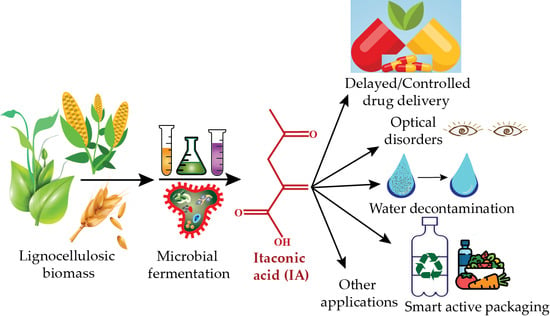
Biomass-Derived Production of Itaconic Acid as a Building Block in Specialty Polymers
Abstract
1. Introduction
2. IA Synthesis by Microbial Fermentation
2.1. Biosynthetic Pathways
2.2. IA Fermentation Using Different Substrates and A. terreus Strains
2.3. IA Fermentation with Other Microorganisms
2.4. Metabolic Engineering
2.5. Consolidated Bioprocessing
2.6. Recovery and Purification of IA
3. Major Applications of IA
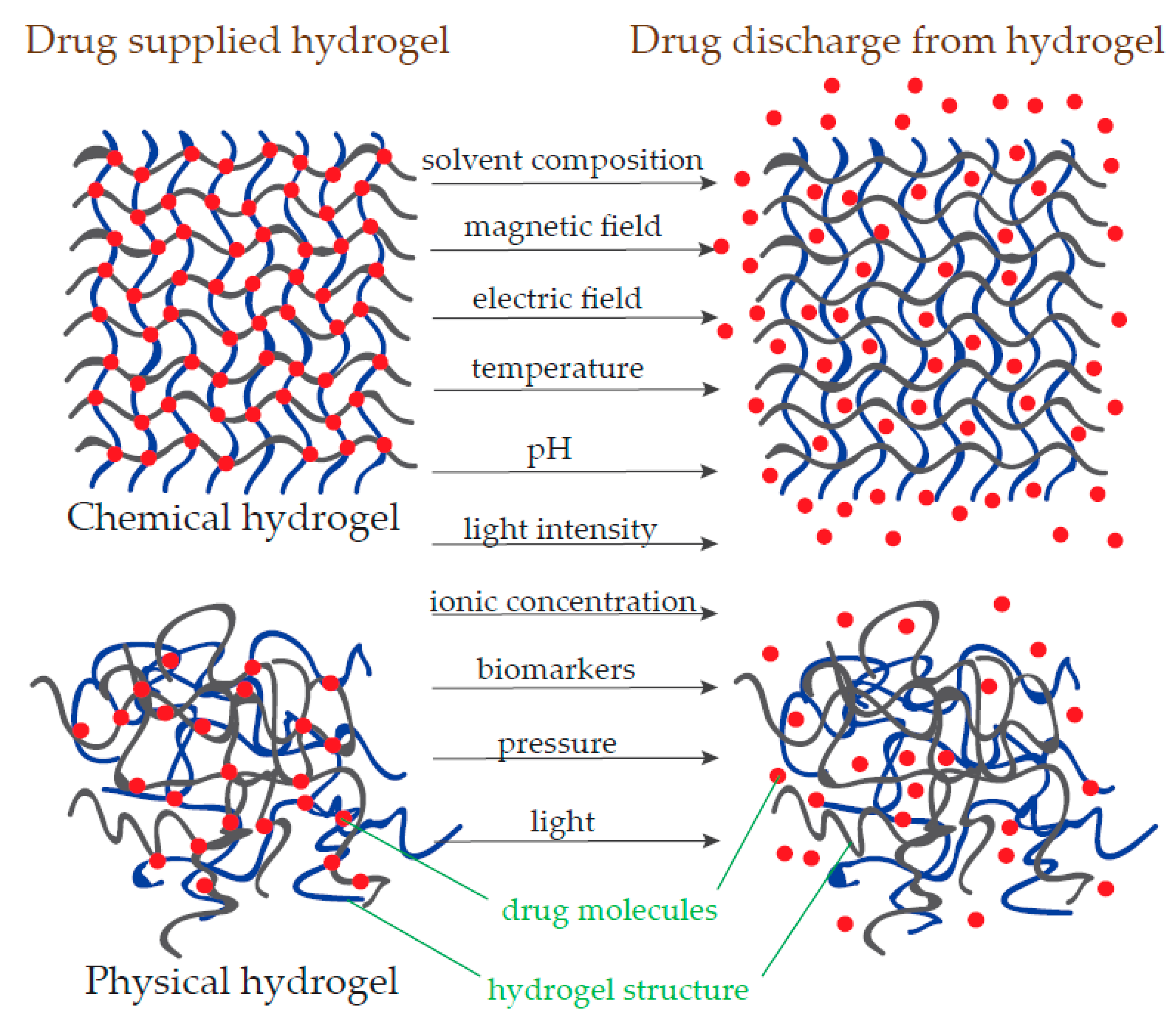
3.1. IA in Polymeric Hydrogels
3.1.1. IA Hydrogels Used in Water Decontamination
3.1.2. Bio-Based Smart Nanohydrogels for Food Applications
3.1.3. Nanohydrogel Application in the Pharmaceutical Industry
3.2. Antimicrobial Agent and Medical Applications
3.3. Other Applications
4. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rodrigues, N.M. Exploring Saccharomyces Cerevisiae to Improve Microbe-Based Production of Itaconic Acid. Master’s Thesis, Tecnico Lisboa, Lisboa, Portugal, 2014. [Google Scholar]
- Becker, J.; Lange, A.; Fabarius, J.; Wittmann, C. Top value platform chemicals: Bio-based production of organic acids. Curr. Opin. Biotechnol. 2015, 36, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Szabo, K.; Cătoi, A.F.; Vodnar, D.C. Bioactive Compounds Extracted from Tomato Processing by-Products as a Source of Valuable Nutrients. Plant Foods Hum. Nutr. 2018, 73, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Vodnar, D.C.; Călinoiu, L.F.; Dulf, F.V.; Ştefănescu, B.E.; Crişan, G.; Socaciu, C. Identification of the bioactive compounds and antioxidant, antimutagenic and antimicrobial activities of thermally processed agro-industrial waste. Food Chem. 2017, 231, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Sims, R.E.H.; Bassam, N. El Chapter 1 Biomass and Resources. In Bioenergy Options for a Cleaner Environment; Elsevier: Amsterdam, The Netherlands, 2004; pp. 1–28. [Google Scholar]
- Lee, S.Y.; Kim, H.U.; Chae, T.U.; Cho, J.S.; Kim, J.W.; Shin, J.H.; Kim, D.I.; Ko, Y.-S.; Jang, W.D.; Jang, Y.-S. A comprehensive metabolic map for production of bio-based chemicals. Nat. Catal. 2019, 2, 18–33. [Google Scholar] [CrossRef]
- Popović, I.; Katsikas, L. The thermal degradation of some polymeric di-alkyl esters of itaconic acid. J. Serbian Chem. Soc. 2013, 78, 2179–2200. [Google Scholar] [CrossRef]
- Ishii-Hyakutake, M.; Mizuno, S.; Tsuge, T. Biosynthesis and Characteristics of Aromatic Polyhydroxyalkanoates. Polymers (Basel) 2018, 10, 1267. [Google Scholar] [CrossRef]
- Sun, J.; Shen, J.; Chen, S.; Cooper, M.A.; Fu, H.; Wu, D.; Yang, Z. Nanofiller reinforced biodegradable PLA/PHA composites: Current status and future trends. Polymers (Basel) 2018, 10, 505. [Google Scholar] [CrossRef]
- Weastra S.r.o. WP 8.1. Determination of Market Potential for Selected Platform Chemicals Market Study on Succinic Acid, Itaconic Acid and 2,5-Furandicarboxylic Acid; Weastra s.r.o.: Bratislava, Slovakia, 2011. [Google Scholar]
- De Carvalho, J.C.; Magalhaes, A.I.; Soccol, C.R. (PDF) Biobased itaconic acid market and research trends—Is it really a promising chemical? Chim. Oggi-Chem. Today 2018, 36, 56–58. [Google Scholar]
- Mondala, A.H. Direct fungal fermentation of lignocellulosic biomass into itaconic, fumaric, and malic acids: Current and future prospects. J. Ind. Microbiol. Biotechnol. 2015, 42, 487–506. [Google Scholar] [CrossRef]
- Robert, T.; Friebel, S. Itaconic acid-a versatile building block for renewable polyesters with enhanced functionality. Green Chem. 2016, 18, 2922–2934. [Google Scholar] [CrossRef]
- Yang, W.; Hu, Y.; Chen, Z.; Jiang, X.; Wang, J.; Wang, R. Solubility of itaconic acid in different organic solvents: Experimental measurement and thermodynamic modeling. Fluid Phase Equilib. 2012, 314, 180–184. [Google Scholar] [CrossRef]
- Fawcett, H.H. Kirk-Othmer concise encyclopedia of chemical technology. J. Hazard. Mater. 1985, 24–25. [Google Scholar] [CrossRef]
- Raghu, C.; Raghuveer, P. Itaconic acid Production—A short review. Int. J. Adv. Eng. Technol. Manag. Appl. Sci. 2017, 4, 8–15. [Google Scholar]
- Willke, T.; Vorlop, K.D. Biotechnological production of itaconic acid. Appl. Microbiol. Biotechnol. 2001, 56, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Larsen, H.; Eimhjellen, K.E.K. The mechanism of itaconic acid formation by Aspergillus terreus. 1. The effect of acidity. Biochem. J. 1955, 6217, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Baup, S. Ueber eine neue Pyrogen-Citronensäure, und über Benennung der Pyrogen-Säuren überhaupt. Ann. Chim. Phys. 1837, 39–41. [Google Scholar] [CrossRef]
- Kinoshita, K. Über die Produktion von Itaconsäure und Mannit durch einen neuen Schimmelpilz, Aspergillus itaconicus. Acta Phytochim. 1932, 5, 271–287. [Google Scholar]
- Nelson, G.E.N.; Traufler, D.H.; Kelley, S.E.; Lockwood, L.B. Production of Itaconic Acid by Aspergillus terreus in 20-Liter Fermentors. Ind. Eng. Chem. 2005, 44, 1166–1168. [Google Scholar] [CrossRef]
- Eimhjellen, K.E.; Larsen, H. The mechanism of itaconic acid formation by Aspergillus terreus. 2. The effect of substrates and inhibitors. Biochem. J. 1955, 60, 139–147. [Google Scholar] [CrossRef]
- Da Cruz, J.C.; Camporese Sérvulo, E.F.; de Castro, A.M. Microbial Production of Itaconic Acid. In Microbial Production of Food Ingredients and Additives; Elsevier: Amsterdam, The Netherlands, 2017; pp. 291–316. ISBN 9780128115206. [Google Scholar]
- Hegde, K.; Prabhu, A.; Sarma, S.J.; Brar, S.K.; Venkata Dasu, V. Potential Applications of Renewable Itaconic Acid for the Synthesis of 3-Methyltetrahydrofuran; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128029800. [Google Scholar]
- Tate, B.E. Polymerization of itaconic acid and derivatives BT-Fortschritte der Hochpolymeren-Forschung. Adv. Polym. Sci. 1967, 5, 214–232. [Google Scholar]
- Bafana, R.; Pandey, R.A. New approaches for itaconic acid production: Bottlenecks and possible remedies. Crit. Rev. Biotechnol. 2017, 38, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, A.I.; de Carvalho, J.C.; Thoms, J.F.; Medina, J.D.C.; Soccol, C.R. Techno-economic analysis of downstream processes in itaconic acid production from fermentation broth. J. Clean. Prod. 2019, 206, 336–348. [Google Scholar] [CrossRef]
- Boruta, T.; Bizukojc, M. Production of lovastatin and itaconic acid by Aspergillus terreus: A comparative perspective. World J. Microbiol. Biotechnol. 2017, 33, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.C. Emerging biotechnologies for production of itaconic acid and its applications as a platform chemical. J. Ind. Microbiol. Biotechnol. 2017, 44, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Lu, X.; Zong, H.; Li, J.; Zhuge, B. Itaconic acid production in micro-organisms. Biotechnol. Lett. 2018. [Google Scholar] [CrossRef]
- Yamamoto, K.; Nagata, K.; Ohara, H.; Aso, Y. Challenges in the production of itaconic acid by metabolically. Bioengineered 2015, 6, 303–306. [Google Scholar] [CrossRef]
- Cunha da Cruz, J.; Machado de Castro, A.; Camporese Sérvulo, E.F. World market and biotechnological production of itaconic acid. 3 Biotech 2018, 8, 138. [Google Scholar] [CrossRef]
- Kumar, S.; Krishnan, S.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Itaconic acid used as a versatile building block for the synthesis of renewable resource based Resins and Polyesters for Future Prospective: A Review. Polym. Int. 2017, 66, 1349–1363. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas Top Value Added Chemicals From Biomass Volume I: Results of Screening for Potential Candidates; U.S. Department of Energy, Energy Efficiency and Renewable Energy: Washington, DC, USA, 2004; Volume I.
- Klement, T.; Büchs, J. Itaconic acid—A biotechnological process in change. Bioresour. Technol. 2013, 135, 422–431. [Google Scholar] [CrossRef]
- Okabe, M.; Lies, D.; Kanamasa, S.; Park, E.Y. Biotechnological production of itaconic acid and its biosynthesis in Aspergillus terreus. Appl. Microbiol. Biotechnol. 2009, 84, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Global Industry Analysts. The Global Itaconic Acid Market—Trends, Drivers & Projections. 2016. Available online: https://www.strategyr.com/MarketResearch/Itaconic_Acid_IA_Market_Trends.asp (accessed on 19 April 2019).
- Sudip, S. Itaconic acid Market Is Expected to Reach USD 204.6 Million by 2023. Available online: http://www.transparencymarketresearch.com/pressrelease/itaconic-acid-market.htm (accessed on 21 January 2019).
- Karaffa, L.; Kubicek, C.P. Citric acid and itaconic acid accumulation: Variations of the same story? Appl. Microbiol. Biotechnol. 2019, 103, 2889–2902. [Google Scholar] [CrossRef] [PubMed]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Van der Straat, L.; Vernooij, M.; Lammers, M.; van den Berg, W.; Schonewille, T.; Cordewener, J.; van der Meer, I.; Koops, A.; De Graaff, L.H. Expression of the Aspergillus terreus itaconic acid biosynthesis cluster in Aspergillus niger. Microb. Cell Fact. 2014, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.H.; Li, A.; Brickwedde, A.; Wilms, L.; Caspers, M.; Overkamp, K.; Punt, P.J. Rewiring a secondary metabolite pathway towards itaconic acid production in Aspergillus niger. Microb. Cell Fact. 2016, 15, 130. [Google Scholar] [CrossRef] [PubMed]
- Molnár, Á.P.; Németh, Z.; Kolláth, I.S.; Fekete, E.; Flipphi, M.; Ág, N.; Soós, Á.; Kovács, B.; Sándor, E.; Kubicek, C.P.; et al. High oxygen tension increases itaconic acid accumulation, glucose consumption, and the expression and activity of alternative oxidase in Aspergillus terreus. Appl. Microbiol. Biotechnol. 2018, 102, 8799–8808. [Google Scholar] [CrossRef]
- Krull, S.; Eidt, L.; Hevekerl, A.; Kuenz, A.; Prüße, U. Itaconic acid production from wheat chaff by Aspergillus terreus. Process Biochem. 2017, 63, 169–176. [Google Scholar] [CrossRef]
- Carstensen, F.; Klement, T.; Büchs, J.; Melin, T.; Wessling, M. Continuous production and recovery of itaconic acid in a membrane bioreactor. Bioresour. Technol. 2013, 137, 179–187. [Google Scholar] [CrossRef]
- Geiser, E.; Przybilla, S.K.; Buckel, W.; Wierckx, N.; Lars, M.; Bölker, M. Ustilago maydis produces itaconic acid via the unusual intermediate trans -aconitate. Microb. Biotechnol. 2016, 9, 116–126. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4720413/ (accessed on 24 January 2019). [CrossRef]
- Levinson, W.E.; Kurtzman, C.P.; Kuo, T.M. Production of itaconic acid by Pseudozyma antarctica NRRL Y-7808 under nitrogen-limited growth conditions. Enzyme Microb. Technol. 2006, 39, 824–827. [Google Scholar] [CrossRef]
- Blazeck, J.; Hill, A.; Jamoussi, M.; Pan, A.; Miller, J.; Alper, H.S. Metabolic engineering of Yarrowia lipolytica for itaconic acid production. Metab. Eng. 2015, 32, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Huang, X.; Zhong, C.; Li, J.; Lu, X. Identification of an itaconic acid degrading pathway in itaconic acid producing Aspergillus terreus. Appl. Microbiol. Biotechnol. 2016, 100, 7541–7548. [Google Scholar] [CrossRef]
- Li, A.; van Luijk, N.; ter Beek, M.; Caspers, M.; Punt, P.; van der Werf, M. A clone-based transcriptomics approach for the identification of genes relevant for itaconic acid production in Aspergillus. Fungal Genet. Biol. 2011, 48, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Michelucci, A.; Cordes, T.; Ghelfi, J.; Pailot, A.; Reiling, N.; Goldmann, O.; Binz, T.; Wegner, A.; Tallam, A.; Rausell, A.; et al. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc. Natl. Acad. Sci. USA 2013, 110, 7820–7825. [Google Scholar] [CrossRef] [PubMed]
- Diskin, C.; Pålsson-McDermott, E.M. Metabolic modulation in macrophage effector function. Front. Immunol. 2018, 9, 270. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Huang, S.C.C.; Sergushichev, A.; Lampropoulou, V.; Ivanova, Y.; Loginicheva, E.; Chmielewski, K.; Stewart, K.M.; Ashall, J.; Everts, B.; et al. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 2015, 42, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Strelko, C.L.; Lu, W.; Dufort, F.J.; Seyfried, T.N.; Chiles, T.C.; Rabinowitz, J.D.; Roberts, M.F. Itaconic acid is a mammalian metabolite induced during macrophage activation. J. Am. Chem. Soc. 2011, 133, 16386–16389. [Google Scholar] [CrossRef]
- Park, H.S.; Jun, S.C.; Han, K.H.; Hong, S.B.; Yu, J.H. Diversity, Application, and Synthetic Biology of Industrially Important Aspergillus Fungi. Adv. Appl. Microbiol. 2017, 100, 161–202. [Google Scholar] [PubMed]
- Slade, R.; Bauen, A.; Gross, R. Global bioenergy resources. Nat. Clim. Chang. 2014, 4, 99–105. [Google Scholar] [CrossRef]
- Ben, H.; Wu, Z.; Han, G.; Jiang, W.; Ragauskas, A. Pyrolytic behavior of major biomass components in waste biomass. Polymers (Basel) 2019, 11, 324. [Google Scholar] [CrossRef]
- Van Meerbeek, K.; Muys, B.; Hermy, M. Lignocellulosic biomass for bioenergy beyond intensive cropland and forests. Renew. Sustain. Energy Rev. 2019, 102, 139–149. [Google Scholar] [CrossRef]
- Regestein, L.; Klement, T.; Grande, P.; Kreyenschulte, D.; Heyman, B.; Maßmann, T.; Eggert, A.; Sengpiel, R.; Wang, Y.; Wierckx, N.; et al. From beech wood to itaconic acid: Case study on biorefinery process integration. Biotechnol. Biofuels 2018, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Bafana, R.; Sivanesan, S.; Pandey, R.A. Itaconic Acid Production by Filamentous Fungi in Starch-Rich Industrial Residues. Indian J. Microbiol. 2017, 57, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, M.; Lu, X.; Li, Y.; Li, X.; Li, J.J. Direct production of itaconic acid from liquefied corn starch by genetically engineered Aspergillus terreus. Microb. Cell Fact. 2014, 13, 108. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, G.B.; Montipó, S.; Mario, D.A.N.; Alves, S.H.; Martins, A.F. Building block itaconic acid from left-over biomass. Biomass Convers. Biorefinery 2017, 7, 23–35. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Q.; Deng, Y.; Li, J.; Chen, X.; Gu, Y.; Lv, X.; Zheng, Z.; Jiang, S.; Li, X. Production of itaconic acid by biotransformation of wheat bran hydrolysate with Aspergillus terreus CICC40205 mutant. Bioresour. Technol. 2017, 241, 25–34. [Google Scholar] [CrossRef]
- Madadi, M.; Tu, Y.; Abbas, A. Recent Status on Enzymatic Saccharification of Lignocellulosic Biomass for Bioethanol Production Recent Status on Enzymatic Saccharification of Lignocellulosic Biomass for Bioethanol Production. Eletronic J. Biol. 2017, 13, 135–143. [Google Scholar]
- Kuenz, A.; Gallenmüller, Y.; Willke, T.; Vorlop, K.D. Microbial production of itaconic acid: Developing a stable platform for high product concentrations. Appl. Microbiol. Biotechnol. 2012, 96, 1209–1216. [Google Scholar] [CrossRef]
- Saha, B.C.; Kennedy, G.J. Mannose and galactose as substrates for production of itaconic acid by Aspergillus terreus. Lett. Appl. Microbiol. 2017, 65, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Hevekerl, A.; Kuenz, A.; Vorlop, K.-D. Influence of the pH on the itaconic acid production with Aspergillus terreus. Appl. Microbiol. Biotechnol. 2014, 98, 10005–10012. [Google Scholar] [CrossRef] [PubMed]
- Kuenz, A.; Krull, S. Biotechnological production of itaconic acid—Things you have to know. Appl. Microbiol. Biotechnol. 2018, 102, 3901–3914. [Google Scholar] [CrossRef]
- Huang, X.; Lu, X.; Li, Y.; Li, X.; Li, J.J. Improving itaconic acid production through genetic engineering of an industrial Aspergillus terreus strain. Microb. Cell Fact. 2014, 13, 119. [Google Scholar] [CrossRef] [PubMed]
- Horitsu, H.; Takahashi, Y.; Tsuda, J.; Kawai, K.; Kawano, Y. Production of itaconic acid by Aspergillus terreus immobilized in polyacrylamide gels. Eur. J. Appl. Microbiol. Biotechnol. 1983, 18, 358–360. [Google Scholar] [CrossRef]
- Yahiro, K.; Shibata, S.; Jia, S.; Park, Y. Efficient Itaconic Acid Production from Raw Corn Starch. J. Ferment. Bioeng. 1997, 84, 375–377. [Google Scholar] [CrossRef]
- Rao, D.M.; Hussain, S.M.D.J.; Rangadu, V.P.; Subramanyam, K.; Krishna, G.S.; Swamy, A.V.N. Fermentatative production of itaconic acid by Aspergillus terreus using Jatropha seed cake. Afr. J. Biotechnol. 2007, 6, 2140–2142. [Google Scholar]
- Yoshinoubu, K.; Aki, K.; Eisuke, F. USE of Itaconic Acid for Regulation of Glycolytic Metabolism—Suntory Limited. Europe Patent EP1062954A1, 27 December 2000. [Google Scholar]
- Krull, S.; Hevekerl, A.; Kuenz, A.; Prüße, U. Process development of itaconic acid production by a natural wild type strain of Aspergillus terreus to reach industrially relevant final titers. Appl. Microbiol. Biotechnol. 2017, 101, 4063–4072. [Google Scholar] [CrossRef] [PubMed]
- Kreyenschulte, D.; Heyman, B.; Eggert, A.; Maßmann, T.; Kalvelage, C.; Kossack, R.; Regestein, L.; Jupke, A.; Büchs, J. In situ reactive extraction of itaconic acid during fermentation of Aspergillus terreus. Biochem. Eng. J. 2018, 135, 133–141. [Google Scholar] [CrossRef]
- Saha, B.C.; Kennedy, G.J. Ninety six well microtiter plate as microbioreactors for production of itaconic acid by six Aspergillus terreus strains. J. Microbiol. Methods 2018, 144, 53–59. [Google Scholar] [CrossRef]
- Reddy, C.S.K.; Singh, R.P. Enhanced production of itaconic acid from corn starch and market refuse fruits by genetically manipulated Aspergillus terreus SKR10. Bioresour. Technol. 2002, 85, 69–71. [Google Scholar] [CrossRef]
- Klement, T.; Milker, S.; Jäger, G.; Grande, P.M.; De María, P.D.; Büchs, J. Biomass pretreatment affects Ustilago maydis in producing itaconic acid. Microb. Cell Fact. 2012, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, T.; Sugisawa, T.; Ishidori, T.; Nakahara, T.; Sugiyama, J. Itaconic acid fermentation by a yeast belonging to the genus candida. Agric. Biol. Chem. 1981, 45, 475–479. [Google Scholar] [CrossRef]
- Otten, A.; Brocker, M.; Bott, M. Metabolic engineering of Corynebacterium glutamicum for the production of itaconate. Metab. Eng. 2015, 30, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Zambanini, T.; Hosseinpour Tehrani, H.; Geiser, E.; Merker, D.; Schleese, S.; Krabbe, J.; Buescher, J.M.; Meurer, G.; Wierckx, N.; Blank, L.M. Efficient itaconic acid production from glycerol with Ustilago vetiveriae TZ1. Biotechnol. Biofuels 2017, 10, 131. [Google Scholar] [CrossRef] [PubMed]
- Geiser, E.; Reindl, M.; Blank, L.M.; Feldbrügge, M.; Wierckx, N.; Schipper, K. Activating Intrinsic Carbohydrate-Active Enzymes of the Smut Fungus Ustilago maydis for the Degradation of Plant Cell Wall Components. Appl. Environ. Microbiol. 2016, 82, 5174–5185. [Google Scholar] [CrossRef]
- Harder, B.-J.; Bettenbrock, K.; Klamt, S. Model-based metabolic engineering enables high yield itaconic acid production by Escherichia coli. Metab. Eng. 2016, 38, 29–37. [Google Scholar] [CrossRef]
- Okamoto, S.; Chin, T.; Nagata, K.; Takahashi, T.; Ohara, H.; Aso, Y. Production of itaconic acid in Escherichia coli expressing recombinant α-amylase using starch as substrate. J. Biosci. Bioeng. 2015, 119, 548–553. [Google Scholar] [CrossRef]
- Li, A.; Pfelzer, N.; Zuijderwijk, R.; Punt, P. Enhanced itaconic acid production in Aspergillus niger using genetic modification and medium optimization. BMC Biotechnol. 2012, 12, 57. [Google Scholar] [CrossRef]
- Van Der Straat, L.; Tamayo-ramos, J.A.; Schonewille, T.; Graaff, L.H. De Overexpression of a modified 6-phosphofructo-1-kinase results in an increased itaconic acid productivity in Aspergillus niger. AMB Express 2013, 3, 1. [Google Scholar] [CrossRef]
- Yin, X.; Shin, H.; Li, J.; Du, G.; Liu, L.; Chen, J. Pgas, a Low-pH-Induced Promoter, as a Tool for Dynamic Control of Gene Expression for Metabolic Engineering of Aspergillus niger. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef]
- Mitrea, L.; Trif, M.; Cătoi, A.F.; Vodnar, D.C. Utilization of biodiesel derived-glycerol for 1,3-PD and citric acid production. Microb. Cell Fact. 2017, 16, 190. [Google Scholar] [CrossRef]
- Mitrea, L.; Călinoiu, L.-F.; Precup, G.; Bindea, M.; Rusu, B.; Trif, M.; Ştefănescu, B.-E.; Pop, I.-D.; Vodnar, D.-C. Isolated Microorganisms for Bioconversion of Biodiesel-Derived Glycerol Into 1,3-Propanediol. Bull. UASVM Food Sci. Technol. 2017, 74, 43–49. [Google Scholar] [CrossRef]
- Dulf, E.H.; Vodnar, D.C.; Dulf, F.V. Modeling tool using neural networks for l (+)- lactic acid production by pellet—Form Rhizopus oryzae NRRL 395 on biodiesel crude glycerol. Chem. Cent. J. 2018, 12, 124. [Google Scholar] [CrossRef] [PubMed]
- Bindea, M.; Rusu, B.; Rusu, A.; Trif, M.; Leopold, L.F.; Dulf, F.; Vodnar, D.C. Valorification of crude glycerol for pure fractions of docosahexaenoic acid and β-carotene production by using Schizochytrium limacinum and Blakeslea trispora. Microb. Cell Fact. 2018, 17, 97. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Zhao, H. Metabolic Engineering of Oleaginous Yeasts for Production of Fuels and Chemicals. Front. Microbiol. 2017, 8, 2185. [Google Scholar] [CrossRef] [PubMed]
- Vuoristo, K.S.; Mars, A.E.; Sangra, J.V.; Springer, J.; Eggink, G.; Sanders, J.P.M. Metabolic engineering of the mixed-acid fermentation pathway of Escherichia coli for anaerobic production of glutamate and itaconate. AMB Express 2015, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Rau, M.H.; Calero, P.; Lennen, R.M.; Long, K.S.; Nielsen, A.T. Genome-wide Escherichia coli stress response and improved tolerance towards industrially relevant chemicals. Microb. Cell Fact. 2016, 15, 176. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Seo, H.; Bhatia, S.K.; Song, H.; Kim, J.; Jeon, J.; Choi, K.; Kim, W.; Yoon, J.; Kim, Y. Production of itaconate by whole-cell bioconversion of citrate mediated by expression of multiple genes in Escherichia coli. Nat. Publ. Gr. 2017, 7, 39786. [Google Scholar]
- Chang, P.; Chen, G.S.; Chu, H.Y.; Lu, K.W.; Shen, C.R. Engineering efficient production of itaconic acid from diverse substrates in Escherichia coli. J. Biotechnol. 2017, 249, 73–81. [Google Scholar] [CrossRef]
- Moon, Y.-M.; Gurav, R.; Kim, J.; Hong, Y.-G.; Bhatia, S.K.; Jung, H.-R.; Hong, J.-W.; Choi, T.R.; Yang, S.Y.; Park, H.Y.; et al. Whole-cell Immobilization of Engineered Escherichia coli JY001 with Barium-alginate for Itaconic Acid Production. Biotechnol. Bioprocess Eng. 2018, 23, 442–447. [Google Scholar] [CrossRef]
- Li, A.; Pfelzer, N.; Zuijderwijk, R.; Brickwedde, A.; Van Zeijl, C.; Punt, P. Reduced by-product formation and modified oxygen availability improve itaconic acid production in Aspergillus niger. Appl. Microbiol. Biotechnol. 2013, 97, 3901–3911. [Google Scholar] [CrossRef]
- Steiger, M.G.; Blumhoff, M.L.; Mattanovich, D.; Sauer, M. Biochemistry of microbial itaconic acid production. Front. Microbiol. 2013, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Agbor, V.; Carere, C.; Cicek, N.; Sparling, R.; Levin, D. Biomass pretreatment for consolidated bioprocessing (CBP). In Advances in Biorefineries: Biomass and Waste Supply Chain Exploitation; Elsevier: Amsterdam, The Netherlands, 2014; pp. 234–258. ISBN 9780857095213. [Google Scholar]
- Den Haan, R.; van Rensburg, E.; Rose, S.H.; Görgens, J.F.; van Zyl, W.H. Progress and challenges in the engineering of non-cellulolytic microorganisms for consolidated bioprocessing. Curr. Opin. Biotechnol. 2015, 33, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Antonov, E.; Schlembach, I.; Regestein, L.; Rosenbaum, M.A.; Büchs, J. Process relevant screening of cellulolytic organisms for consolidated bioprocessing. Biotechnol. Biofuels 2017, 10, 106. [Google Scholar] [CrossRef] [PubMed]
- Geiser, E.; Wiebach, V.; Wierckx, N.; Blank, L.M. Prospecting the biodiversity of the fungal family Ustilaginaceae for the production of value-added chemicals. Fungal Biol. Biotechnol. 2014, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Guevarra, E.D.; Tabuchi, T. Accumulation of itaconic, 2-hydroxyparaconic, itatartaric, and malic acids by strains of the genus ustilago. Agric. Biol. Chem. 1990, 54, 2353–2358. [Google Scholar] [CrossRef][Green Version]
- Zambanini, T.; Kleineberg, W.; Sarikaya, E.; Buescher, J.M.; Meurer, G.; Wierckx, N.; Blank, L.M. Enhanced malic acid production from glycerol with high-cell density Ustilago trichophora TZ1 cultivations. Biotechnol. Biofuels 2016, 9, 135. [Google Scholar] [CrossRef] [PubMed]
- Schute, K.; Detoni, C.; Kann, A.; Jung, O.; Palkovits, R.; Rose, M. Separation in biorefineries by liquid phase adsorption: Itaconic acid as case study. ACS Sustain. Chem. Eng. 2016, 4, 5921–5928. [Google Scholar] [CrossRef]
- Magalhães, A.I.; de Carvalho, J.C.; Medina, J.D.C.; Soccol, C.R. Downstream process development in biotechnological itaconic acid manufacturing. Appl. Microbiol. Biotechnol. 2017, 101, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dwiarti, L.; Otsuka, M.; Miura, S.; Yaguchi, M.; Okabe, M. Itaconic acid production using sago starch hydrolysate by Aspergillus terreus TN484-M1. Bioresour. Technol. 2007, 98, 3329–3337. [Google Scholar] [CrossRef] [PubMed]
- Urbanus, J.; Roelands, C.P.M.; Verdoes, D.; ter Horst, J.H. Intensified crystallization in complex media: Heuristics for crystallization of platform chemicals. Chem. Eng. Sci. 2012, 77, 18–25. [Google Scholar] [CrossRef]
- Eggert, A.; Maßmann, T.; Kreyenschulte, D.; Becker, M.; Heyman, B.; Büchs, J.; Jupke, A. Integrated in-situ product removal process concept for itaconic acid by reactive extraction, pH-shift back extraction and purification by pH-shift crystallization. Sep. Purif. Technol. 2019, 463–472. [Google Scholar] [CrossRef]
- Raghuwanshi, S.S.; Rathore, A.K.; Pal, S. Recovery of Itaconic Acid From An Aqueous Solution By Using Chemically Modified Vegetable Oil As Diluent. Res. J. Pharm. Biol. Chem. Sci. 2018, 9, 81–86. [Google Scholar]
- Kaur, G.; Maesen, M.; Garcia-Gonzalez, L.; De Wever, H.; Elst, K. Novel Intensified Back Extraction Process for Itaconic Acid: Toward in Situ Product Recovery for Itaconic Acid Fermentation. ACS Sustain. Chem. Eng. 2018, 6, 7403–7411. [Google Scholar] [CrossRef]
- Gorden, J.; Geiser, E.; Wierckx, N.; Blank, L.M.; Zeiner, T.; Brandenbusch, C. Integrated process development of a reactive extraction concept for itaconic acid and application to a real fermentation broth. Eng. Life Sci. 2017, 17, 809–816. [Google Scholar] [CrossRef]
- Varga, V.; Bélafi-Bakó, K.; Vozik, D.; Nemestóthy, N. Recovery of Itaconic Acid by Electrodialysis. Hungarian J. Ind. Chem. 2019, 46, 43–46. [Google Scholar] [CrossRef]
- Qi, P.; Chen, H.L.; Nguyen, H.T.H.; Lin, C.C.; Miller, S.A. Synthesis of biorenewable and water-degradable polylactam esters from itaconic acid. Green Chem. 2016, 18, 4170–4175. [Google Scholar] [CrossRef]
- Lei, W.; Yang, X.; Qiao, H.; Shi, D.; Wang, R.; Zhang, L. Renewable resource-based elastomer nanocomposite derived from myrcene, ethanol, itaconic acid and nanosilica: Design, preparation and properties. Eur. Polym. J. 2018, 106, 1–8. [Google Scholar] [CrossRef]
- Louven, Y.; Schute, K.; Palkovits, R. Ruthenium Catalyzed Reductive Transformation of Itaconic Acid and Ammonia Into 3- and 4-Methyl-pyrrolidone. ChemCatChem 2019, 11, 439–442. [Google Scholar] [CrossRef]
- Park, D.S.; Abdelrahman, O.A.; Vinter, K.P.; Howe, P.M.; Bond, J.Q.; Reineke, T.M.; Zhang, K.; Dauenhauer, P.J. Multifunctional Cascade Catalysis of Itaconic Acid Hydrodeoxygenation to 3-Methyl-tetrahydrofuran. ACS Sustain. Chem. Eng. 2018, 6, 9394–9402. [Google Scholar] [CrossRef]
- Kassi, E.; Loizou, E.; Porcar, L.; Patrickios, C.S. Di(n-butyl) itaconate end-functionalized polymers: Synthesis by group transfer polymerization and solution characterization. Eur. Polym. J. 2011, 47, 816–822. [Google Scholar] [CrossRef]
- Gumare, N. Global Industry Analysis, Size, Share, Growth, Trends, and Forecast 2015–2023 Report. Itaconic Acid Market for Synthetic Latex, Unsaturated Polyester Resins, Detergents, Superabsorbent Polymers (SAP), and Other Applications; U.S.A. 2015. Available online: https://www.transparencymarketresearch.com/itaconic-acid-market.html (accessed on 15 March 2019).
- Fuciños, C.; Fuciños, P.; Amado, I.R.; Míguez, M.; Fajardo, P.; Pastrana, L.M.; Rúa, M.L. Smart Nanohydrogels for Controlled Release of Food Preservatives; Elsevier Inc.: Amsterdam, The Netherlands, 2016; ISBN 9780128007235. [Google Scholar]
- Tomić, S.L.; Suljovrujić, E.H.; Filipović, J.M. Biocompatible and bioadhesive hydrogels based on 2-hydroxyethyl methacrylate, monofunctional poly(alkylene glycol)s and itaconic acid. Polym. Bull. 2006, 57, 691–702. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Bahram, M.; Mohseni, N.; Moghtader, M. An Introduction to Hydrogels and Some Recent Applications. In Emerging Concepts in Analysis and Applications of Hydrogels Chemical; Majee, S.B., Ed.; Intech: Rijeka, Croatia, 2016; pp. 10–38. ISBN 978-953-51-2510-5. [Google Scholar]
- Athukoralalage, S.S.; Rajkamal, B.; Naba, D.K.; Choudhury, N.R. 3D Bioprinted Nanocellulose-Based Hydrogels for Tissue Engineering Applications: A Brief Review. Polymers 2019, 11, 898. [Google Scholar] [CrossRef] [PubMed]
- Dannert, C.; Stokke, B.T.; Dias, R.S. Nanoparticle-Hydrogel Composites: From Molecular Interactions to Macroscopic Behavior. Polymers (Basel) 2019, 11, 275. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M.M. Hydrogel Preparation Technologies: Relevance Kinetics, Thermodynamics and Scaling up Aspects. J. Polym. Environ. 2019, 27, 871–891. [Google Scholar] [CrossRef]
- Harrison, I.P.; Spada, F. Hydrogels for atopic dermatitis and wound management: A superior drug delivery vehicle. Pharmaceutics 2018, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Chirani, N.; Yahia, L.; Gritsch, L.; Motta, F.L.; Chirani, S.; Faré, S. History and Applications of Hydrogels. J. Biomed. Sci. 2015, 4, 2. [Google Scholar]
- Stanojević, M.; Krušić, M.K.; Filipović, J.; Parojčić, J.; Stupar, M. An investigation into the influence of hydrogel composition on swelling behavior and drug release from poly(acrylamide-co-itaconic acid) hydrogels in various media. Drug Deliv. J. Deliv. Target. Ther. Agents 2006, 13, 1–7. [Google Scholar] [CrossRef]
- El-Halah, A.; Machado, D.; González, N.; Contreras, J.; López-Carrasquero, F. Use of super absorbent hydrogels derivative from acrylamide with itaconic acid and itaconates to remove metal ions from aqueous solutions. J. Appl. Polym. Sci. 2019, 136, 46999. [Google Scholar] [CrossRef]
- Amonpattaratkit, P.; Khunmanee, S.; Kim, D.H.; Park, H. Synthesis and Characterization of Gelatin-Based Crosslinkers for the Fabrication of Superabsorbent Hydrogels. Materials (Basel) 2017, 10, 826. [Google Scholar] [CrossRef]
- Mohammadzadeh Pakdel, P.; Peighambardoust, S.J. A review on acrylic based hydrogels and their applications in wastewater treatment. J. Environ. Manag. 2018, 217, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Foungfung, D.; Phattanarudee, S.; Seetapan, N.; Kiatkamjornwong, S. Acrylamide-itaconic acid superabsorbent polymers and superabsorbent polymer/mica nanocomposites. Polym. Adv. Technol. 2011, 22, 635–647. [Google Scholar] [CrossRef]
- Thakur, S.; Chaudhary, J.; Kumar, V.; Thakur, V.K. Progress in pectin based hydrogels for water purification: Trends and challenges. J. Environ. Manage. 2019, 238, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Elgadir, M.A.; Akanda, M.J.H.; Ferdosh, S.; Mehrnoush, A.; Karim, A.A.; Noda, T.; Sarker, M.Z.I. Mixed biopolymer systems based on starch. Molecules 2012, 17, 584–597. [Google Scholar] [CrossRef] [PubMed]
- Soto, D.; Urdaneta, J.; Pernía, K.; Leõn, O.; Muñoz-Bonilla, A.; Fernandez-García, M. Removal of heavy metal ions in water by starch esters. Starch/Staerke 2016, 68, 37–46. [Google Scholar] [CrossRef]
- Soto, D.; Urdaneta, J.; Pernia, K.; León, O.; Muñoz-Bonilla, A.; Fernández-García, M. Itaconic Acid Grafted Starch Hydrogels as Metal Remover: Capacity, Selectivity and Adsorption Kinetics. J. Polym. Environ. 2016, 24, 343–355. [Google Scholar] [CrossRef]
- De Peña, Y.P.; López, W.; Burguera, M.; Burguera, J.L.; López-Carrasquero; Carrillo, M. Flow injection system for cadmium preconcentration on poly(octadecyl diitaconate) (PDI-18) and atomic absorption spectrometry detection. Anal. Chim. Acta 2001, 438, 259–266. [Google Scholar] [CrossRef]
- Ge, H.; Hua, T.; Wang, J. Preparation and characterization of poly (itaconic acid)-grafted crosslinked chitosan nanoadsorbent for high uptake of Hg2+ and Pb2+. Int. J. Biol. Macromol. 2017, 95, 954–961. [Google Scholar] [CrossRef]
- Çavuş, S.; Gürdaǧ, G.L. Noncompetitive removal of heavy metal ions from aqueous solutions by poly[2-(acrylamido)-2-methyl-1-propanesulfonic acid-co-itaconic acid] hydrogel. Ind. Eng. Chem. Res. 2009, 48, 2652–2658. [Google Scholar] [CrossRef]
- Sharma, N.; Tiwari, A. Nanomagnetite-loaded poly (acrylamide-co-itaconic acid) hydrogel as adsorbent for effective removal of Mn2+ from contaminated water. Desalin. Water Treat. 2016, 57, 5654–5672. [Google Scholar] [CrossRef]
- Milašinović, N.; Jakovetić, S.; Knežević-Jugović, Z.; Milosavljević, N.; Lučić, M.; Filipović, J.; Kalagasidis Krušić, M. Catalyzed Ester Synthesis Using Candida rugosa Lipase Entrapped by Poly(N-isopropylacrylamide-co-itaconic Acid) Hydrogel. Sci. World J. 2014, 2014, 142123. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Lo, I.M.C. A holistic review of hydrogel applications in the adsorptive removal of aqueous pollutants: Recent progress, challenges, and perspectives. Water Res. 2016, 106, 259–271. [Google Scholar] [CrossRef]
- Fucinõs, C.; Fucinõs, P.; Míguez, M.; Katime, I.; Pastrana, L.M.; Ŕua, M.L. Temperature- and pH-sensitive nanohydrogels of poly(N-isopropylacrylamide) for food packaging applications: Modelling the swelling-collapse behaviour. PLoS ONE 2014, 9, e87190. [Google Scholar] [CrossRef] [PubMed]
- Călinoiu, L.-F.; Ştefănescu, B.; Pop, I.; Muntean, L.; Vodnar, D. Chitosan Coating Applications in Probiotic Microencapsulation. Coatings 2019, 9, 194. [Google Scholar] [CrossRef]
- Trif, M.; Vodnar, D.; Mitrea, L.; Rusu, A.; Socol, C. Design and Development of Oleoresins Rich in Carotenoids Coated Microbeads. Coatings 2019, 9, 235. [Google Scholar] [CrossRef]
- Mehtiö, T.; Anghelescu-Hakala, A.; Hartman, J.; Kunnari, V.; Harlin, A. Crosslinkable poly(lactic acid)-based materials: Biomass-derived solution for barrier coatings. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Cespugli, M.; Lotteria, S.; Navarini, L.; Lonzarich, V.; Del Terra, L.; Vita, F.; Zweyer, M.; Baldini, G.; Ferrario, V.; Ebert, C.; et al. Rice Husk as an Inexpensive Renewable Immobilization Carrier for Biocatalysts Employed in the Food, Cosmetic and Polymer Sectors. Catalysts 2018, 8, 471. [Google Scholar] [CrossRef]
- Ma, L.; Feng, S.; La Fuente-Núñez, C.D.; Hancock, R.E.W.; Lu, X. Development of Molecularly Imprinted Polymers to Block Quorum Sensing and Inhibit Bacterial Biofilm Formation. ACS Appl. Mater. Interfaces 2018, 10, 18450–18457. [Google Scholar] [CrossRef]
- Mathers, C.; Boerma, T.; Ma Fat, D. The Global Burden of Disease: 2004 Update; WHO: Geneva, Switzerland, 2008. [Google Scholar]
- Vos, T.; Barber, R.M.; Bell, B.; Bertozzi-Villa, A.; Biryukov, S.; Bolliger, I.; Charlson, F.; Davis, A.; Degenhardt, L.; Dicker, D.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef]
- Yang, K.; Han, Q.; Chen, B.; Zheng, Y.; Zhang, K.; Li, Q.; Wang, J. Antimicrobial hydrogels: Promising materials for medical application. Int. J. Nanomed. 2018, 13, 2217–2263. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Nan, S.; Zhang, L.; Huang, H.; Wang, J. Synthesis and characterization of pH-sensitive poly(itaconic acid)-poly(ethylene glycol)-folate-poly(L-histidine) micelles for enhancing tumor therapy and tunable drug release. J. Colloid Interface Sci. 2015, 458, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Li, A.; Zhang, A.; Zheng, Y.; Liu, J. Recent Advances in Functional-Polymer-Decorated Transition-Metal Nanomaterials for Bioimaging and Cancer Therapy. ChemMedChem 2018, 13, 2134–2149. [Google Scholar] [CrossRef] [PubMed]
- Raţă, D.M.; Chailan, J.F.; Peptu, C.A.; Costuleanu, M.; Popa, M. Chitosan: Poly(N-vinylpyrrolidone-alt-itaconic anhydride) nanocapsules—A promising alternative for the lung cancer treatment. J. Nanopart. Res. 2015, 17, 316. [Google Scholar] [CrossRef]
- Milašinović, N.; Kalagasidis Krušić, M.; Knežević-Jugović, Z.; Filipović, J. Hydrogels of N-isopropylacrylamide copolymers with controlled release of a model protein. Int. J. Pharm. 2010, 383, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Sakthivel, M.; Franklin, D.S.; Sudarsan, S.; Chitra, G.; Sridharan, T.B.; Guhanathan, S. Investigation on pH/salt-responsive multifunctional itaconic acid based polymeric biocompatible, antimicrobial and biodegradable hydrogels. React. Funct. Polym. 2018, 122, 9–21. [Google Scholar] [CrossRef]
- Pathania, D.; Verma, C.; Negi, P.; Tyagi, I.; Asif, M.; Kumar, N.S.; Al-Ghurabi, E.H.; Agarwal, S.; Gupta, V.K. Novel nanohydrogel based on itaconic acid grafted tragacanth gum for controlled release of ampicillin. Carbohydr. Polym. 2018, 196, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Petan, T.; Jarc, E.; Jusović, M. Lipid Droplets in Cancer: Guardians of Fat in a Stressful World. Molecules 2018, 23, 1941. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, S.; Hamishehkar, H.; Ghorbani, M. A novel smart PEGylated gelatin nanoparticle for co-delivery of doxorubicin and betanin: A strategy for enhancing the therapeutic efficacy of chemotherapy. Mater. Sci. Eng. C 2019, 97, 833–841. [Google Scholar] [CrossRef]
- Nita, L.E.; Chiriac, A.P.; Bercea, M.; Ghilan, A.; Rusu, A.G.; Dumitriu, R.P.; Mititelu-Tartau, L. Multifunctional hybrid 3D network based on hyaluronic acid and a copolymer containing pendant spiroacetal moieties. Int. J. Biol. Macromol. 2019, 125, 191–202. [Google Scholar] [CrossRef]
- Dong, K.; Lei, Q.; Qi, H.; Zhang, Y.; Cui, N.; Wu, X.; Xie, L.; Yan, X.; Lu, T. Amplification of Oxidative Stress in MCF-7 Cells by a Novel pH-Responsive Amphiphilic Micellar System Enhances Anticancer Therapy. Mol. Pharm. 2019, 16, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Heng, C.; Zhou, X.; Zheng, X.; Liu, M.; Wen, Y.; Huang, H.; Fan, D.; Hui, J.; Zhang, X.; Wei, Y. Surface grafting of rare-earth ions doped hydroxyapatite nanorods (HAp:Ln(Eu/Tb)) with hydrophilic copolymers based on ligand exchange reaction: Biological imaging and cancer treatment. Mater. Sci. Eng. C 2018, 91, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, G.; Agrawal, R.; Pich, A. Dual Responsive Poly(N-vinylcaprolactam) Based Degradable Microgels for Drug Delivery. Part. Part. Syst. Charact. 2017, 34, 1700132. [Google Scholar] [CrossRef]
- Eroglu, B.; Dalgakiran, D.; Inan, T.; Kurkcuoglu, O.; Güner, F.S. A computational and experimental approach to develop minocycline-imprinted hydrogels and determination of their drug delivery performances. J. Polym. Res. 2018, 25, 258. [Google Scholar] [CrossRef]
- Valente, M.; Vedelago, J.; Chacón, D.; Mattea, F.; Velásquez, J.; Pérez, P. Water-equivalence of gel dosimeters for radiology medical imaging. Appl. Radiat. Isot. 2018, 141, 193–198. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations The Review on Antimicrobial Resistance Chaired. HM Gov. Wellcome Trust 2014, 1–20. Available online: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf (accessed on 02 May 2019).
- Riga, E.K.; Vöhringer, M.; Widyaya, V.T.; Lienkamp, K. Polymer-Based Surfaces Designed to Reduce Biofilm Formation: From Antimicrobial Polymers to Strategies for Long-Term Applications. Macromol. Rapid Commun. 2017, 38. [Google Scholar] [CrossRef] [PubMed]
- Boschert, D.; Schneider-Chaabane, A.; Himmelsbach, A.; Eickenscheidt, A.; Lienkamp, K. Synthesis and Bioactivity of Polymer-Based Synthetic Mimics of Antimicrobial Peptides (SMAMPs) Made from Asymmetrically Disubstituted Itaconates. Chemistry 2018, 24, 8217–8227. [Google Scholar] [CrossRef] [PubMed]
- Kiso, Y.; Kosumoto, A.; Furuya, E. Use of Itaconic Acid for Regulation of Glycolytic Metabolism. European Patent EP1062954B1, 30 May 2007. [Google Scholar]
- De Seymour, J.V.; Conlon, C.A.; Sulek, K.; Villas Bôas, S.G.; McCowan, L.M.E.; Kenny, L.C.; Baker, P.N. Early pregnancy metabolite profiling discovers a potential biomarker for the subsequent development of gestational diabetes mellitus. Acta Diabetol. 2014, 51, 887–890. [Google Scholar] [CrossRef]
- Meiser, J.; Kraemer, L.; Jaeger, C.; Madry, H.; Link, A.; Lepper, P.M.; Hiller, K.; Schneider, J.G. Itaconic acid indicates cellular but not systemic immune system activation. Oncotarget 2018, 9, 32098–32107. [Google Scholar] [CrossRef]
- Sakai, A.; Kusumoto, A.; Kiso, Y.; Furuya, E. Itaconate reduces visceral fat by inhibiting fructose 2,6-bisphosphate synthesis in rat liver. Nutrition 2004, 20, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.L.; Ryan, D.G.; Prag, H.A.; Dikovskaya, D.; Menon, D.; Zaslona, Z.; Jedrychowski, M.P.; Costa, A.S.H.; Higgins, M.; Hams, E.; et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 2018, 556, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Poillucci, R.A.; Hansen, C.J. Reducing Use of Styrene Monomer in Unsaturated Polyester Resins; TURI: Lowell, MA, USA, 2013. [Google Scholar]
- Panic, V.V.; Seslija, S.I.; Popovic, I.G.; Spasojevic, V.D.; Popovic, A.R.; Nikolic, V.B.; Spasojevic, P.M. Simple One-Pot Synthesis of Fully Biobased Unsaturated Polyester Resins Based on Itaconic Acid. Biomacromolecules 2017, 18, 3881–3891. [Google Scholar] [CrossRef] [PubMed]
- European Commission. A European Strategy for Plastics in a Circular Economy; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Fidanovski, B.Z.; Popovic, I.G.; Radojevic, V.J.; Radisavljevic, I.Z.; Perisic, S.D.; Spasojevic, P.M. Composite materials from fully bio-based thermosetting resins and recycled waste poly(ethylene terephthalate). Compos. Part B Eng. 2018, 153, 117–123. [Google Scholar] [CrossRef]






| Substrate | A. terreus | IA [g/L] | pH | T [°C] | Method | OP [g/L/h] | Y [g/gTS] | Ref. |
|---|---|---|---|---|---|---|---|---|
| glucose | NRRL 1960 | 24.7–49.5 | 1.8–2.0 | 34 | BF | 0.33–0.44 | N.A. | [22] |
| carbon sources | NRRL 1960 | 129 | 2.1–6.0 | N.A. | SF | N.A. | N.A. | [19] |
| glucose | 2.5 | 35 | BR | N.A. | N.A. | [71] | ||
| corn starch | TN-484 | 62 | 1.5 | 30 | SF/ALB | N.A. | N.A. | [72] |
| 61 | 2.0 | 30 | ||||||
| 59 | 2.5 | 30 | ||||||
| 57 | 3.0 | 30 | ||||||
| Jathropa seed cake | 24.46 | 3.5 | 32 | SF | N.A. | N.A. | [73] | |
| glucose | 86.2 | 3.1 | 33 | STR | 0.51 | 0.62 | [66] | |
| 90 | N.A. | 0.58 | ||||||
| corn starch | CICC 40205 | 77.6 | 4.0 | 37 | SF | N.A. | N.A. | [62] |
| glucose | DSM 23081 | 129 | 3.1 | 33 | STR | N.A. | N.A. | [68] |
| 87 | N.A. | N.A. | ||||||
| 146 | 3.0 | N.A. | ||||||
| rice husk | ATCC 10020 | 1.9 | 6.0 | 30 | SF | N.A. | N.A. | [63] |
| wheat chaff | DSM 23081 | 27.7 | 3.1 | 33 | SF | 0.19 | 0.41 | [45] |
| artif. wheat chaff | 51.5 | 3.1 | 33 | 0.31 | 0.59 | |||
| potato starch | C1 | 30.8 | N.A. | 35 | SF | N.A. | N.A. | [61] |
| C2 | 23.4 | N.A. | N.A. | |||||
| wheat bran | CICC 40205 | 49.65 | 7.0 | 32 | SF | N.A. | N.A. | [74] |
| mannose | NRRL 1971 | 36.4 | 3.1 | 33 | SF | N.A. | 0.46 | [30] |
| glucose | 42.6 | N.A. | ||||||
| xylose | 30.5 | |||||||
| arabinose | 25.8 | |||||||
| galactose | DSM 23081 | 9.1 | ||||||
| glucose | DSM 23081 | 129 | 3.0 | 35 | SGR | 0.61 | 0.57 | [75] |
| 138 | 3.2 | 0.82 | N.A. | |||||
| 162 | 3.4 | 0.99 | 0.46 | |||||
| 150 | 3.0 | STR | N.A. | 0.56 | ||||
| glucose | DSM 23081 | 70 | 3.1 | 33 | Fl | N.A. | N.A. | [76] |
| glucose | DSM 23081 | 105 | 3.1 | 33 | ||||
| glucose | NRRL 1960 | 51.9 | 3.1 | 33 | SF | N.A. | N.A. | [77] |
| xylose | DSM 23081 | 38.7 | ||||||
| arabinose | NRRL 1961 | 34.8 | ||||||
| GXA | 33.2 | |||||||
| glucose | NRRL 1960 | 73.6 | 3.0 | 33 | BF | N.A. | 0.85 | [44] |
| fruit waste | SKR10 | 20 | 3.0 | 34 | SF | N.A. | 0.22 | [78] |
| Corn starch | 28.5–31.0 | N.A. | 0.26 |
| Substrate | Strain | IA [g/L] | pH | T [°C] | Method | OP [g/L/h] | Y [g/gTS] | Ref. |
|---|---|---|---|---|---|---|---|---|
| glucose | Yarrowia lipolytica | 4.6 | 3.5– 5.0 | 28 | BR | 0.045 | 0.058 | [49] |
| glucose | Candida sp. | 30-35 | 3 | 26 | flask | N.A. | N.A. | [80] |
| glucose | C. glutamicum strains | 1.4–3.6 | 7.0 | 30 | BSF | N.A. | 0.011 | [81] |
| urea | 13.3–59.0 | 0.23 | ||||||
| glycerol | U. vetiveriae TZ1 | 34.7 | 6.5 | N.A. | SF | 0.09 | N.A. | [82] |
| glucose | U. maydis MB 215 | 4 | 6 | 30 | CF | 0.8 | N.A. | [46] |
| potato starch | U. maydis | 34.52 | N.A. | N.A. | N.A. | N.A. | N.A. | [61] |
| glucose | U. maydis MB215 | 20 | N.A. | 30 | SF | 0.27 | 0.17 | [79] |
| cellobiose | U. maydis strains | N.A. | 6.5 | 30 | SF | N.A. | N.A. | [83] |
| glycerol | Eng. E. coli | 22 | 5–5.5 | 37 | BSF | 0.6 | 0.55 | [74] |
| xylose | 20 | 0.6 | 0.51 | |||||
| glucose | 18 | 0.43 | 0.36 | |||||
| glucose | E. coli strains | 2.27 | N.A. | 37 | SF | N.A. | 0.77 | [84] |
| 32 | N.A. | 30 | BR | N.A. | 0.68 | |||
| starch | E. coli | 0.15–0.62 | 6.8 | 28 | JF | N.A. | N.A. | [85] |
| glucose | A. niger strains | 26.2 | 3.5 | 33 | BF | 0.35 | N.A. | [43] |
| glucose | A. niger strains | 0.26–0.29 | 3.5 | 33 | BF | N.A. | N.A. | [86] |
| sorbitol | A. niger CAD4 | 3–8 | N.A. | 30 | flask | N.A. | N.A. | [87] |
| sorbitol + xylose | Eng. A. niger + cadA | 54.3 | N.A. | 30 | F | N.A. | N.A. | [42] |
| glucose | Eng. A. niger strains | 0.82–4.92 | 3.1 | 35 | SF | N.A. | N.A. | [88] |
| Method | Media | Yield [%] | Disadvantage | Ref. |
|---|---|---|---|---|
| Crystallization | FB | 80 | - high thermal energy input required - reduced final purity > additional purification step | [37] |
| Crystallization | FB | 51 | - low yield - high thermal energy input required | [109] |
| Crystallization | FB | 23 | - low yield - change in fermentation temperature | [110] |
| Adsorption | AS | 100 | - high waste-water quantity | [107] |
| Reactive extraction/back extraction/pH- shift crystallization | FB | 99 | - NaCl salt by-product formation | [111] |
| Reactive extraction | AS | 94.7 | - decreased toxicity due to usage of vegetable oil | [112] |
| Back extraction | 80 | - high energy demand - unwanted side products | [113] | |
| Reactive extraction | FB | 91 | - mass transfer area limitations | [76] |
| Reactive extraction | AS | 80 | - process under study | [114] |
| Electrodialysis | AS | 50 | - low efficiency - competitive only with a yield of 98% | [115] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teleky, B.-E.; Vodnar, D.C. Biomass-Derived Production of Itaconic Acid as a Building Block in Specialty Polymers. Polymers 2019, 11, 1035. https://doi.org/10.3390/polym11061035
Teleky B-E, Vodnar DC. Biomass-Derived Production of Itaconic Acid as a Building Block in Specialty Polymers. Polymers. 2019; 11(6):1035. https://doi.org/10.3390/polym11061035
Chicago/Turabian StyleTeleky, Bernadette-Emőke, and Dan Cristian Vodnar. 2019. "Biomass-Derived Production of Itaconic Acid as a Building Block in Specialty Polymers" Polymers 11, no. 6: 1035. https://doi.org/10.3390/polym11061035
APA StyleTeleky, B.-E., & Vodnar, D. C. (2019). Biomass-Derived Production of Itaconic Acid as a Building Block in Specialty Polymers. Polymers, 11(6), 1035. https://doi.org/10.3390/polym11061035