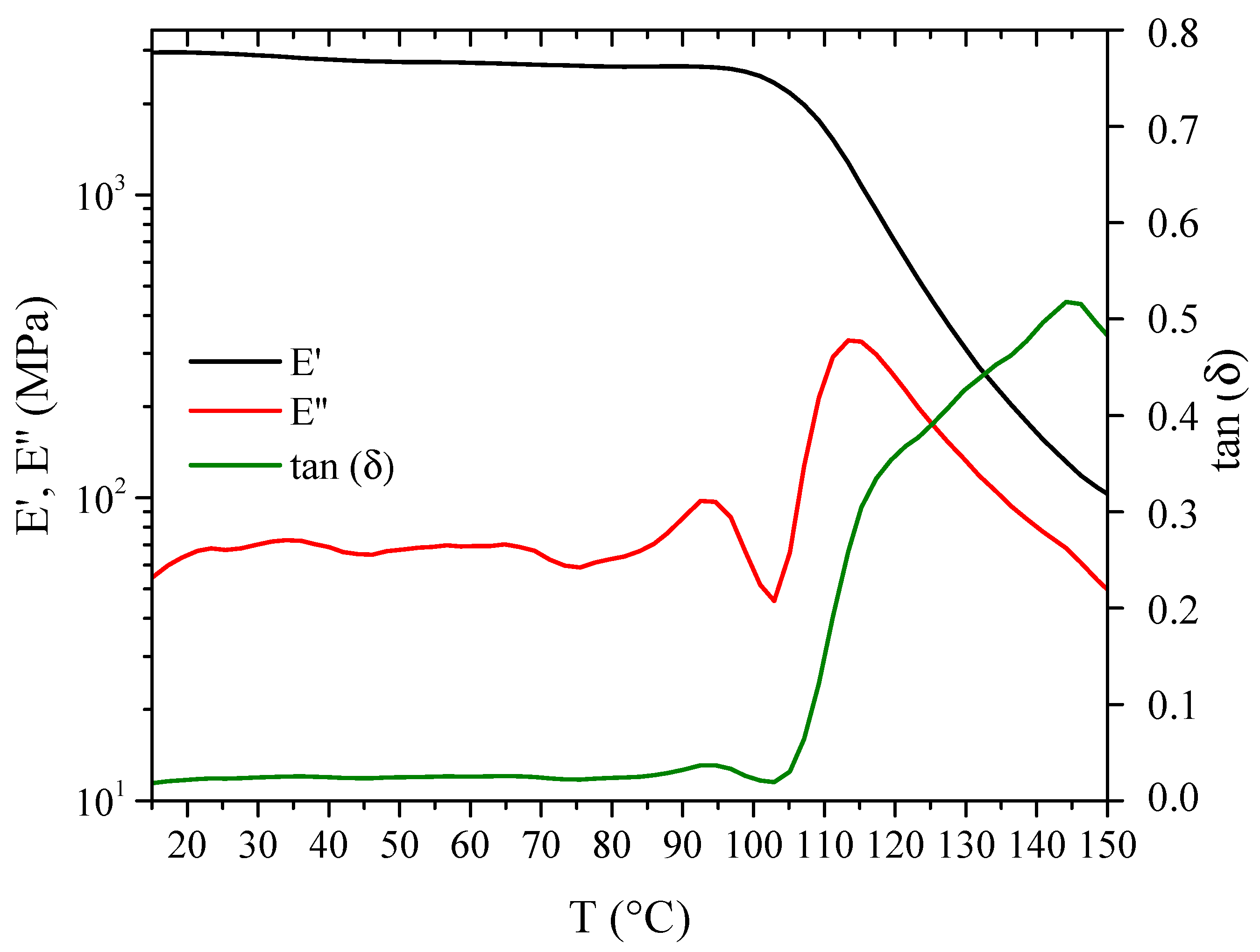
3.2. Thermal Reversibility
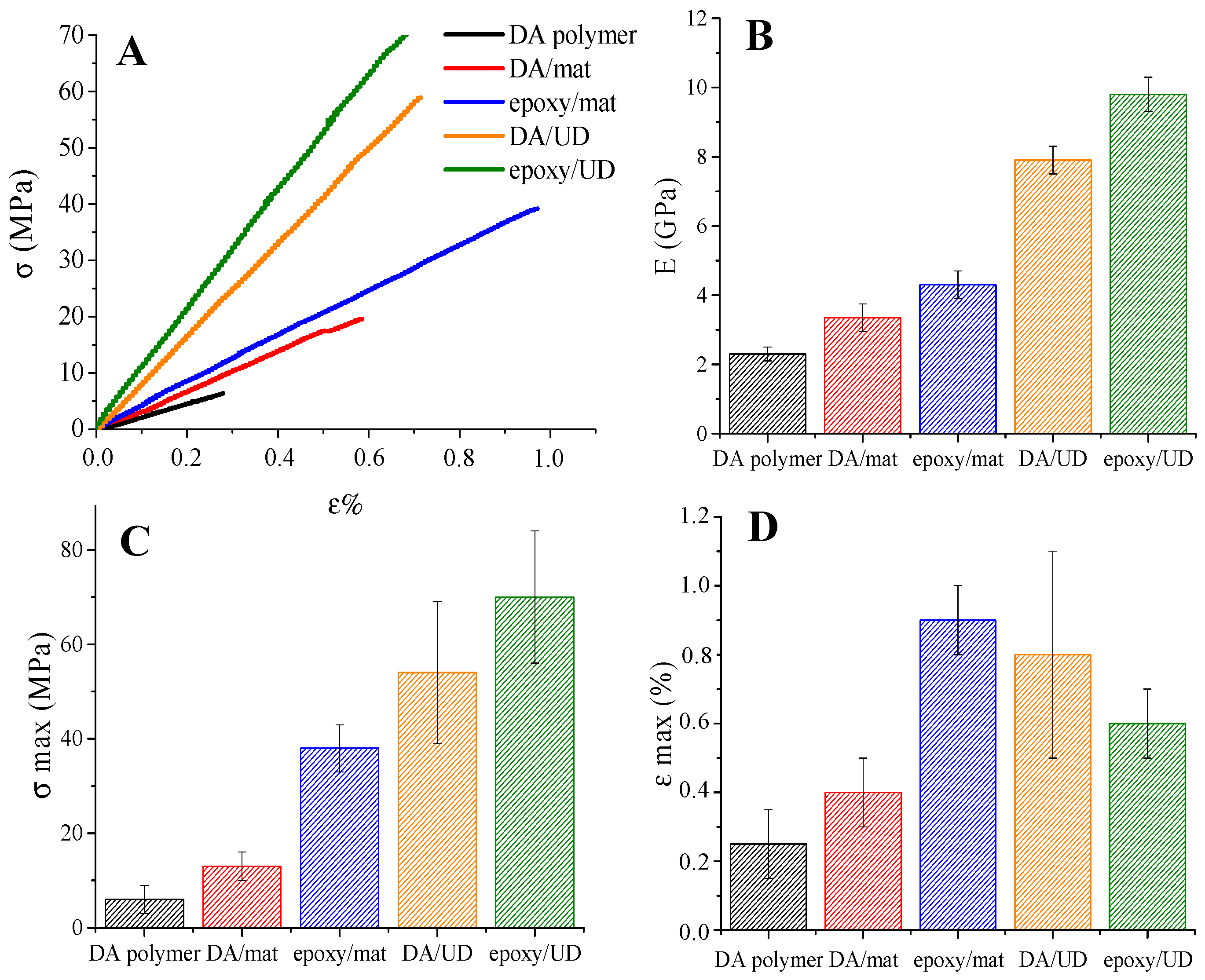
3.3. Composites Preparation and Characterization
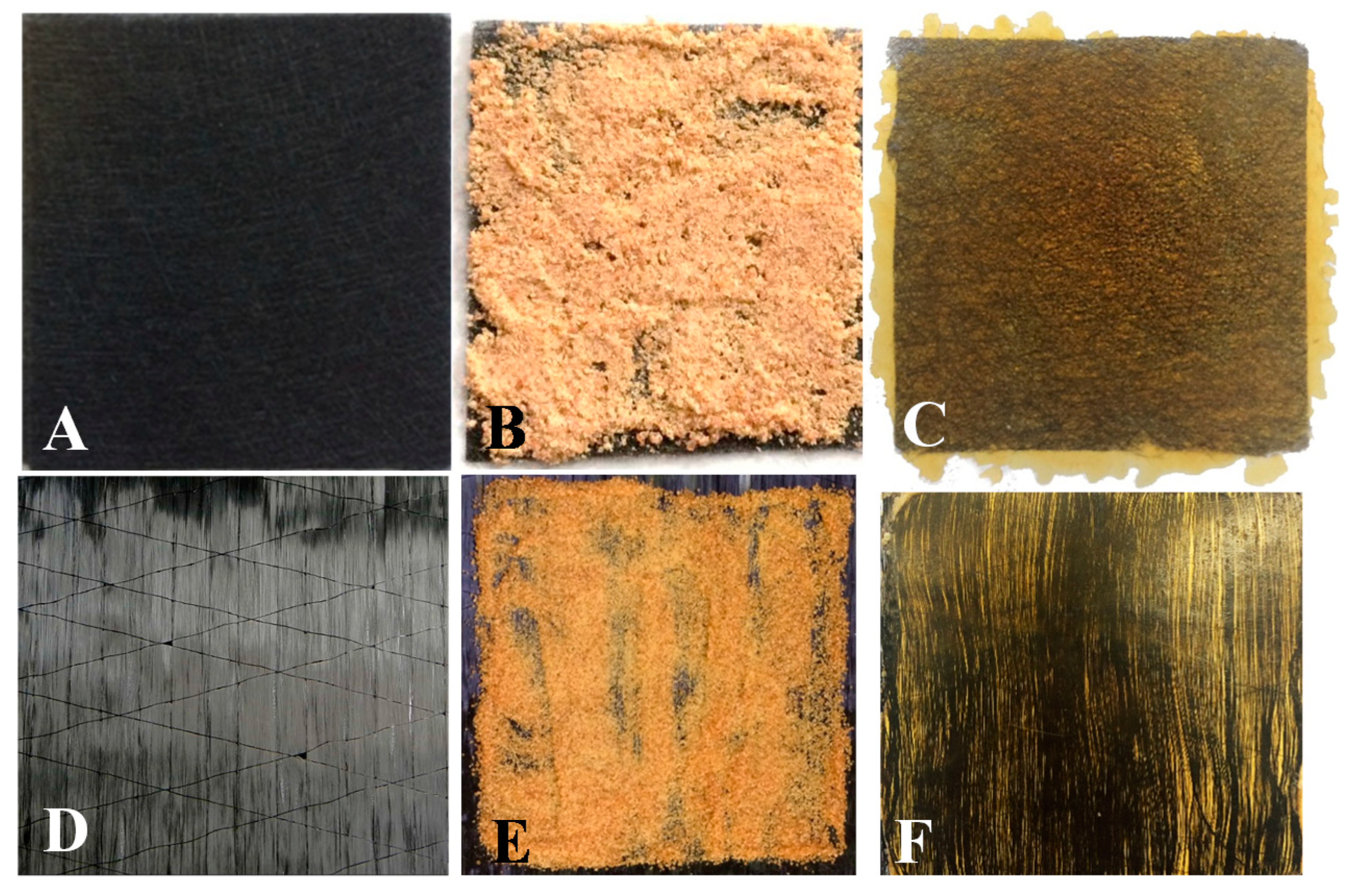
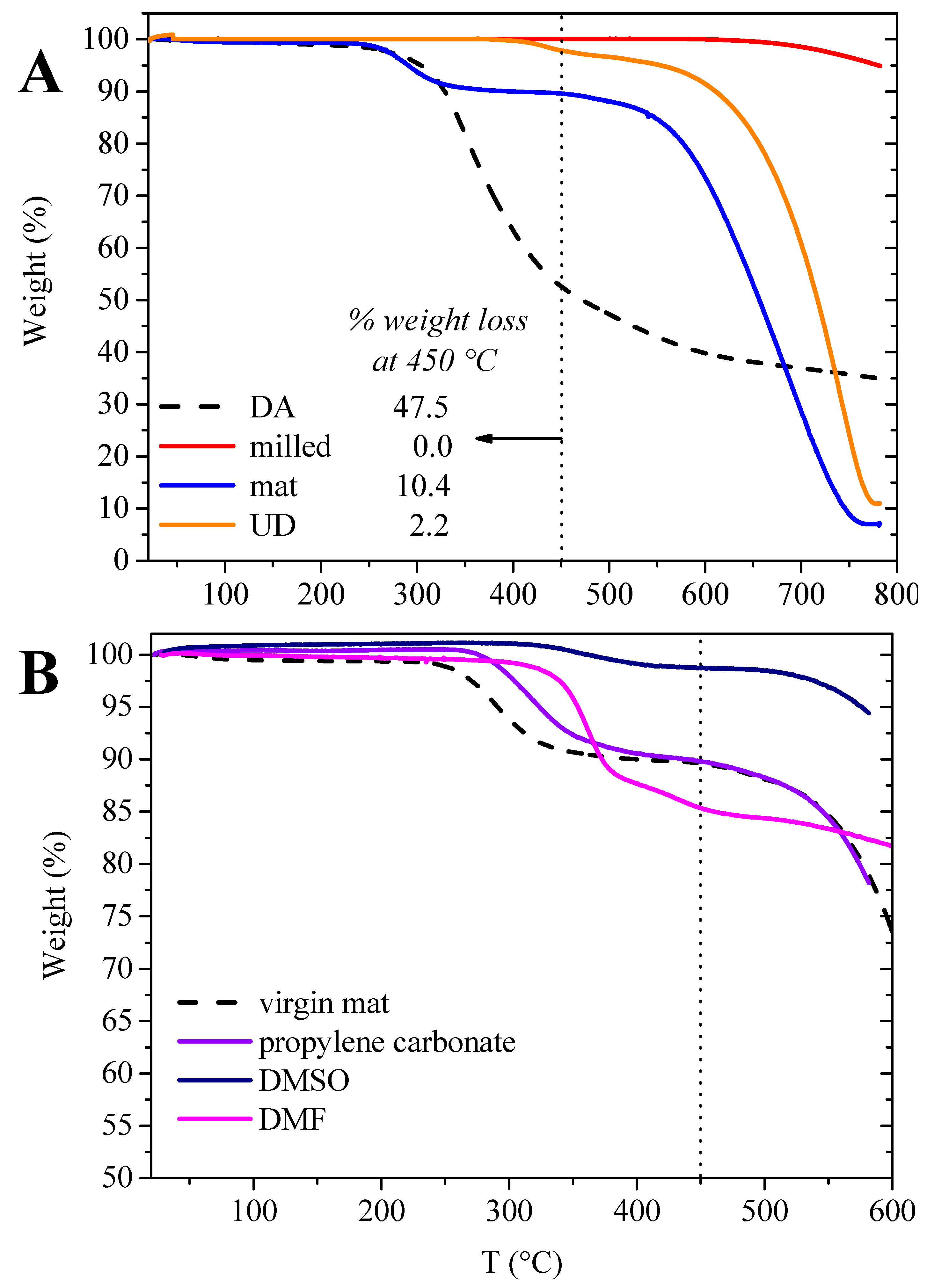
3.4. Fiber Recovery through Solvolysis and Reuse
3.5. Matrix Reuse as a Smart Coating
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mazumdar, S. 2019 State of the Industry Report. A Snapshot of Key Materials and Markets in the Global Composite Industry. Available online: http://compositesmanufacturingmagazine.com/2019/01/2019-state-of-the-industry-report/2/ (accessed on 20 March 2019).
- Jacob, A. Composites can be recycled. Reinf. Plast. 2011, 55, 45–46. [Google Scholar] [CrossRef]
- Oliveux, G.; Dandy, L.O.; Leeke, G.A. Current status of recycling of fibre reinforced polymers: Review of technologies, reuse and resulting properties. Prog. Mater Sci. 2015, 72, 61–99. [Google Scholar] [CrossRef] [Green Version]
- Pickering, S.J. Recycling technologies for thermoset composite materials—Current status. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1206–1215. [Google Scholar] [CrossRef]
- Dang, W.; Kubouchi, M.; Yamamoto, S.; Sembokuya, H.; Tsuda, K. An approach to chemical recycling of epoxy resin cured with amine using nitric acid. Polymer 2002, 43, 2953–2958. [Google Scholar] [CrossRef]
- Morin, C.; Loppinet-Serani, A.; Cansell, F.; Aymonier, C. Near- and supercritical solvolysis of carbon fibre reinforced polymers (CFRPs) for recycling carbon fibers as a valuable resource: State of the art. J. Supercrit. Fluids 2012, 66, 232–240. [Google Scholar] [CrossRef] [Green Version]
- Okajima, I.; Sako, T. Recycling of carbon fiber-reinforced plastic using supercritical and subcritical fluids. J. Mater. Cycles Waste Manag. 2017, 19, 15. [Google Scholar] [CrossRef]
- Banatao, D.R.; Kosinski, S.; Pastine, S.J. Recyclable by design: A chemical approach to recyclable epoxy composites. In Proceedings of the CAMX 2014—Composites and Advanced Materials Expo: Combined Strength. Unsurpassed Innovation, Orlando, FL, USA, 13 October 2014. [Google Scholar]
- Pastine, S.J. Sustainable by design: Introducing recyclable epoxytechnology. In Proceedings of the Society of Plastics Engineers—13th Annual Automotive Composites Conference and Exhibition (ACCE 2013), Novi, MI, USA, 11–13 September 2013; pp. 369–376. [Google Scholar]
- Cicala, G.; Mannino, S.; La Rosa, A.D.; Banatao, D.R.; Pastine, S.J.; Kosinski, S.T.; Scarpa, F. Hybrid biobased recyclable epoxy composites for mass production. Polym. Compos. 2018, 39, 2217–2225. [Google Scholar] [CrossRef]
- La Rosa, A.D.; Banatao, D.R.; Pastine, S.J.; Latteri, A.; Cicala, G. Recycling treatment of carbon fibre/epoxy composites: Materials recovery and characterization and environmental impacts through life cycle assessment. Compos. Part B Eng. 2016, 104, 17–25. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Cui, X.J.; Ge, H.; Yang, Y.X.; Wang, Y.X.; Zhang, C.; Li, J.J.; Deng, T.S.; Qin, Z.F.; Hou, X.L. Chemical Recycling of Carbon Fiber Reinforced Epoxy Resin Composites via Selective Cleavage of the Carbon-Nitrogen Bond. ACS Sustain. Chem. Eng. 2015, 3, 3332–3337. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Hashimoto, T.; Kakichi, Y.; Urushisaki, M.; Sakaguchi, T.; Kawabe, K.; Kondo, K.; Iyo, H. Recyclable Carbon Fiber-Reinforced Plastics (CFRP) Containing Degradable Acetal Linkages: Synthesis, Properties, and Chemical Recycling. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1052–1059. [Google Scholar] [CrossRef]
- Kloxin, C.J.; Scott, T.F.; Adzima, B.J.; Bowman, C.N. Covalent Adaptable Networks (CANs): A Unique Paradigm in Crosslinked Polymers. Macromolecules 2010, 43, 2643–2653. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Shi, Q.; Dunn, M.L.; Wang, T.; Qi, H.J. Carbon Fiber Reinforced Thermoset Composite with Near 100% Recyclability. Adv. Funct. Mater. 2016, 26, 6098–6106. [Google Scholar] [CrossRef]
- Wang, S.J.; Xing, X.L.; Zhang, X.T.; Wang, X.; Jing, X.L. Room-temperature fully recyclable carbon fibre reinforced phenolic composites through dynamic covalent boronic ester bonds. J. Mater. Chem. A 2018, 6, 10868–10878. [Google Scholar] [CrossRef]
- Gopinathan, J.; Noh, I. Click Chemistry-Based Injectable Hydrogels and Bioprinting Inks for Tissue Engineering Applications. Tissue Eng. Regen. Med. 2018, 15, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Vauthier, M.; Jierry, L.; Oliveira, J.C.; Hassouna, L.; Roucoules, V.; Bally-Le Gall, F. Interfacial Thermoreversible Chemistry on Functional Coatings: A Focus on the Diels–Alder Reaction. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- Bednarek, M.; Kubisa, P. Reversible networks of degradable polyesters containing weak covalent bonds. Polym. Chem. 2019, 10, 1848–1872. [Google Scholar] [CrossRef]
- Gandini, A. The furan/maleimide Diels-Alder reaction: A versatile click-unclick tool in macromolecular synthesis. Prog. Polym. Sci. 2013, 38, 1–29. [Google Scholar] [CrossRef]
- Toncelli, C.; de Reus, D.C.; Broekhuis, A.A.; Picchioni, F. Thermoreversibility in Polymeric Systems: Chemical and Physical Aspects. In Self-Healing at the Nanoscale: Mechanisms and Key Concepts of Natural and Artificial Systems; Amendola, V., Meneghetti, M., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 199–248. [Google Scholar]
- Turkenburg, D.H.; Fischer, H.R. Diels-Alder based, thermo-reversible cross-linked epoxies for use in self-healing composites. Polymer 2015, 79, 187–194. [Google Scholar] [CrossRef]
- Heo, Y.; Sodano, H.A. Thermally responsive self-healing composites with continuous carbon fiber reinforcement. Compos. Sci. Technol. 2015, 118, 244–250. [Google Scholar] [CrossRef]
- Zhang, W.; Duchet, J.; Gérard, J.F. Self-healable interfaces based on thermo-reversible Diels-Alder reactions in carbon fiber reinforced composites. J. Colloid Interface Sci. 2014, 430, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Fang, L.; Chen, T.; Sun, M.; Lu, C.; Xu, Z. Near-infrared light and solar light activated self-healing epoxy coating having enhanced properties using MXene flakes as multifunctional fillers. Polymers 2018, 10, 474. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.W.; Feng, L.B.; Shi, X.T.; Wang, Y.P.; Liu, Y.H. Synthesis and healing behavior of thermo-reversible self-healing epoxy resins. Acta Polym. Sin. 2018, 395–401. [Google Scholar] [CrossRef]
- Štirn, Ž.; Ručigaj, A.; Karger-Kocsis, J.; Krajnc, M. Effects of Diels–Alder Adduct and Lass Transition on the Repeated Self-Healing of Aliphatic Amine-Cured Epoxy Resin. Macromol. Mater. Eng. 2018, 303. [Google Scholar] [CrossRef]
- Moazzen, K.; Zohuriaan-Mehr, M.J.; Jahanmardi, R.; Kabiri, K. Toward poly(furfuryl alcohol) applications diversification: Novel self-healing network and toughening epoxy–novolac resin. J. Appl. Polym. Sci. 2018, 135. [Google Scholar] [CrossRef]
- Li, M.; Liu, N.; Chen, J.; Shi, K.; Li, Q. Development of reprocessable novel sulfur-containing epoxy based on thermal treatment. RSC Adv. 2018, 8, 28386–28394. [Google Scholar] [CrossRef] [Green Version]
- Karami, Z.; Zohuriaan-Mehr, M.J.; Rostami, A. Biobased Diels-Alder engineered network from furfuryl alcohol and epoxy resin: Preparation and mechano-physical characteristics. ChemistrySelect 2018, 3, 40–46. [Google Scholar] [CrossRef]
- Xu, X.; Fan, P.; Ren, J.; Cheng, Y.; Ren, J.; Zhao, J.; Song, R. Self-healing thermoplastic polyurethane (TPU)/polycaprolactone (PCL) /multi-wall carbon nanotubes (MWCNTs) blend as shape-memory composites. Compos. Sci. Technol. 2018, 168, 255–262. [Google Scholar] [CrossRef]
- Fang, L.; Chen, J.; Zou, Y.; Xu, Z.; Lu, C. Thermally-induced self-healing behaviors and properties of four epoxy coatings with different network architectures. Polymers 2017, 9, 333. [Google Scholar] [CrossRef]
- Dello Iacono, S.; Martone, A.; Pastore, A.; Filippone, G.; Acierno, D.; Zarrelli, M.; Giordano, M.; Amendola, E. Thermally activated multiple self-healing diels-alder epoxy system. Polym. Eng. Sci. 2017, 57, 674–679. [Google Scholar] [CrossRef]
- Coope, T.S.; Turkenburg, D.H.; Fischer, H.R.; Luterbacher, R.; Van Bracht, H.; Bond, I.P. Novel Diels-Alder based self-healing epoxies for aerospace composites. Smart Mater. Struct. 2016, 25. [Google Scholar] [CrossRef]
- Postiglione, G.; Turri, S.; Levi, M. Effect of the plasticizer on the self-healing properties of a polymer coating based on the thermoreversible Diels-Alder reaction. Prog. Org. Coat. 2015, 78, 526–531. [Google Scholar] [CrossRef]
- Li, J.; Zhang, G.; Deng, L.; Jiang, K.; Zhao, S.; Gao, Y.; Sun, R.; Wong, C. Thermally reversible and self-healing novolac epoxy resins based on Diels-Alder chemistry. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Kuang, X.; Liu, G.; Dong, X.; Liu, X.; Xu, J.; Wang, D. Facile fabrication of fast recyclable and multiple self-healing epoxy materials through diels-alder adduct cross-linker. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2094–2103. [Google Scholar] [CrossRef]
- Bai, N.; Simon, G.P.; Saito, K. Characterisation of the thermal self-healing of a high crosslink density epoxy thermoset. New J. Chem. 2015, 39, 3497–3506. [Google Scholar] [CrossRef]
- Luo, K.; Xie, T.; Rzayev, J. Synthesis of thermally degradable epoxy adhesives. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4992–4997. [Google Scholar] [CrossRef]
- Bai, N.; Saito, K.; Simon, G.P. Synthesis of a diamine cross-linker containing Diels-Alder adducts to produce self-healing thermosetting epoxy polymer from a widely used epoxy monomer. Polym. Chem. 2013, 4, 724–730. [Google Scholar] [CrossRef]
- Scheltjens, G.; Brancart, J.; De Graeve, I.; Van Mele, B.; Terryn, H.; Van Assche, G. Self-healing property characterization of reversible thermoset coatings. J. Therm. Anal. Calorim. 2011, 105, 805–809. [Google Scholar] [CrossRef]
- Tian, Q.; Rong, M.Z.; Zhang, M.Q.; Yuan, Y.C. Synthesis and characterization of epoxy with improved thermal remendability based on Diels-Alder reaction. Polym. Int. 2010, 59, 1339–1345. [Google Scholar] [CrossRef]
- Liu, Y.L.; Hsieh, C.Y. Crosslinked epoxy materials exhibiting thermal remendablility and removability from multifunctional maleimide and furan compounds. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 905–913. [Google Scholar] [CrossRef]
- Araya-Hermosilla, R.; Fortunato, G.; Pucci, A.; Raffa, P.; Polgar, L.; Broekhuis, A.A.; Pourhossein, P.; Lima, G.M.R.; Beljaars, M.; Picchioni, F. Thermally reversible rubber-toughened thermoset networks via Diels–Alder chemistry. Eur. Polym. J. 2016, 74, 229–240. [Google Scholar] [CrossRef]
- Liu, X.; Du, P.; Liu, L.; Zheng, Z.; Wang, X.; Joncheray, T.; Zhang, Y. Kinetic study of Diels–Alder reaction involving in maleimide–furan compounds and linear polyurethane. Polym. Bull. 2013, 70, 2319–2335. [Google Scholar] [CrossRef]
- Flory, P.J. Molecular Size Distribution in Three Dimensional Polymers. I. Gelation. JACS 1941, 63, 3083–3090. [Google Scholar] [CrossRef]
- Toncelli, C.; De Reus, D.C.; Picchioni, F.; Broekhuis, A.A. Properties of Reversible Diels–Alder Furan/Maleimide Polymer Networks as Function of Crosslink Density. Macromol. Chem. Phys. 2012, 213, 157–165. [Google Scholar] [CrossRef]
- Canadell, J.; Fischer, H.; De With, G.; van Benthem, R.A.T.M. Stereoisomeric effects in thermo-remendable polymer networks based on Diels–Alder crosslink reactions. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 3456–3467. [Google Scholar] [CrossRef]
- Tian, Q.; Yuan, Y.C.; Rong, M.Z.; Zhang, M.Q. A thermally remendable epoxy resin. J. Mater. Chem. 2009, 19, 1289–1296. [Google Scholar] [CrossRef]
- Chawla, K.K. Composite Materials: Science and Engineering; Springer-Verlag: New York, NY, USA, 1987. [Google Scholar]
- Bellenger, V.; Fontaine, E.; Fleishmann, A.; Saporito, J.; Verdu, J. Thermogravimetric study of amine cross-linked epoxies. Polym. Degrad. Stab. 1984, 9, 195–208. [Google Scholar] [CrossRef]
- Meyer, L.O.; Schulte, K.; Grove-Nielsen, E. CFRP-Recycling Following a Pyrolysis Route: Process Optimization and Potentials. J. Compos. Mater. 2009, 43, 1121. [Google Scholar] [CrossRef]
- Yang, P.; Zhou, Q.; Li, X.-Y.; Yang, K.-K.; Wang, Y.-Z. Chemical recycling of fiber-reinforced epoxy resin using a polyethylene glycol/NaOH system. J. Reinf. Plast. Compos. 2014, 33, 2106–2114. [Google Scholar] [CrossRef]
- Leroux, F.; Stimpfling, T.; Hintze-Bruening, H. Relevance and Performance of LDH Platelets in Coatings. Recent Patents Nanotechnol. 2012, 6, 238–248. [Google Scholar] [CrossRef]
- Keith, M.J.; Román-Ramírez, L.A.; Leeke, G.; Ingram, A. Recycling a carbon fibre reinforced polymer with a supercritical acetone/water solvent mixture: Comprehensive analysis of reaction kinetics. Polym. Degrad. Stab. 2019, 161, 225–234. [Google Scholar] [CrossRef]
- Oliveux, G.; Bailleul, J.-L.; Gillet, A.; Mantaux, O.; Leeke, G.A. Recovery and reuse of discontinuous carbon fibres by solvolysis: Realignment and properties of remanufactured materials. Compos. Sci. Technol. 2017, 139, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Keith, M.J.; Oliveux, G.; Leeke, G.A. Optimisation of solvolysis for recycling carbon fibre reinforced composites. In Proceedings of the ECCM 2016—17th European Conference on Composite Materials, Budapest, Hungary, 26–30 June 2016. [Google Scholar]
- Henry, L.; Schneller, A.; Doerfler, J.; Mueller, W.M.; Aymonier, C.; Horn, S. Semi-continuous flow recycling method for carbon fibre reinforced thermoset polymers by near- and supercritical solvolysis. Polym. Degrad. Stab. 2016, 133, 264–274. [Google Scholar] [CrossRef]
- Kuang, X.; Zhou, Y.; Shi, Q.; Wang, T.; Qi, H.J. Recycling of Epoxy Thermoset and Composites via Good Solvent Assisted and Small Molecules Participated Exchange Reactions. ACS Sustain. Chem. Eng. 2018, 6, 9189–9197. [Google Scholar] [CrossRef]
- Iacono, S.D.; Martone, A.; Amendola, E. Corrosion-resistant self-healing coatings. AIP Conf. Proc. 2018, 1990, 020010. [Google Scholar] [CrossRef]




| Sample | 2F:3F (mol:mol) | fav | f | ρ | pca | DA Conv b (%) | Gel Content (%) |
|---|---|---|---|---|---|---|---|
| M25 | 1:1 | 2.5 | 3 | 0.60 | 0.79 | 70 ± 1 | 98.5 ± 0.5 |
| M22 | 4:1 | 2.2 | 3 | 0.27 | 0.89 | 85 ± 2 | 98.6 ± 0.8 |
| M21 | 9:1 | 2.1 | 3 | 0.14 | 0.94 | 85 ± 2 | 96.5 ± 0.5 |
| Fiber Type | Solvent | Weight Loss at 450 °C (%) | Estimated Matrix Residue (%) |
|---|---|---|---|
| milled | DMF | 2.1 | 4.4 |
| mat | DMF | 14.7 | 31.0 |
| mat | propylene carbonate | 10.2 | 21.5 |
| mat | DMSO | 2.3 | 4.9 |
| UD | propylene carbonate | 17.7 | 37.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fortunato, G.; Anghileri, L.; Griffini, G.; Turri, S. Simultaneous Recovery of Matrix and Fiber in Carbon Reinforced Composites through a Diels–Alder Solvolysis Process. Polymers 2019, 11, 1007. https://doi.org/10.3390/polym11061007
Fortunato G, Anghileri L, Griffini G, Turri S. Simultaneous Recovery of Matrix and Fiber in Carbon Reinforced Composites through a Diels–Alder Solvolysis Process. Polymers. 2019; 11(6):1007. https://doi.org/10.3390/polym11061007
Chicago/Turabian StyleFortunato, Giovanni, Luca Anghileri, Gianmarco Griffini, and Stefano Turri. 2019. "Simultaneous Recovery of Matrix and Fiber in Carbon Reinforced Composites through a Diels–Alder Solvolysis Process" Polymers 11, no. 6: 1007. https://doi.org/10.3390/polym11061007







