Essential Oils-Loaded Polymer Particles: Preparation, Characterization and Antimicrobial Property
Abstract
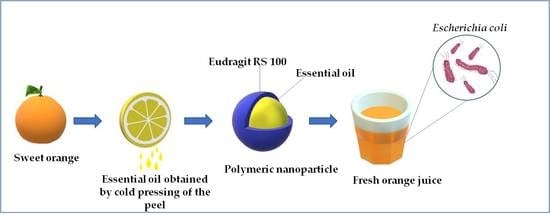
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Gas Chromatography of Citrus Essential Oils
2.2.2. Identification of Citrus Essential Oils Components by GC-FID
2.3. Nanoparticles Preparation via Nanoprecipitation Process
2.4. Orange Juice Preparation
2.5. Nanoparticles Characterization
2.5.1. Hydrodynamic Size and Zeta Potential
2.5.2. Colloidal Stability Study
2.5.3. Fluorescence Microscopy Analysis
2.5.4. Scanning Electron Microscopy (SEM)
2.5.5. Atomic Force Microscopy (AFM)
2.5.6. Encapsulation Efficiency
Encapsulation Efficiency Determination Using Gas Chromatography
Encapsulation Efficiency Determination Using UV Spectrophotometer
2.6. Antibacterial Activity Study
3. Results and Discussions
3.1. Hydrodynamic Size and Zeta Potential as Function of pH and Salinity
3.2. Colloidal Stability Study
3.3. Fluorescence Microscopy Analysis
3.4. Scanning Electron Microscopy Analysis
3.5. Atomic Force Microscopy (AFM)
3.6. Gas Chromatography Analysis of Sweet Orange and Bergamot Essential Oils
3.7. Encapsulation Efficiency
3.8. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Navarra, M.; Mannucci, C.; Delbò, M.; Calapai, G. Citrus bergamia essential oil: From basic research to clinical application. Front. Pharmacol. 2015, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.; Phillips, C. Potential antimicrobial uses of essential oils in food: Is citrus the answer? Trends Food Sci. Technol. 2008, 19, 156–164. [Google Scholar] [CrossRef]
- O’Bryan, C.A.; Crandall, P.G.; Chalova, V.I.; Ricke, S.C. Orange essential oils antimicrobial activities against Salmonella spp. J. Food Sci. 2008, 73, M264–M267. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, L.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Physical properties of edible chitosan films containing bergamot essential oil and their inhibitory action on Penicillium Italicum. Carbohydr. Polym. 2010, 82, 277–283. [Google Scholar] [CrossRef]
- Tao, N.; Liu, Y.; Zhang, M. Chemical composition and antimicrobial activities of essential oil from the peel of bingtang sweet orange (Citrus sinensis Osbeck). Int. J. Food Sci. Technol. 2009, 44, 1281–1285. [Google Scholar] [CrossRef]
- Deans, S.G.; Ritchie, G. Antibacterial properties of plant essential oils. Int. J. Food Microbiol. 1987, 5, 165–180. [Google Scholar] [CrossRef]
- Kollanoor-Johny, A.; Mattson, T.; Baskaran, S.A.; Amalaradjou, M.A.; Babapoor, S.; March, B.; Valipe, S.; Darre, M.; Hoagland, T.; Schreiber, D.; et al. Reduction of Salmonella enterica serovar Enteritidis colonization in 20-day-old broiler chickens by the plant-derived compounds trans-cinnamaldehyde and eugenol. Appl. Environ. Microbiol. 2012, 78, 2981–2987. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, K.; Deng, W.; Zhong, B.; Yang, W.; Chun, J. Chemical composition and antimicrobial activity of Gannan navel orange (Citrus sinensis Osbeck cv. Newhall) peel essential oils. Food Sci. Nutr. 2018, 6, 1431–1437. [Google Scholar] [CrossRef]
- Sanei-Dehkordi, A.; Vatandoost, H.; Abaei, M.R.; Davari, B.; Sedaghat, M.M. Chemical composition and larvicidal activity of Bunium persicum essential oil against two important mosquitoes vectors. J. Essent. Oil Bear. Plants 2016, 19, 349–357. [Google Scholar] [CrossRef]
- Donsì, F.; Annunziata, M.; Sessa, M.; Ferrari, G. Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT-Food Sci. Technol. 2011, 44, 1908–1914. [Google Scholar] [CrossRef]
- Pignatello, R.; Bucolo, C.; Ferrara, P.; Maltese, A.; Puleo, A.; Puglisi, G. Eudragit RS100® nanosuspensions for the ophthalmic controlled delivery of ibuprofen. Eur. J. Pharm. Sci. 2002, 16, 53–61. [Google Scholar] [CrossRef]
- Fessi, H.P.F.D.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Miladi, K.; Ibraheem, D.; Iqbal, M.; Sfar, S.; Fessi, H.; Elaissari, A. Particles from preformed polymers as carriers for drug delivery. EXCLI J. 2014, 13, 28–57. [Google Scholar] [PubMed]
- Miladi, K.; Sfar, S.; Fessi, H.; Elaissari, A. Nanoprecipitation process: From particle preparation to in vivo applications. In Polymer Nanoparticles Nanomedicines; Springer: Cham, Switzerland, 2016; pp. 17–53. [Google Scholar]
- Rivas, C.J.M.; Tarhini, M.; Badri, W.; Miladi, K.; Greige-Gerges, H.; Nazari, Q.A.; Galindo Rodríguez, S.A.; Román, R.Á.; Fessi, H.; Elaissari, A.; et al. Nanoprecipitation process: From encapsulation to drug delivery. Int. J. Pharm. 2017, 532, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.B.; Cook, V.; Tighe, R.; Schüller, S. Enterohemorrhagic Escherichia coli colonization of human colonic epithelium in vitro and ex vivo. Infect. Immun. 2015, 83, 942–949. [Google Scholar] [CrossRef]
- Polydera, A.C.; Stoforos, N.G.; Taoukis, P.S. Comparative shelf life study and vitamin C loss kinetics in pasteurised and high pressure processed reconstituted orange juice. J. Food Eng. 2003, 60, 21–29. [Google Scholar] [CrossRef]
- Emamifar, A.; Kadivar, M.; Shahedi, M.; Soleimanian-Zad, S. Evaluation of nanocomposite packaging containing Ag and ZnO on shelf life of fresh orange juice. Innov. Food Sci. Emerg. Technol. 2010, 11, 742–748. [Google Scholar] [CrossRef]
- Badri, W.; El Asbahani, A.; Miladi, K.; Baraket, A.; Agusti, G.; Nazari, Q.A.; Errachid, A.; Fessi, H.; Elaissari, A. Poly (ε-caprolactone) nanoparticles loaded with indomethacin and Nigella sativa L. essential oil for the topical treatment of inflammation. J. Drug Deliv. Sci. Tec. 2018, 46, 234–242. [Google Scholar] [CrossRef]
- Nathalie, D.; Yannick, G.; Caroline, B.; Sandrine, D.; Claude, F.; Corinne, C.; Pierre-Jacques, F. Assessment of the phototoxic hazard of some essential oils using modified 3T3 neutral red uptake assay. Toxicol. In Vitro 2006, 20, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Tirado, C.B.; Stashenko, E.E.; Combariza, M.Y.; Martinez, J.R. Comparative study of Colombian citrus oils by high-resolution gas chromatography and gas chromatography-mass spectrometry. J. Chromatogr. 1995, 697, 501–513. [Google Scholar] [CrossRef]
- Mondello, L.; Casilli, A.; Tranchida, P.Q.; Cicero, L.; Dugo, P.; Dugo, G. Comparison of fast and conventional GC analysis for citrus essential oils. J. Agric. Food Chem. 2003, 51, 5602–5606. [Google Scholar] [CrossRef] [PubMed]
- Sawamura, M.; Onishi, Y.; Ikemoto, J.; Tu, N.T.M.; Phi, N.T.L. Characteristic odour components of bergamot (Citrus bergamia Risso) essential oil. Flavour Fragrance J. 2006, 21, 609–615. [Google Scholar] [CrossRef]
- Ishii, Y.; Ohtori, N. Molecular insights into the boundary conditions in the Stokes-Einstein relation. Phys. Rev. 2016, 93, 050104-1–050104-4. [Google Scholar] [CrossRef]
- Sze, A.; Erickson, D.; Ren, L.; Li, D. Zeta-potential measurement using the Smoluchowski equation and the slope of the current–time relationship in electroosmotic flow. J. Colloid Interface Sci. 2003, 261, 402–410. [Google Scholar] [CrossRef]
- Prata, A.; Grosso, C. Production of microparticles with gelatin and chitosan. Carbohydr. Polym. 2015, 116, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Nabzar, L.; Duracher, D.; Elaïssari, A.; Chauveteau, G.; Pichot, C. Electrokinetic Properties and Colloidal Stability of Cationic Amino-Containing N-Isopropylacrylamide- Styrene Copolymer Particles Bearing Different Shell Structures. Langmuir 1998, 14, 5062–5069. [Google Scholar] [CrossRef]
- Berg, J.M.; Romoser, A.; Banerjee, N.; Zebda, R.; Sayes, C.M. The relationship between pH and zeta potential of 30 nm metal oxide nanoparticle suspensions relevant to in vitro toxicological evaluations. Nanotox 2009, 3, 276–283. [Google Scholar] [CrossRef]
- Bougrini, M.; Baraket, A.; Jamshaid, T.; Elaissari, A.; Bausells, J.; Zabala, M.; El Bari, N.; Bouchikhi, B.; Jaffrezic-Renaulta, N.; Errachid, A.; et al. Development of a novel capacitance electrochemical biosensor based on silicon nitride for ochratoxin A detection. Sens. Actuators B Chem. 2016, 234, 446–452. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid. Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef] [PubMed]










| Component | Bergamot (%) | Sweet Orange (%) |
|---|---|---|
| Limonene | 20.19 | 95.53 |
| Myrcene | - | 2.53 |
| Linalool | 8.08 | - |
| Linalyl acetate | 5.92 | - |
| γ-terpinen | 5.15 | - |
| Sample | Essential Oil Amount (mg/mL) | Entrapment Efficiency % (± SD) | Essential Oil Entrapped in mg/mg of Polymer |
|---|---|---|---|
| 5 mg OEO | 0.1 | 56 ± 0.2 | 0.014 |
| 10 mg OEO | 0.2 | 62 ± 0.2 | 0.031 |
| 25 mg OEO | 0.5 | 60 ± 1.2 | 0.075 |
| 50 mg OEO | 1 | 48 ± 0.8 | 0.12 |
| 100 mg OEO | 2 | 80 ± 1.5 | 0.4 |
| 200 mg OEO | 4 | 74 ± 2.3 | 0.74 |
| 300 mg OEO | 6 | 96 ± 1.8 | 1.44 |
| 400 mg OEO | 8 | 63 ± 0.9 | 1.26 |
| 50 mg BEO | 1 | 28 ± 0.7 | 0.07 |
| 100 mg BEO | 2 | 46 ± 0.5 | 0.23 |
| 200 mg BEO | 4 | 84 ± 0.8 | 0.84 |
| 300 mg BEO | 6 | 68 ± 2.8 | 1.02 |
| 400 mg BEO | 8 | 58 ± 1.5 | 1.16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Froiio, F.; Ginot, L.; Paolino, D.; Lebaz, N.; Bentaher, A.; Fessi, H.; Elaissari, A. Essential Oils-Loaded Polymer Particles: Preparation, Characterization and Antimicrobial Property. Polymers 2019, 11, 1017. https://doi.org/10.3390/polym11061017
Froiio F, Ginot L, Paolino D, Lebaz N, Bentaher A, Fessi H, Elaissari A. Essential Oils-Loaded Polymer Particles: Preparation, Characterization and Antimicrobial Property. Polymers. 2019; 11(6):1017. https://doi.org/10.3390/polym11061017
Chicago/Turabian StyleFroiio, Francesca, Lorianne Ginot, Donatella Paolino, Noureddine Lebaz, Abderrazzak Bentaher, Hatem Fessi, and Abdelhamid Elaissari. 2019. "Essential Oils-Loaded Polymer Particles: Preparation, Characterization and Antimicrobial Property" Polymers 11, no. 6: 1017. https://doi.org/10.3390/polym11061017
APA StyleFroiio, F., Ginot, L., Paolino, D., Lebaz, N., Bentaher, A., Fessi, H., & Elaissari, A. (2019). Essential Oils-Loaded Polymer Particles: Preparation, Characterization and Antimicrobial Property. Polymers, 11(6), 1017. https://doi.org/10.3390/polym11061017