Replication of Mesoscale Pore One-dimensional Nanostructures: Surface-induced Phase Separation of Polystyrene/Poly(vinyl alcohol) (PS/PVA) Blends
Abstract
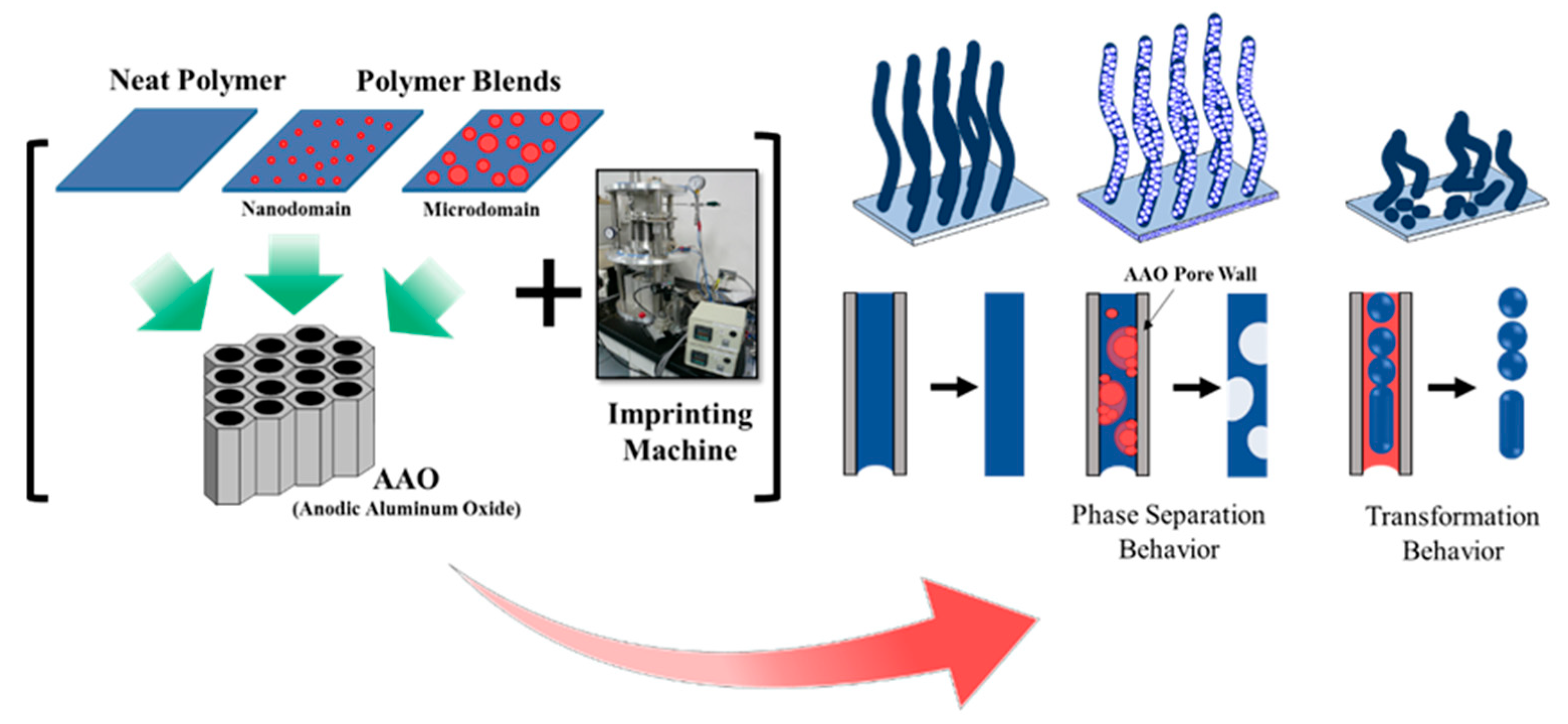
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of AAOs
2.3. Preparation of Polymer Blends
2.4. Preparation of Polymer Films
2.5. Fabrication of Nanostructures by Thermal Nanoimprint
2.6. Characterization
3. Results and Discussion
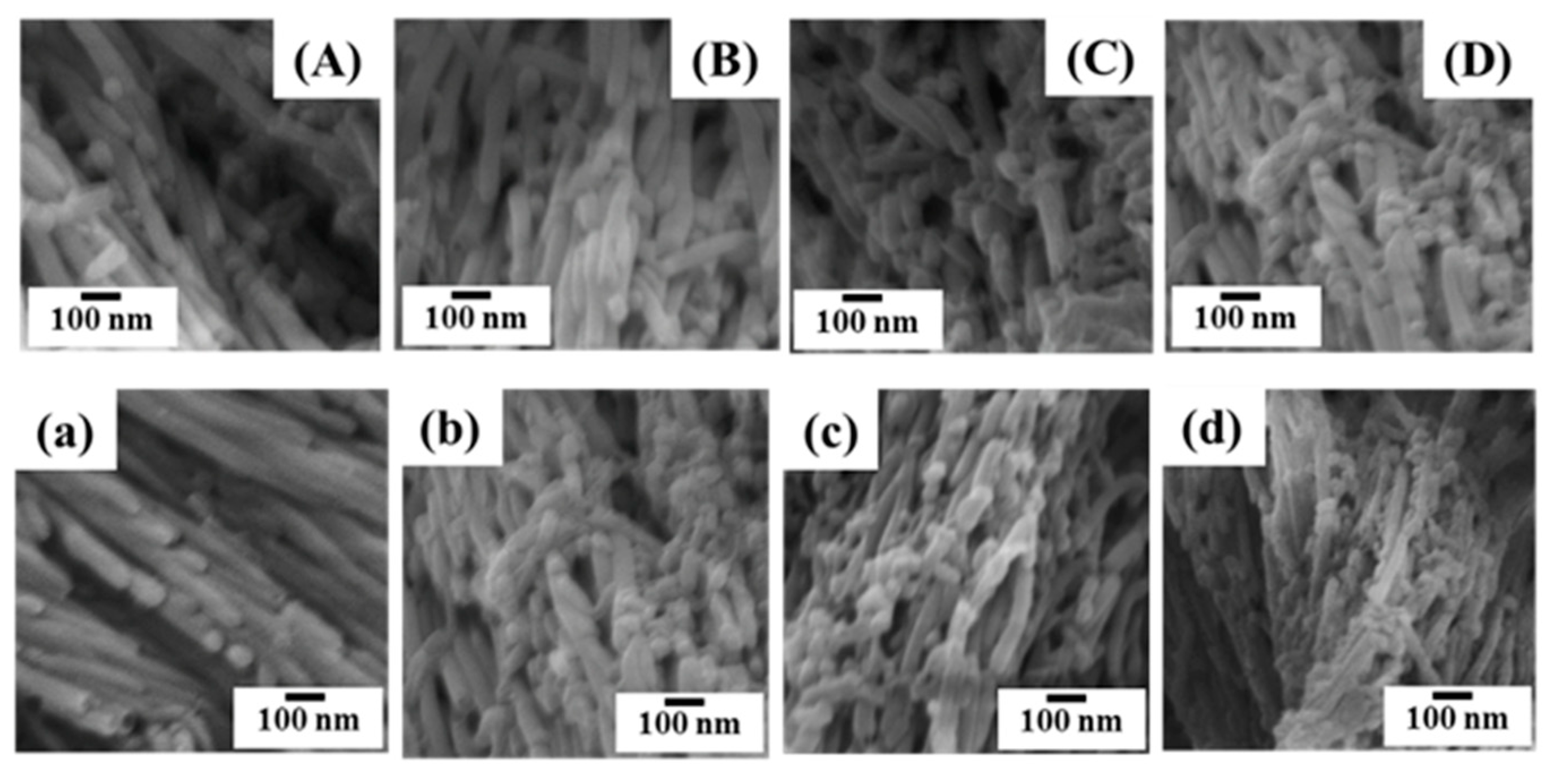
3.1. Characterization of AAOs
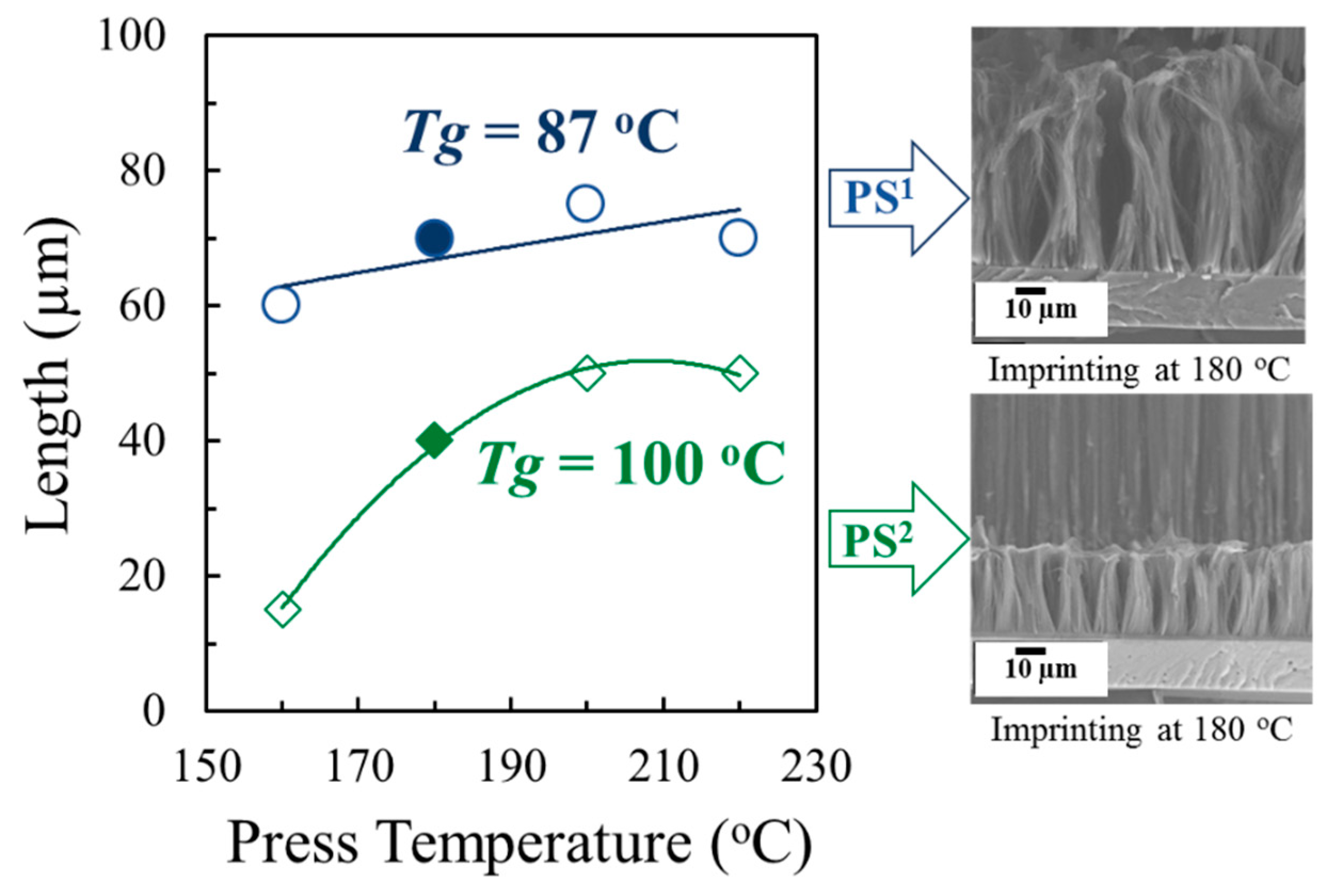
3.2. Fabrication of One–dimensional Nanostructures by Thermal Nanoimprint
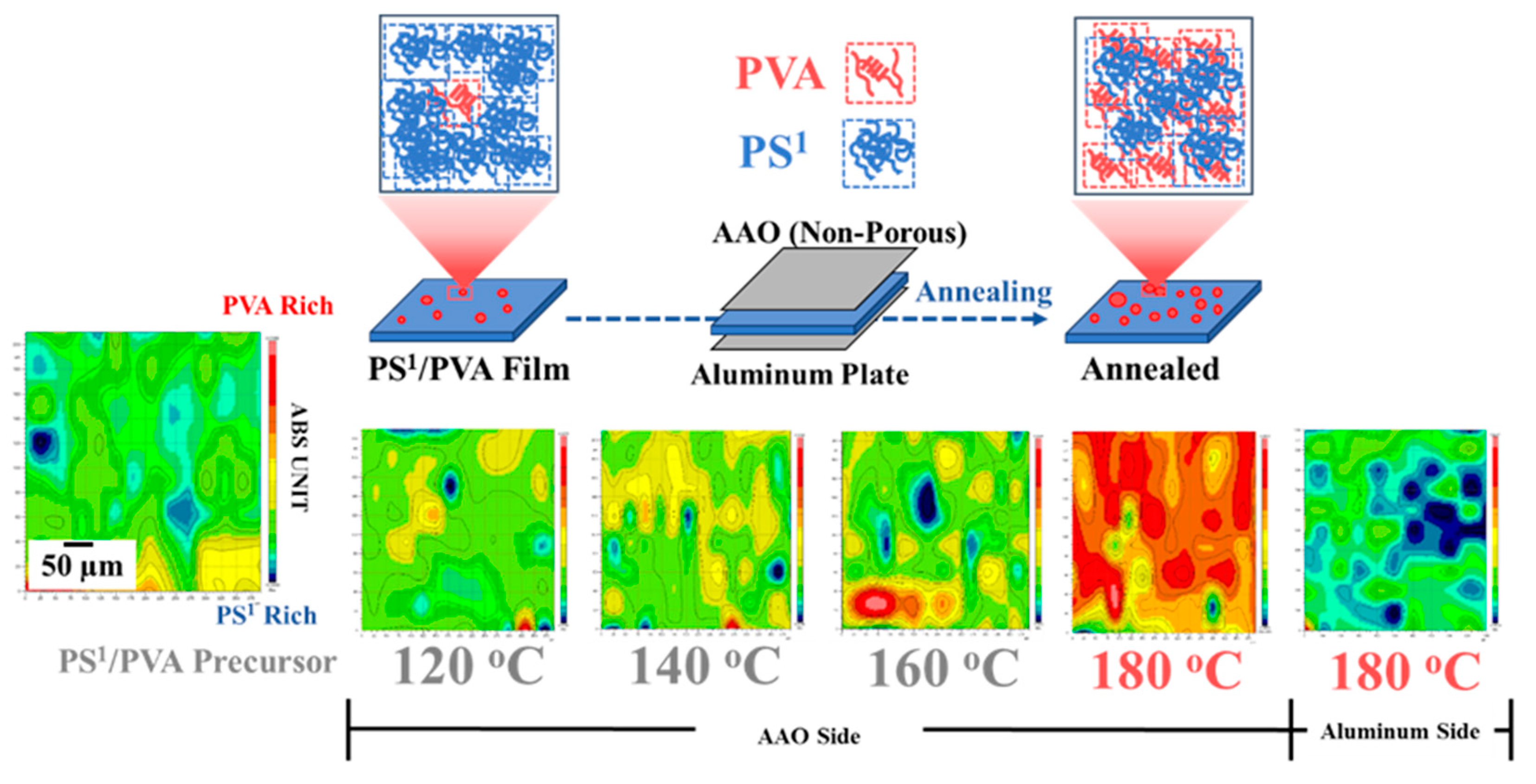
3.3. Phase Separation and Structural Transformation of Polymer Blends at the Nanoscale
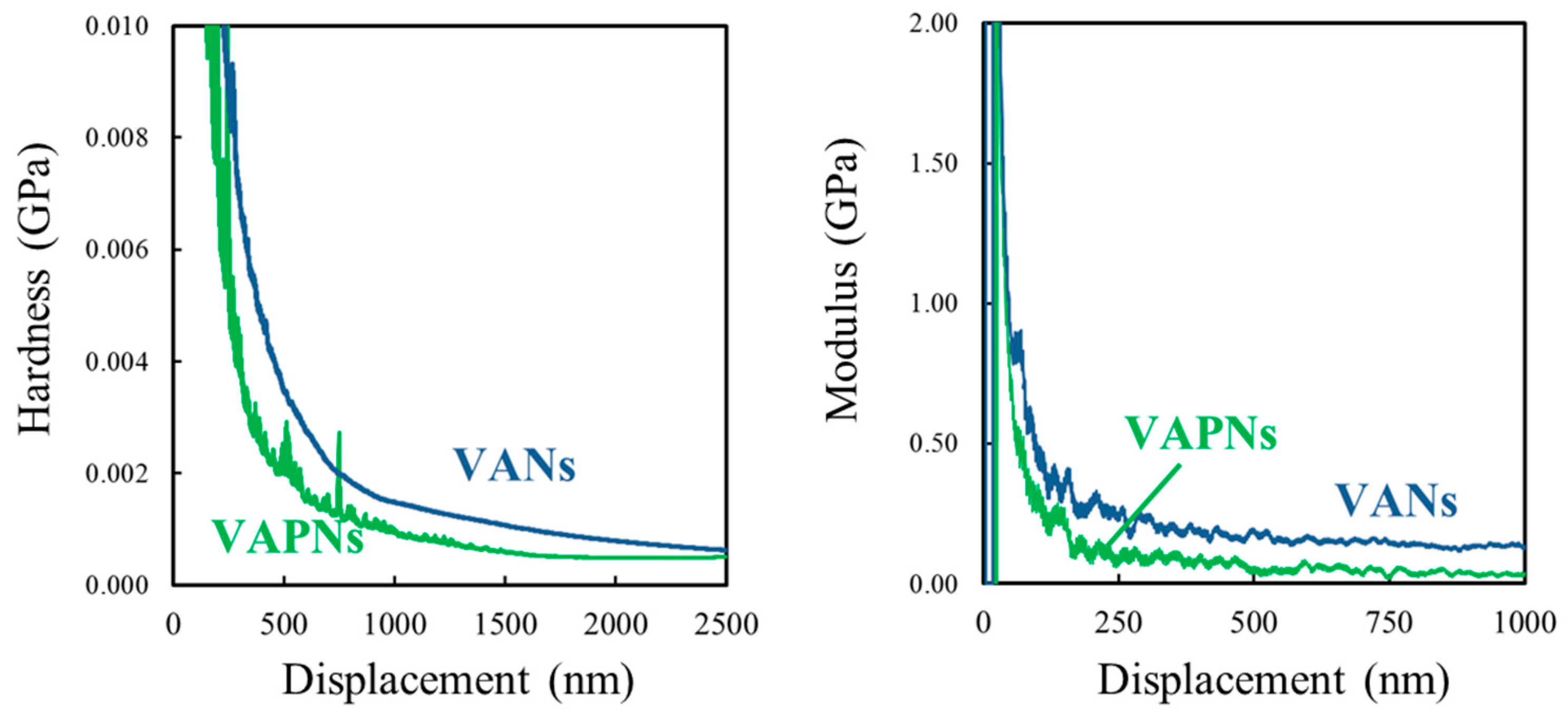
3.4. Surface Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, S.; Xia, S. Rational Design and Nanofabrication of Gecko-Inspired Fibrillar Adhesives. Small 2012, 8, 2464–2468. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Zhang, J. Superhydrophobic Behaviors of Polymeric Surfaces with Aligned Nanofibers. Langmuir 2009, 25, 6916–6922. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Bougher, T.L.; Weathers, A.; Cai, Y.; Bi, K.; Pettes, M.T.; McMenamin, S.A.; Lv, W.; Resler, D.P.; Gattuso, T.R.; et al. High Thermal Conductivity of Chain-Oriented Amorphous Polythiophene. Nat. Nanotechnol. 2014, 9, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Limanto, F.; Lee, D.H.; Fearing, R.S.; Maboudian, R. Role of Counter-substrate Surface Energy in Macroscale Friction of Nanofiber Arrays. Langmuir 2012, 28, 2922–2927. [Google Scholar] [CrossRef] [PubMed]
- Mijangos, C.; Hernández, R.; Martín, J. A Review on the Progress of Polymer Nanostructures with Modulated Morphologies and Properties, Using Nanoporous AAO Templates. Prog. Polym. Sci. 2016, 54–55, 148–182. [Google Scholar] [CrossRef]
- Hernández, J.J.; Monclús, M.A.; Navarro-Baena, I.; Viela, F.; Molina-Aldareguia, J.M.; Rodríguez, I. Multifunctional Nano-engineered Polymer Surfaces with Enhanced Mechanical Resistance and Superhydrophobicity. Sci. Rep. 2017, 7, 43450. [Google Scholar] [CrossRef] [Green Version]
- Dickson, M.N.; Liang, E.I.; Rodriguez, L.A.; Vollereaux, N.; Yee, A.F. Nanopatterned Polymer Surfaces with Bactericidal Properties. Biointerphases 2015, 10, 021010. [Google Scholar] [CrossRef]
- Li, X.; Cheung, G.S.; Watson, G.S.; Watson, J.A.; Lin, S.; Schwarzkopf, L.; Green, D.W. The Nanotipped Hairs of Gecko Skin and Biotemplated Replicas Impair and/or Kill Pathogenic Bacteria with High Efficiency. Nanoscale 2016, 8, 18860–18869. [Google Scholar] [CrossRef]
- Xue, L.; Kovalev, A.; Dening, K.; Eichler-Volf, A.; Eickmeier, H.; Haase, M.; Enke, D.; Steinhart, M.; Gorb, S.N. Reversible Adhesion Switching of Porous Fibrillar Adhesive Pads by Humidity. Nano Lett. 2013, 13, 5541–5548. [Google Scholar] [CrossRef]
- Xue, L.; Kovalev, A.; Eichler-Volf, A.; Steinhart, M.; Gorb, S.N. Humidity-Enhanced Wet Adhesion on Insect-Inspired Fibrillar Adhesive Pads. Nat. Commun. 2015, 6, 6621. [Google Scholar] [CrossRef]
- Huang, L.-B.; Zhou, Y.; Han, S.-T.; Yan, Y.; Zhou, L.; Roy, V.A.L. The Role of Nanoparticle Monolayer on the Flowing Behaviour of Polymer Melts in Nanochannels. Nanoscale 2014, 6, 11013–11018. [Google Scholar] [CrossRef] [PubMed]
- Yung, K.-L.; Kong, J.; Xu, Y. Studies on Flow Behaviors of Polymer Melts in Nanochannels by Wetting Actions. Polymer 2007, 48, 7645–7652. [Google Scholar] [CrossRef]
- Ali, S.; Tian, W.; Ali, N.; Shi, L.; Kong, J.; Ali, N. Polymer Melt Flow through Nanochannels: From Theory and Fabrication to Application. RSC Adv. 2015, 5, 7160–7172. [Google Scholar] [CrossRef]
- Mei, S.; Feng, X.; Jin, Z. Polymer Nanofibers by Controllable Infiltration of Vapour Swollen Polymers into Cylindrical Nanopores. Soft Matter 2013, 9, 945–951. [Google Scholar] [CrossRef]
- Muanchan, P.; Suzuki, S.; Kyotani, T.; Ito, H. One-dimensional Polymer Nanofiber Arrays with High Aspect Ratio Obtained by Thermal Nanoimprint Method. Polym. Eng. Sci. 2017, 57, 214–223. [Google Scholar] [CrossRef]
- Wu, H.; Su, Z.; Takahara, A. Characterization of An Isotactic Polystyrene/Poly(2,6 dimethylphenylene oxide) Nanorod Blend with Gradient Composition and Crystallinity. RSC Adv. 2012, 2, 8707–8712. [Google Scholar] [CrossRef]
- Wu, H.; Su, Z.; Takahara, A. Gradient Composition Distribution in Poly(2,6-dimethylphenylene oxide)/Polystyrene Blend Nanorods. Soft Matter 2011, 7, 1868–1873. [Google Scholar] [CrossRef]
- Wu, H.; Su, Z.; Takahara, A. Molecular Composition Distribution of Polycarbonate/Polystyrene Blends in Cylindrical Nanopores. Polym. J. 2011, 43, 600–605. [Google Scholar] [CrossRef]
- Wei, T.-H.; Chi, M.-H.; Tsai, C.-C.; Ko, H.-W.; Chen, J.-T. Porous Polymer Nanostructures Fabricated by the Surface-Induced Phase Separation of Polymer Solutions in Anodic Aluminum Oxide Templates. Langmuir 2013, 29, 9972–9978. [Google Scholar] [CrossRef]
- Chi, M.-H.; Chang, C.-W.; Ko, H.-W.; Su, C.-H.; Lee, C.-W.; Peng, C.-H.; Chen, J.-T. Solvent-Induced Dewetting on Curved Substrates: Fabrication of Porous Polymer Nanotubes by Anodic Aluminum Oxide Templates. Macromolecule 2015, 48, 6241–6250. [Google Scholar] [CrossRef]
- Chen, D.; Chen, J.-T.; Glogowski, E.; Emrick, T.; Russell, T.P. Thin Film Instabilities in Blends under Cylindrical Confinement. Macromol. Rapid Commun. 2009, 30, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-W.; Wei, T.-H.; Chang, C.-W.; Chen, J.-T. Effect of Nonsolvent on the Formation of Polymer Nanomaterials in the Nanopores of Anodic Aluminum Oxide Templates. Macromol. Rapid Commun. 2012, 33, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-T.; Lee, C.-W.; Chi, M.-H.; Yao, I.-C. Solvent-Annealing-Induced Nanowetting in Templates: Towards Tailored Polymer Nanostructures. Macromol. Rapid Commun. 2013, 34, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-C.; Fan, P.-W.; Lee, C.-W.; Chu, C.-W.; Tsai, C.-C.; Chen, J.-T. Transformation of Polymer Nanofibers to Nanospheres Driven by the Rayleigh Instability. ACS Appl. Mater. Interfaces 2013, 5, 3134–3142. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-T.; Wei, T.-H.; Chang, C.-W.; Ko, H.-W.; Chu, C.-W.; Chi, M.-H.; Tsai, C.-C. Fabrication of Polymer Nanopeapods in the Nanopores of Anodic Aluminum Oxide Templates Using a Double-Solution Wetting Method. Macromolecules 2014, 47, 5227–5235. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Chen, J.-T. Rayleigh Instability in Polymer Thin Films Coated in the Nanopores of Anodic Aluminum Oxide Templates. Langmuir 2013, 30, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Jin, Z. Spontaneous Formation of Nanoscale Polymer Spheres, Capsules, or Rods by Evaporation of Polymer Solutions in Cylindrical Alumina Nanopores. Macromolecules 2009, 42, 569–572. [Google Scholar] [CrossRef]
- Ko, H.-W.; Chi, M.-H.; Chang, C.-W.; Su, C.-H.; Wei, T.-H.; Tsai, C.-C.; Peng, C.-H.; Chen, J.-T. Fabrication of Multicomponent Polymer Nanostructures Containing PMMA Shells and Encapsulated PS Nanospheres in the Nanopores of Anodic Aluminum Oxide Templates. Macromol. Rapid Commun. 2015, 36, 439–446. [Google Scholar] [CrossRef]
- Feng, X.; Mei, S.; Jin, Z. Wettability Transition Induced Transformation and Entrapment of Polymer Nanostructures in Cylindrical Nanopores. Langmuir 2011, 27, 14240–14247. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Chen, J.-T. Effect of the Polymer Concentration on the Rayleigh-Instability-Type Transformation in Polymer Thin Films Coated in the Nanopores of Anodic Aluminum Oxide Templates. Langmuir 2015, 31, 2569–2575. [Google Scholar] [CrossRef]
- Chen, J.-T.; Zhang, M.; Russell, T.P. Instabilities in Nanoporous Media. Nano Lett. 2007, 7, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Schlitt, S.; Greiner, A.; Wendorff, J.H. Cylindrical Polymer Nanostructures by Solution Template Wetting. Macromolecules 2008, 41, 3228–3234. [Google Scholar] [CrossRef]
- Chu, C.-J.; Cheng, M.-H.; Chung, P.-Y.; Chi, M.-H.; Jeng, K.-S.; Chen, J.-T. Reversible Morphology Control of Three-Dimensional Block Copolymer Nanostructures by the Solvent Annealing-Induced Wetting in Anodic Aluminum Oxide Templates. Int. J. Polym. Mater. 2016, 65, 695–701. [Google Scholar] [CrossRef]
- Zhang, Z.; Ahn, D.U.; Ding, Y. Instabilities of PS/PMMA Bilayer Patterns with a Corrugated Surface and Interface. Macromolecules 2012, 45, 1972–1981. [Google Scholar] [CrossRef]
- Fan, P.-W.; Chen, W.-L.; Lee, T.-H.; Chen, J.-T. Annealing Effect on Electrospun Polymer Fibers and Their Transformation into Polymer Microspheres. Macromol. Rapid Commun. 2012, 33, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.-F.; Cheng, M.-H.; Jeng, K.-S.; Li, J.-W.; Chen, J.-T. Asymmetric Polymer Particles with Anisotropic Curvatures by Annealing Polystyrene Microspheres on Poly(vinyl alcohol) Films. Macromol. Rapid Commun. 2016, 37, 1825–1831. [Google Scholar] [CrossRef] [PubMed]
- Mead-Hunter, R.; King, A.J.C.; Mullins, B.J. Plateau Rayleigh Instability Simulation. Langmuir 2012, 28, 6731–6735. [Google Scholar] [CrossRef]
- Fan, P.-W.; Chen, W.-L.; Lee, T.-H.; Chiu, Y.-J.; Chen, J.-T. Rayleigh-Instability-Driven Morphology Transformation by Thermally Annealing Electrospun Polymer Fibers on Substrates. Macromolecules 2012, 45, 5816–5822. [Google Scholar] [CrossRef]
- Wang, H.; Chang, T.; Li, X.; Zhang, W.; Hu, Z.; Jonas, A.M. Scaled Down Glass Transition Temperature in Confined Polymer Nanofibers. Nanoscale 2016, 8, 14950–14955. [Google Scholar] [CrossRef]
- Tan, A.W.; Torkelson, J.M. Poly(methyl methacrylate) Nanotubes in AAO Templates: Designing Nanotube Thickness and Characterizing the Tg-Confinement Effect by DSC. Polymer 2016, 82, 327–336. [Google Scholar] [CrossRef]
- Li, L.; Zhou, D.; Huang, D.; Xue, G. Double Glass Transition Temperatures of Poly(methyl methacrylate) Confined in Alumina Nanotube Templates. Macromolecules 2014, 47, 297–303. [Google Scholar] [CrossRef]
- Higuchi, T.; Tajima, A.; Yabu, H.; Shimomura, M. Spontaneous Formation of Polymer Nanoparticles with Inner Micro-Phase Separation Structures. Soft Matter 2008, 4, 1302–1305. [Google Scholar] [CrossRef]
- Hou, P.; Fan, H.; Jin, Z. Spiral and Mesoporous Block Polymer Nanofibers Generated in Confined Nanochannels. Macromolecules 2015, 48, 272–278. [Google Scholar] [CrossRef]
- Chu, C.-J.; Chung, P.-Y.; Chi, M.-H.; Kao, Y.-H.; Chen, J.-T. Three-Dimensional Block Copolymer Nanostructures by the Solvent-Annealing-Induced Wetting in Anodic Aluminum Oxide Templates. Macromol. Rapid Commun. 2014, 35, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Xu, Y.; Jin, Z. Interfacial Interaction in Anodic Aluminum Oxide Templates Modifies Morphology, Surface Area, and Crystallization of Polyamide-6 Nanofibers. Langmuir 2016, 32, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.-W.; Cheng, M.-H.; Chi, M.-H.; Chang, C.-W.; Chen, J.-T. Selective Template Wetting Routes to Hierarchical Polymer Films: Polymer Nanotubes from Phase-Separated Films via Solvent Annealing. Langmuir 2016, 32, 2110–2116. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Sheng, Y.; Liu, H.; Zhu, Y.; Jiang, W. Templated Self-Assembly of Block Copolymers and Morphology Transformation Driven by the Rayleigh Instability. Langmuir 2015, 31, 1660–1669. [Google Scholar] [CrossRef]
- Giussi, J.M.; Blaszczyk-Lezak, I.; Cortizo, M.S.; Mijangos, C. In-situ Polymerization of Styrene in AAO Nanocavities. Polymer 2013, 54, 6886–6893. [Google Scholar] [CrossRef]
- Sanz, B.; Blaszczyk-Lezak, I.; Mijangos, C.; Palacios, J.K.; Müller, A.J. New Double-Infiltration Methodology to Prepare PCL-PS Core-Shell Nanocylinders inside Anodic Aluminum Oxide Templates. Langmuir 2016, 32, 7860–7865. [Google Scholar] [CrossRef]
- Liu, Z. One-step Fabrication of Crystalline Metal Nanostructures by Direct Nanoimprinting below Melting Temperatures. Nat. Commun. 2017, 8, 14910. [Google Scholar] [CrossRef]
- Jin, K.; Torkelson, J.M. Tg-confinement Effects in Strongly Miscible Blends of Poly(2,6-Dimethyl-1,4-Phenylene oxide) and Polystyrene: Roles of Bulk Fragility and Chain Segregation. Polymer 2017, 11, 85–96. [Google Scholar] [CrossRef]
- Napolitano, S.; Glynos, E.; Tito, N. Glass Transition of Polymers in Bulk, Confined Geometries, and Near Interfaces. Rep. Prog. Phys. 2017, 80, 036602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Liu, Y.; Yu, J.; Lv, T.; Li, L. Fabrication of Hierarchical Gecko-Inspired Microarrays Using a Three-dimensional Porous Nickel Oxide Template. J. Mater. Chem. B 2015, 3, 6571–6575. [Google Scholar] [CrossRef]
- Zhang, E.; Wang, Y.; Lv, T.; Li, L.; Cheng, Z.; Liu, Y. Bio-inspired design of hierarchical PDMS microstructures with tunable adhesive superhydrophobicity. Nanoscale 2015, 7, 6151–6158. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Lei, B.; Liu, H.; Niu, D.; Zhao, T.; Chen, B.; Yin, L.; Shi, Y.; Liu, X. Fabrication of Directional Nanopillars with High-Aspect-Ratio by Stretching Imprint with Microcavity Mold. Nanoscale 2017, 9, 2172–2177. [Google Scholar] [CrossRef] [PubMed]
- Spear, J.C.; Custer, J.P.; Batteas, J.D. The Influence of Nanoscale Roughness and Substrate Chemistry on the Frictional Properties of Single and Few Layer Graphene. Nanoscale 2015, 7, 10021–10029. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, A.; Licoccia, S.; Traversac, E. Nanomechanics for MEMS: A Structural Design Perspective. Nanoscale 2011, 3, 811–824. [Google Scholar] [CrossRef]










| Anodization | AAO1 | AAO2 | ||
|---|---|---|---|---|
| 1st | 2nd | 1st | 2nd | |
| Electrical Current (A) | 12.1 | 12.1 | 12.1 | 12.1 |
| Applied Voltage (V) | 40 | 40 | 60 | 60 |
| Oxalic Acid (M) | 0.2 | 0.2 | 0.3 | 0.3 |
| Time (h) | 17 | 24 | 17 | 48 |
| Temperature (°C) | 10 | 10 | 5 | 5 |
| Materials | Temperature (°C) | Pressure (MPa) | Press Time (min) | Obtained Nanostructures |
|---|---|---|---|---|
| PS1 | 160−220 | 5 | 30 | Nanopillars |
| PS2 | 160−220 | 5 | 30 | Nanopillars |
| PS1/PVA (90/10 wt%) | 120−160 | 5 | 30 | Nanopillars |
| PS1/PVA (90/10 wt%) | 180 | 5 | 15–30 60–120 | Porous Nanopillars Nanofragments |
| PS1/PVA (70/30 wt%) | 120−180 | 5 | 15−120 | Nanopeapods |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muanchan, P.; Kurose, T.; Ito, H. Replication of Mesoscale Pore One-dimensional Nanostructures: Surface-induced Phase Separation of Polystyrene/Poly(vinyl alcohol) (PS/PVA) Blends. Polymers 2019, 11, 1039. https://doi.org/10.3390/polym11061039
Muanchan P, Kurose T, Ito H. Replication of Mesoscale Pore One-dimensional Nanostructures: Surface-induced Phase Separation of Polystyrene/Poly(vinyl alcohol) (PS/PVA) Blends. Polymers. 2019; 11(6):1039. https://doi.org/10.3390/polym11061039
Chicago/Turabian StyleMuanchan, Paritat, Takashi Kurose, and Hiroshi Ito. 2019. "Replication of Mesoscale Pore One-dimensional Nanostructures: Surface-induced Phase Separation of Polystyrene/Poly(vinyl alcohol) (PS/PVA) Blends" Polymers 11, no. 6: 1039. https://doi.org/10.3390/polym11061039







