Bioapplications of Bacterial Cellulose Polymers Conjugated with Resveratrol for Epithelial Defect Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. BC Fabrication
2.2. Preparation of BC Scaffolds Containing RSV
2.3. Preparation of a Type I COL Scaffold Containing RSV
2.4. Scanning Electron Microscopy (SEM)
2.5. Fourier-Transform Infrared (FT-IR) Spectroscopy
2.6. Efficiency of RSV Release from the Scaffold
2.7. In Vitro Biocompatibility
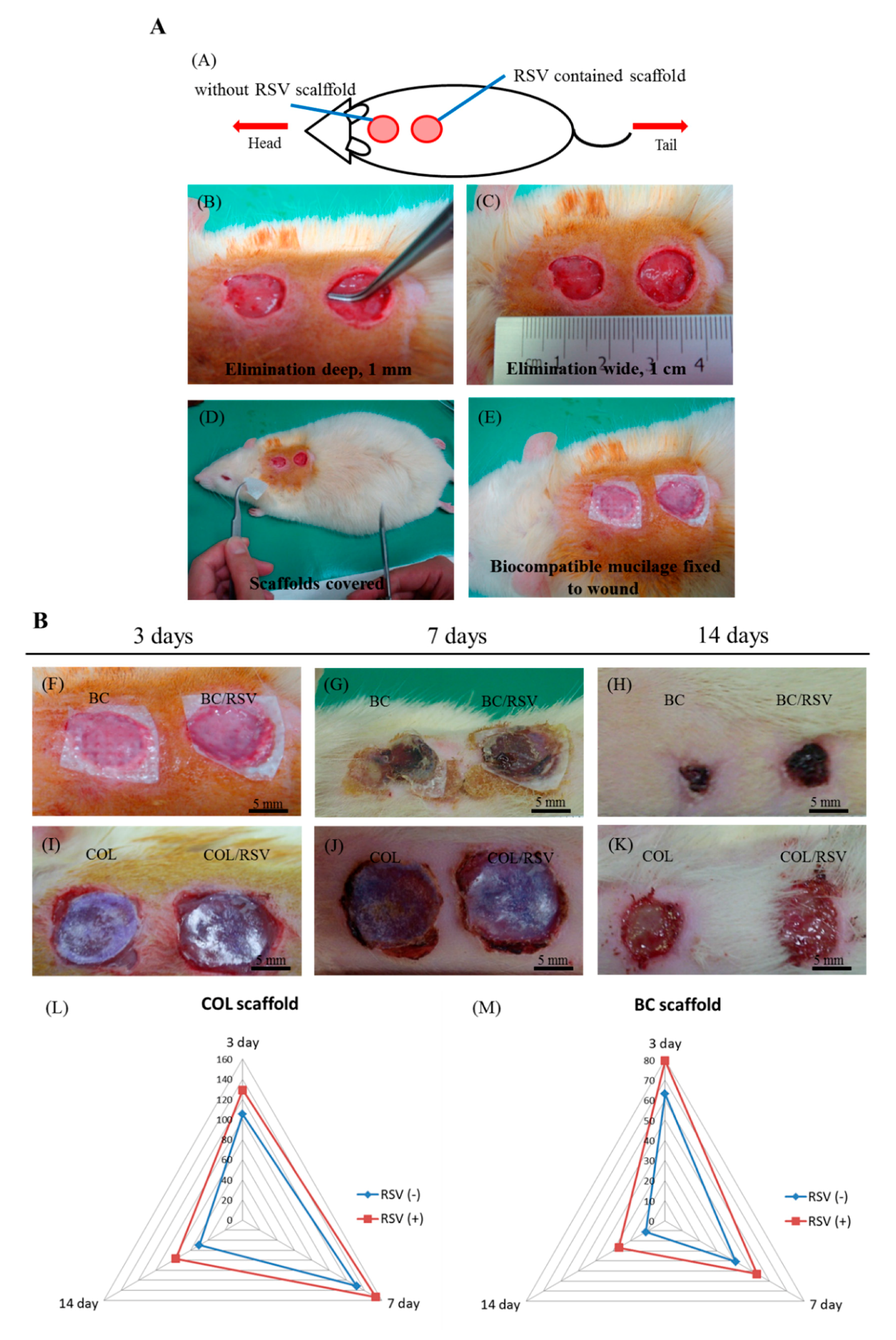
2.8. Animal Model of a Surgical Epidermal Defect
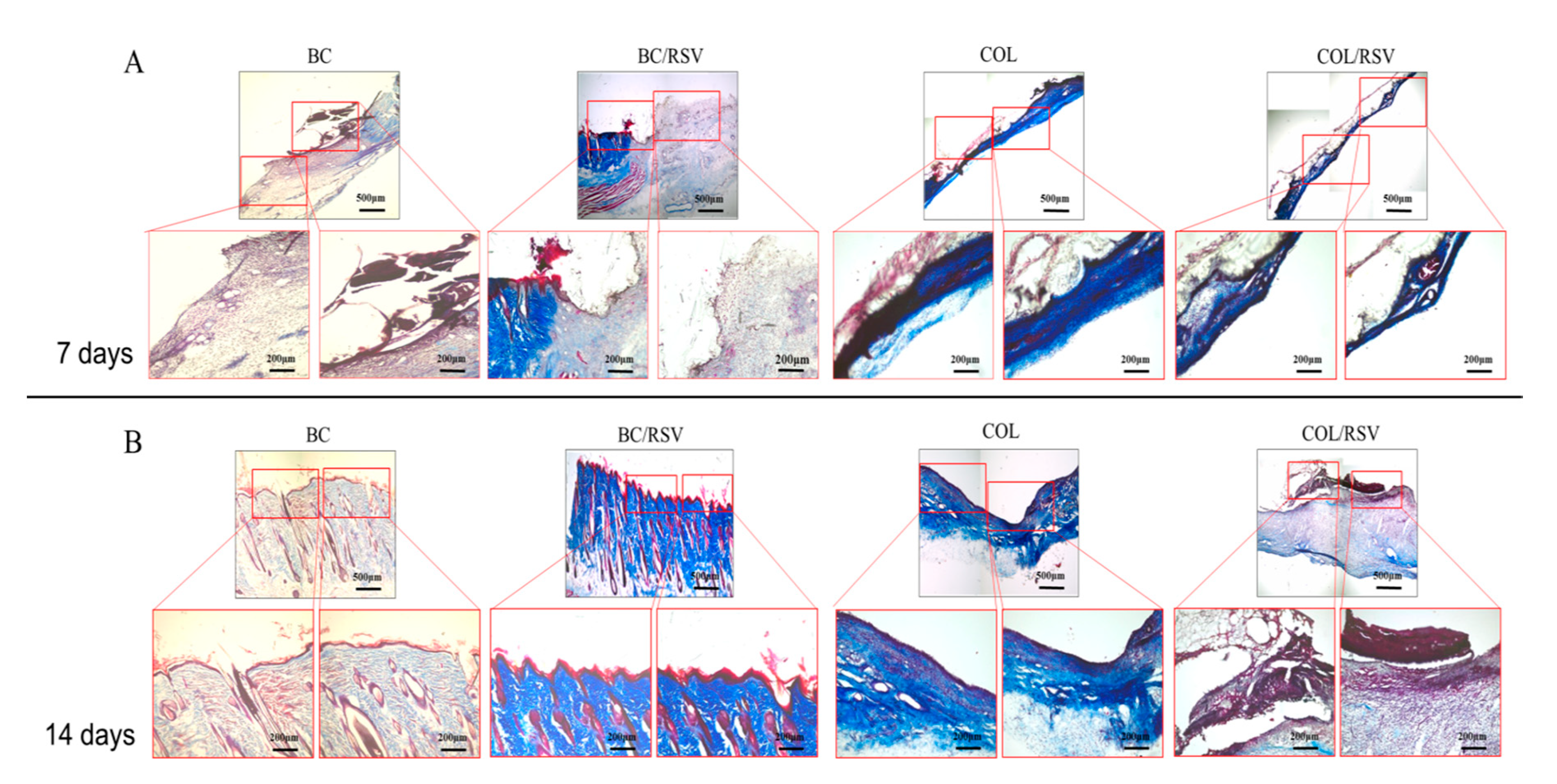
2.9. Histologic Examination
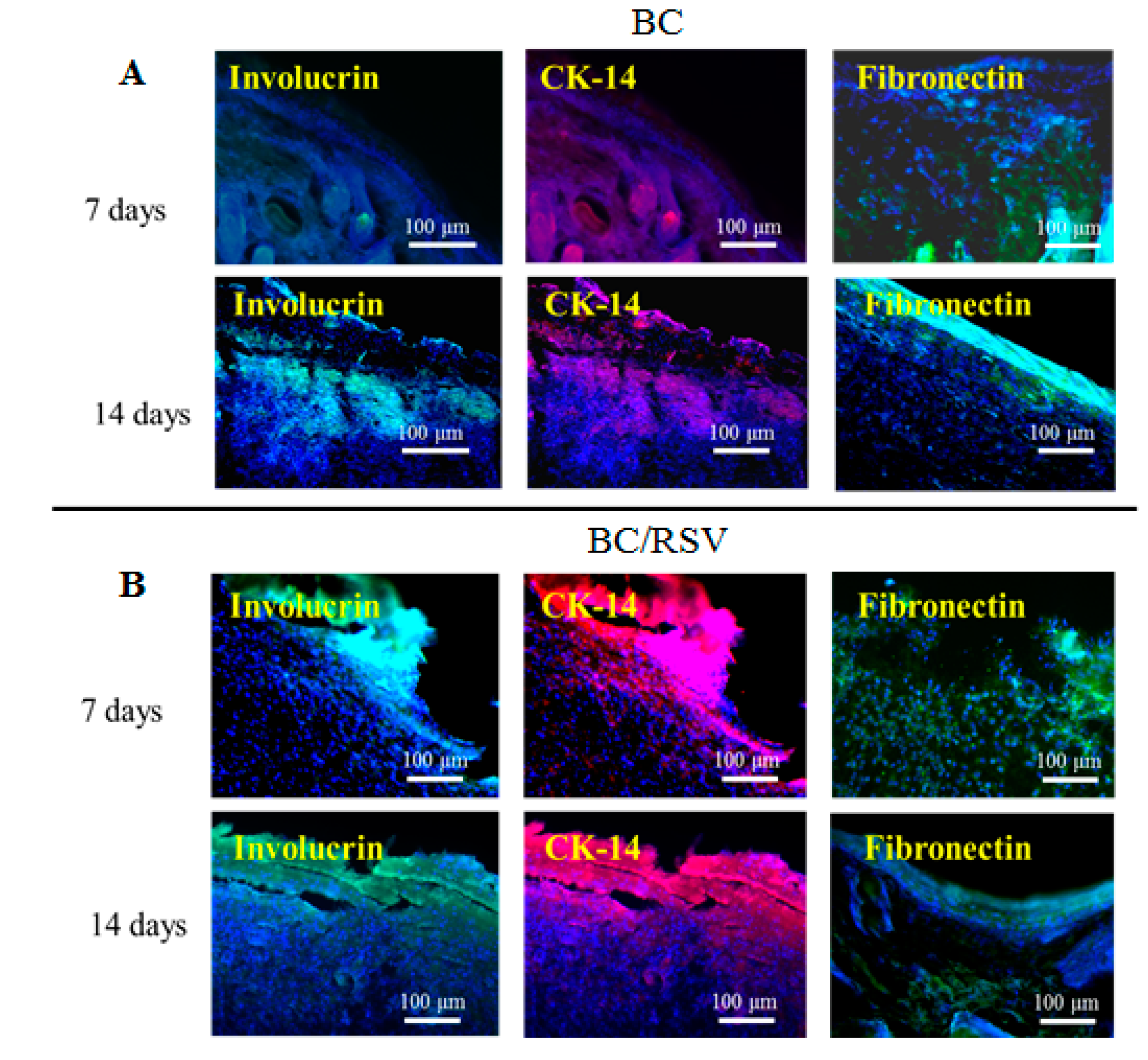
2.10. Immunofluorescence
2.11. Wound-Area Measurements and Statistical Analysis
3. Results and Discussion
3.1. FT-IR
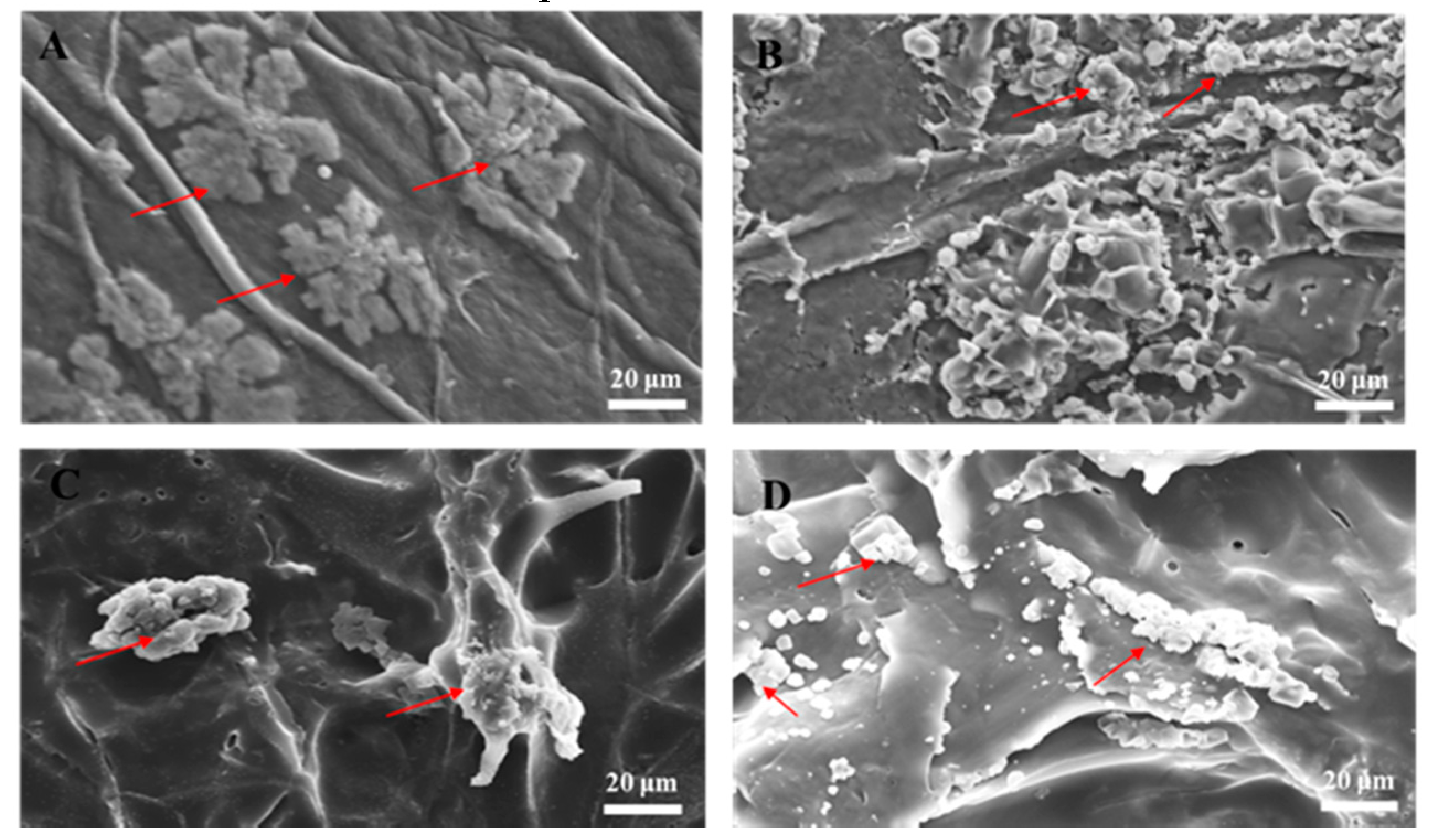
3.2. SEM
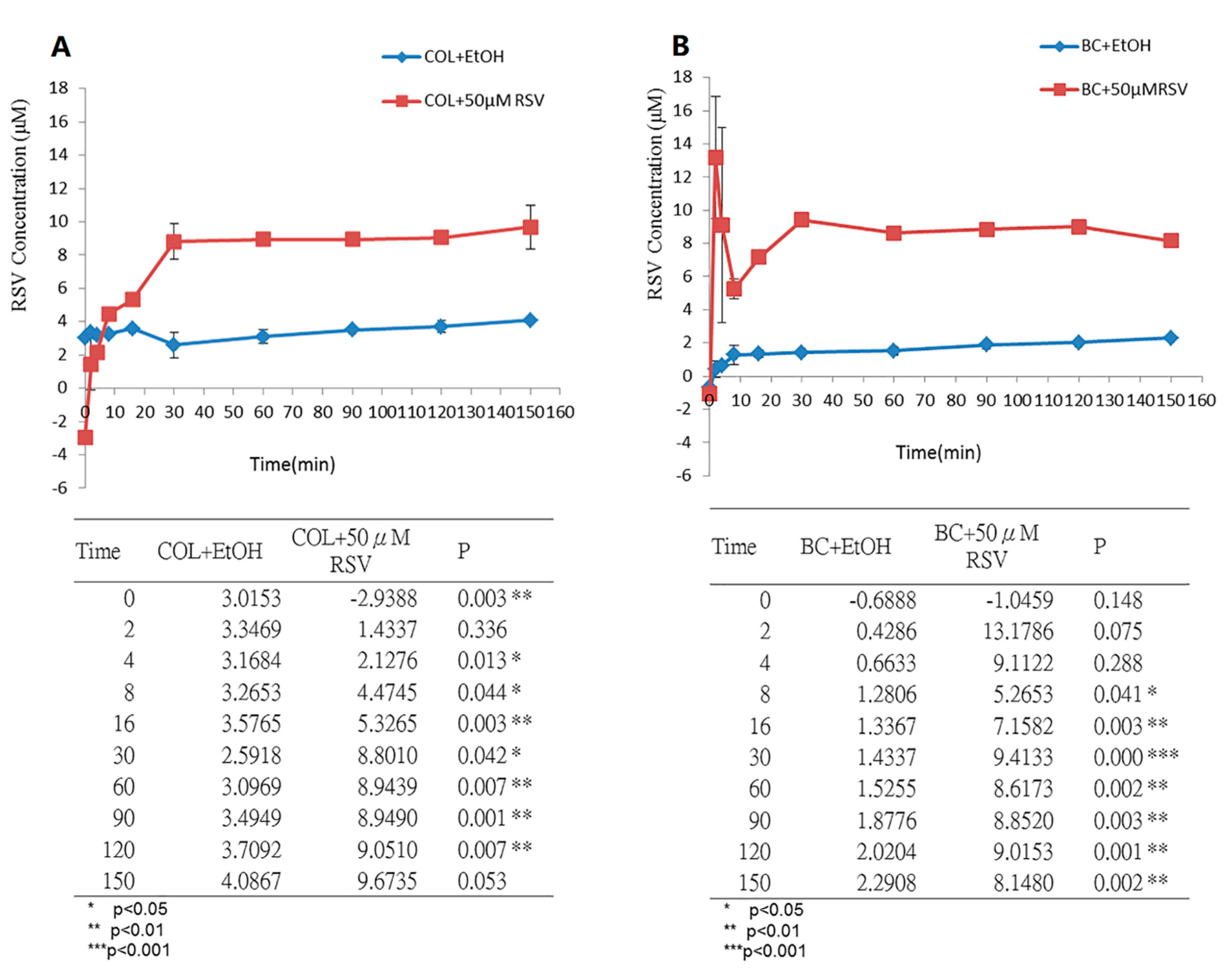
3.3. Measurement of RSV Release over Time
3.4. Functional Biocompatibility In Vitro
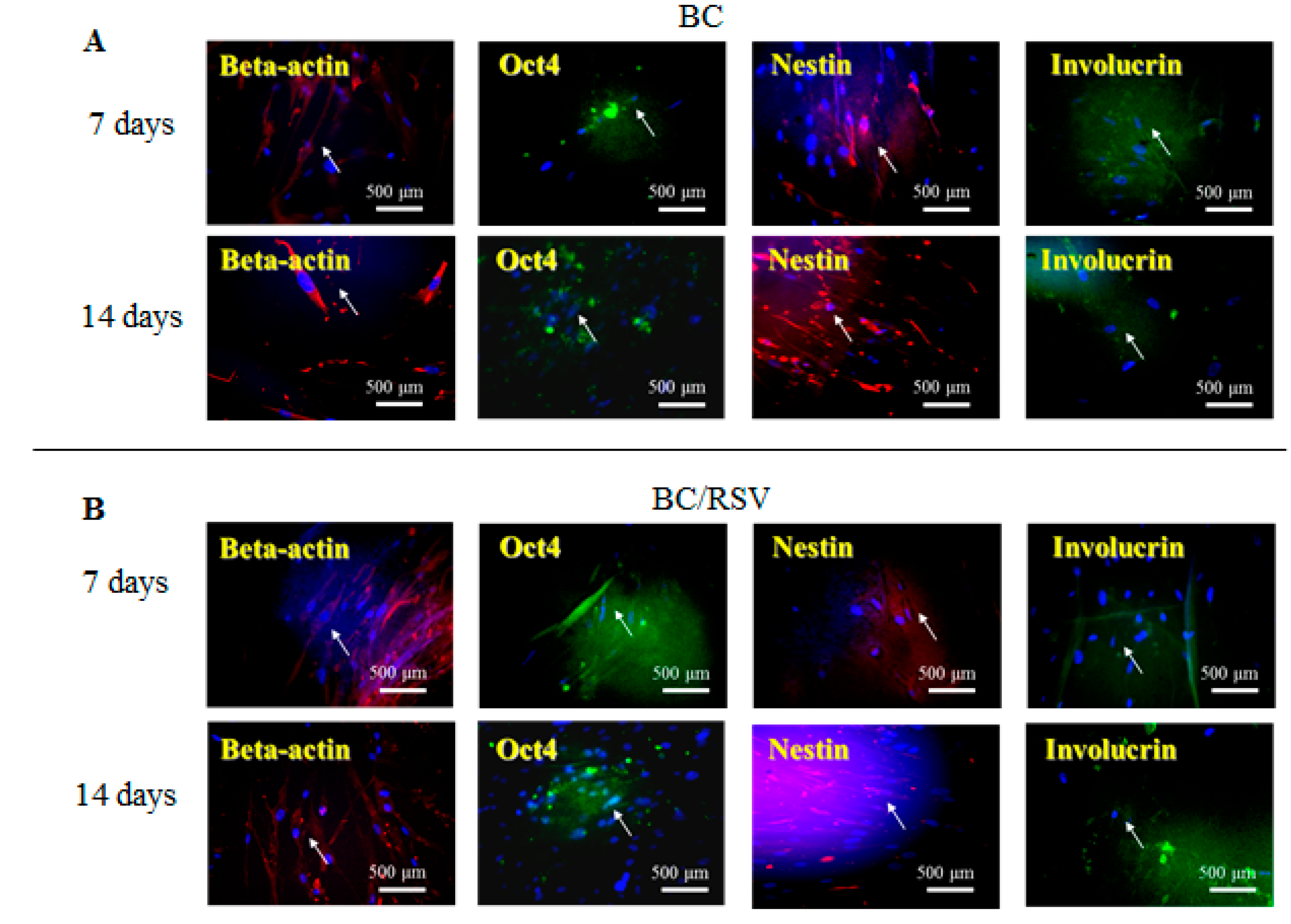
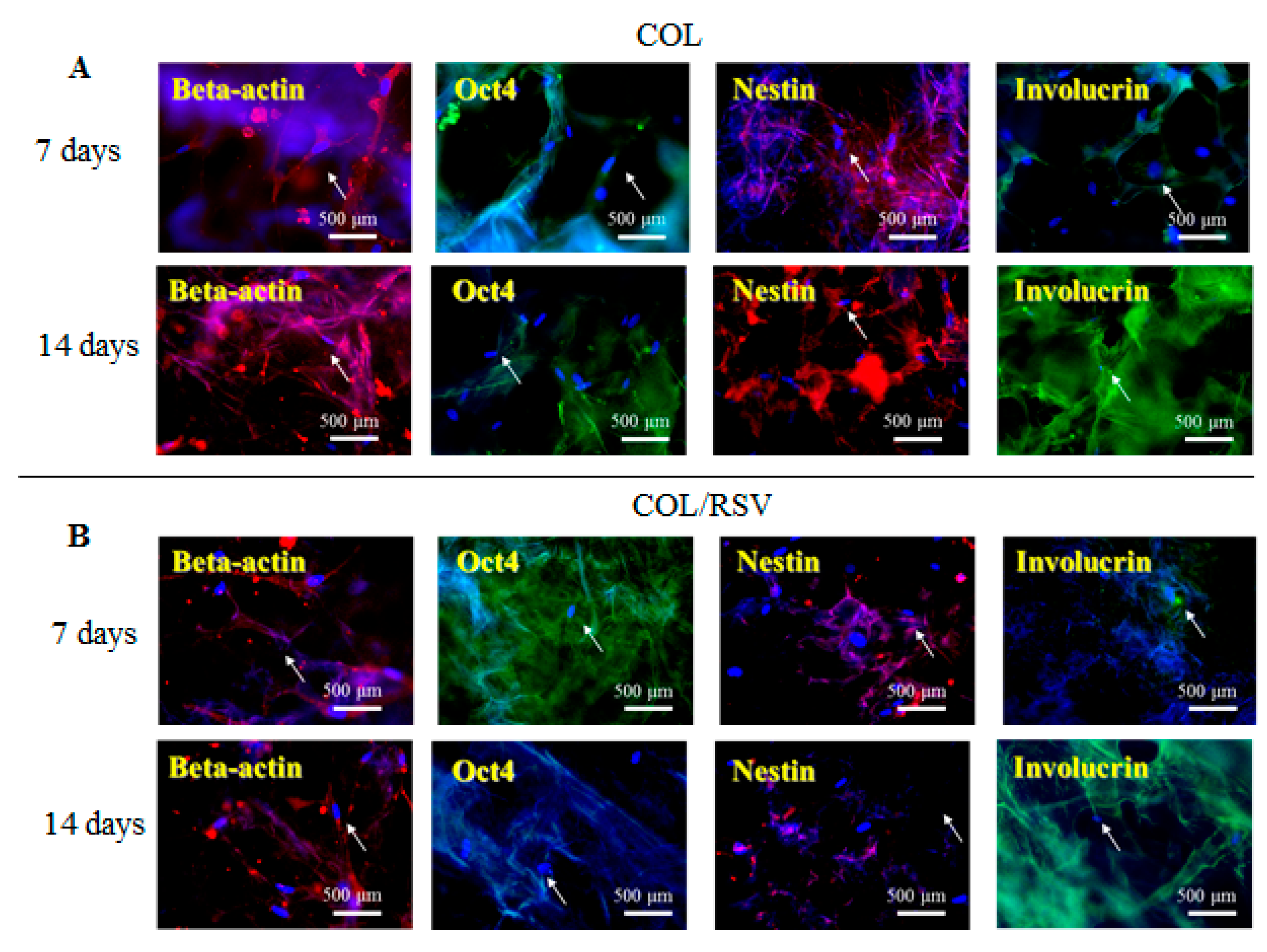
3.5. Immunostaining
3.6. Skin Reconstruction in An Animal Epidermal-Defect Model
3.7. Re-Epithelialization of the Epidermal Debride Area in Skin Reconstitution
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kucińska-Lipka, J.; Gubanska, I.; Janik, H. Bacterial cellulose in the field of wound healing and regenerative medicine of skin: Recent trends and future prospectives. Polym. Bull. 2015, 72, 2399–2419. [Google Scholar] [CrossRef]
- Brown, R.M., Jr.; Willison, J.H.; Richardson, C.L. Cellulose biosynthesis in Acetobacter xylinum: Visualization of the site of synthesis and direct measurement of the in vivo process. Proc. Natl. Acad. Sci. USA 1976, 73, 4565–4569. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Deng, F.; Yeomans, W.G.; Allen, A.L.; Gross, R.A.; Kaplan, D.L. Direct incorporation of glucosamine and n-acetylglucosamine into exopolymers by Gluconacetobacter xylinus (= Acetobacter xylinum) ATCC 10245: Production of chitosan-cellulose and chitin-cellulose exopolymers. Appl. Environ. Microbiol. 2001, 67, 3970–3975. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.; Mayer, R.; Benziman, M. Cellulose biosynthesis and function in bacteria. Microbiol. Rev. 1991, 55, 35–58. [Google Scholar] [PubMed]
- Miyamoto, T.; Takahashi, S.; Ito, H.; Inagaki, H.; Noishiki, Y. Tissue biocompatibility of cellulose and its derivatives. J. Biomed. Mater. Res. 1989, 23, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Svensson, A.; Nicklasson, E.; Harrah, T.; Panilaitis, B.; Kaplan, D.L.; Brittberg, M.; Gatenholm, P. Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 2005, 26, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Bodin, A.; Concaro, S.; Brittberg, M.; Gatenholm, P. Bacterial cellulose as a potential meniscus implant. J. Tissue Eng. Regen. Med. 2007, 1, 406–408. [Google Scholar] [CrossRef] [PubMed]
- Fontana, J.D.; de Souza, A.M.; Fontana, C.K.; Torriani, I.L.; Moreschi, J.C.; Gallotti, B.J.; de Souza, S.J.; Narcisco, G.P.; Bichara, J.A.; Farah, L.F. Acetobacter cellulose pellicle as a temporary skin substitute. Appl. Biochem. Biotechnol. 1990, 24–25, 253–264. [Google Scholar] [CrossRef]
- Novaes, A.B., Jr.; Novaes, A.B. Immediate implants placed into infected sites: A clinical report. Int. J. Oral Maxillofac. Implants 1995, 10, 609–613. [Google Scholar] [PubMed]
- Novaes, A.B., Jr.; Novaes, A.B. Soft tissue management for primary closure in guided bone regeneration: Surgical technique and case report. Int. J. Oral Maxillofac. Implants 1997, 12, 84–87. [Google Scholar]
- Kowalska-Ludwicka, K.; Cala, J.; Grobelski, B.; Sygut, D.; Jesionek-Kupnicka, D.; Kolodziejczyk, M.; Bielecki, S.; Pasieka, Z. Modified bacterial cellulose tubes for regeneration of damaged peripheral nerves. Arch. Med. Sci. 2013, 9, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Moniri, M.; Boroumand Moghaddam, A.; Azizi, S.; Abdul Rahim, R.; Bin Ariff, A.; Zuhainis Saad, W.; Navaderi, M.; Mohamad, R. Production and status of bacterial cellulose in biomedical engineering. Nanomaterials 2017, 7, 257. [Google Scholar] [CrossRef]
- Fu, L.; Zhang, J.; Yang, G. Present status and applications of bacterial cellulose-based materials for skin tissue repair. Carbohydr. Polym. 2013, 92, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Fürsatz, M.; Skog, M.; Sivlér, P.; Palm, E.; Aronsson, C.; Skallberg, A.; Greczynski, G.; Khalaf, H.; Bengtsson, T.; Aili, D. Functionalization of bacterial cellulose wound dressings with the antimicrobial peptide ε-poly-l-lysine. Biomed. Mater. 2018, 13, 025014. [Google Scholar] [CrossRef] [PubMed]
- Sahana, T.G.; Rehka, P.D. Biopolymers: Applications in wound healing and skin tissue engineering. Mol. Biol. Rep. 2018, 45, 2857–2867. [Google Scholar] [CrossRef] [PubMed]
- Chevallay, B.; Herbage, D. Collagen-based biomaterials as 3d scaffold for cell cultures: Applications for tissue engineering and gene therapy. Med. Biol. Eng. Comput. 2000, 38, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Plucknett, D.L.; Smith, N.J. Agricultural research and third world food production. Science 1982, 217, 215–220. [Google Scholar] [CrossRef]
- Burke, J.F.; Yannas, I.V.; Quinby, W.C., Jr.; Bondoc, C.C.; Jung, W.K. Successful use of a physiologically acceptable artificial skin in the treatment of extensive burn injury. Ann. Surg. 1981, 194, 413–428. [Google Scholar] [CrossRef]
- Koopmans, G.; Hasse, B.; Sinis, N. Chapter 19: The role of collagen in peripheral nerve repair. Int. Rev. Neurobiol. 2009, 87, 363–379. [Google Scholar]
- Dutt, J.E.; Ledoux, D.; Baer, H.; Foster, C.S. Collagen abnormalities in conjunctiva of patients with cicatricial pemphigoid. Cornea 1996, 15, 606–611. [Google Scholar] [CrossRef]
- Song, J.; Zhang, P.; Cheng, L.; Liao, Y.; Xu, B.; Bao, R.; Wang, W.; Liu, W. Nano-silver in situ hybridized collagen scaffolds for regeneration of infected full-thickness burn skin. J. Mater. Chem. B 2015, 3, 4231–4241. [Google Scholar] [CrossRef]
- Stiefel, D.; Schiestl, C.; Meuli, M. Integra artificial skin for burn scar revision in adolescents and children. Burns 2010, 36, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Rieu, C.; Picaut, L.; Mosser, G.; Trichet, L. From tendon injury to collagen-based tendon regeneration: Overview and recent advances. Curr. Pharm. Des. 2017, 23, 3483–3506. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Li, W.; Lv, X.; Lei, Z.; Bian, Y.; Deng, H.; Wang, H.; Li, J.; Li, X. Biomimetic lbl structured nanofibrous matrices assembled by chitosan/collagen for promoting wound healing. Biomaterials 2015, 53, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in wound repair: Molecular and cellular mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Bielefeld, K.A.; Amini-Nik, S.; Whetstone, H.; Poon, R.; Youn, A.; Wang, J.; Alman, B.A. Fibronectin and beta-catenin act in a regulatory loop in dermal fibroblasts to modulate cutaneous healing. J. Biol. Chem. 2011, 286, 27687–27697. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.W.; Fourcaudot, A.B.; Yamane, K.; You, T.; Chan, R.K.; Leung, K.P. Exacerbated and prolonged inflammation impairs wound healing and increases scarring. Wound Repair Regen. 2016, 24, 26–34. [Google Scholar] [CrossRef]
- Cottart, C.H.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Beaudeux, J.L. Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res. 2010, 54, 7–16. [Google Scholar] [CrossRef]
- Zhao, P.; Sui, B.D.; Liu, N.; Lv, Y.J.; Zheng, C.X.; Lu, Y.B.; Huang, W.T.; Zhou, C.H.; Chen, J.; Pang, D.L.; et al. Anti-aging pharmacology in cutaneous wound healing: Effects of metformin, resveratrol, and rapamycin by local application. Aging Cell 2017, 16, 1083–1093. [Google Scholar] [CrossRef]
- Surma-Ślusarska, B.; Presler, S.; Danielewicz, D. Characteristics of bacterial cellulose obtained from Acetobacter xylinum culture for application in papermaking. Fibres Text. East Eur. 2008, 16, 108–111. [Google Scholar]
- Jung, H.I.; Lee, O.M.; Jeong, J.H.; Jeon, Y.D.; Park, K.H.; Kim, H.S.; An, W.G.; Son, H.J. Production and characterization of cellulose by Acetobacter sp. V6 using a cost-effective molasses-corn steep liquor medium. Appl. Biochem. Biotechnol. 2010, 162, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Barud, H.S.; de Araújo Júnior, A.M.; Santos, D.B.; de Assunção, R.M.N.; Meireles, C.S.; Cerqueira, D.A.; Rodrigues Filho, G.; Ribeiro, C.A.; Messaddeq, Y.; Ribeiro, S.J.L. Thermal behavior of cellulose acetate produced from homogeneous acetylation of bacterial cellulose. Thermochim. Acta 2008, 471, 61–69. [Google Scholar] [CrossRef]
- Shi, G.; Rao, L.; Yu, H.; Xiang, H.; Yang, H.; Ji, R. Stabilization and encapsulation of photosensitive resveratrol within yeast cell. Int. J. Pharm. 2008, 349, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Belbachir, K.; Noreen, R.; Gouspillou, G.; Petibois, C. Collagen types analysis and differentiation by ftir spectroscopy. Anal. Bioanal. Chem. 2009, 395, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Silva Junior, Z.S.; Botta, S.B.; Ana, P.A.; Franca, C.M.; Fernandes, K.P.; Mesquita-Ferrari, R.A.; Deana, A.; Bussadori, S.K. Effect of papain-based gel on type i collagen--spectroscopy applied for microstructural analysis. Sci. Rep. 2015, 5, 11448. [Google Scholar] [CrossRef] [PubMed]
- Camacho, N.P.; West, P.; Torzilli, P.A.; Mendelsohn, R. FTIR microscopic imaging of collagen and proteoglycan in bovine cartilage. Biopolymers 2001, 62, 1–8. [Google Scholar] [CrossRef]
- Kim, U.J.; Park, J.; Kim, H.J.; Wada, M.; Kaplan, D.L. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials 2005, 26, 2775–2785. [Google Scholar] [CrossRef]
- De Sá Coutinho, D.; Pacheco, T.M.; Frozza, L.R.; Bernardi, A. Anti-inflammatory effects of resveratrol: Mechanistic insights. Int. J. Mol. Sci. 2018, 19, 1812. [Google Scholar] [CrossRef]
- Brumer, H.; Zhou, Q.; Baumann, M.J.; Carlsson, K.; Teeri, T.T. Activation of crystalline cellulose surfaces through the chemoenzymatic modification of xyloglucan. J. Am. Chem. Soc. 2004, 126, 5715–5721. [Google Scholar] [CrossRef]
- Zunino, S.J.; Storms, D.H. Resveratrol alters proliferative responses and apoptosis in human activated b lymphocytes in vitro. J. Nutr. 2009, 139, 1603–1608. [Google Scholar] [CrossRef]
- Peltz, L.; Gomez, J.; Marquez, M.; Alencastro, F.; Atashpanjeh, N.; Quang, T.; Bach, T.; Zhao, Y. Resveratrol exerts dosage and duration dependent effect on human mesenchymal stem cell development. PLoS ONE 2012, 7, e37162. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Gao, D.; Jiang, X.; Hu, S.; Zhang, L.; Fei, Z. Resveratrol inhibits oxygen-glucose deprivation-induced mmp-3 expression and cell apoptosis in primary cortical cells via the nf-kappa-b pathway. Mol. Med. Rep. 2014, 10, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; Cooper, J.A. Actin, a central player in cell shape and movement. Science 2009, 326, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, M.; Jansson, L.; Ketolainen, J.; Pihlajamaki, H.; Suuronen, R.; Skottman, H.; Inzunza, J.; Hovatta, O.; Narkilahti, S. Cd marker expression profiles of human embryonic stem cells and their neural derivatives, determined using flow-cytometric analysis, reveal a novel cd marker for exclusion of pluripotent stem cells. Stem Cell Res. 2009, 2, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Shayan, P.; Busch, F.; Aldinger, C.; Buhrmann, C.; Lueders, C.; Mobasheri, A. Resveratrol mediated modulation of sirt-1/runx2 promotes osteogenic differentiation of mesenchymal stem cells: Potential role of runx2 deacetylation. PLoS ONE 2012, 7, e35712. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Zhao, P.; Sui, B.D.; Liu, N.; Hu, C.H.; Chen, J.; Zheng, C.X.; Liu, A.Q.; Xuan, K.; Pan, Y.P.; et al. Resveratrol enhances the functionality and improves the regeneration of mesenchymal stem cell aggregates. Exp. Mol. Med. 2018, 50, 74. [Google Scholar] [CrossRef] [PubMed]
- Safaeinejad, Z.; Kazeminasab, F.; Kiani-Esfahani, A.; Ghaedi, K.; Nasr-Esfahani, M.H. Multi-effects of resveratrol on stem cell characteristics: Effective dose, time, cell culture conditions and cell type-specific responses of stem cells to resveratrol. Eur. J. Med. Chem. 2018, 155, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Leong, C.W.; Wong, C.H.; Lao, S.C.; Leong, E.C.; Lao, I.F.; Law, P.T.; Fung, K.P.; Tsang, K.S.; Waye, M.M.; Tsui, S.K.; et al. Effect of resveratrol on proliferation and differentiation of embryonic cardiomyoblasts. Biochem. Biophys. Res. Commun. 2007, 360, 173–180. [Google Scholar] [CrossRef]
- Lv, X.; Yang, J.; Feng, C.; Li, Z.; Chen, S.; Xie, M.; Huang, J.; Li, H.; Wang, H.; Xu, Y. Bacterial cellulose-based biomimetic nanofibrous scaffold with muscle cells for hollow organ tissue engineering. ACS Biomater. Sci. Eng. 2016, 2, 19–29. [Google Scholar] [CrossRef]
- Midwood, K.S.; Williams, L.V.; Schwarzbauer, J.E. Tissue repair and the dynamics of the extracellular matrix. Int. J. Biochem. Cell Biol. 2004, 36, 1031–1037. [Google Scholar] [CrossRef]
- Li, B.; Wang, J.H. Fibroblasts and myofibroblasts in wound healing: Force generation and measurement. J. Tissue Viability 2011, 20, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Ratz-Łyko, A.; Arct, J. Resveratrol as an active ingredient for cosmetic and dermatological applications: A review. J. Cosmet. Laser Ther. 2019, 21, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Klinge, C.M.; Bowers, J.L.; Jernigan, S.C.; Tyulmenkov, V.V. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors α and β*. Endocrinology 2000, 141, 3657–3667. [Google Scholar]
- Gauglitz, G.G.; Korting, H.C.; Pavicic, T.; Ruzicka, T.; Jeschke, M.G. Hypertrophic scarring and keloids: Pathomechanisms and current and emerging treatment strategies. Mol. Med. 2011, 17, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Yates, C.C.; Whaley, D.; Hooda, S.; Hebda, P.A.; Bodnar, R.J.; Wells, A. Delayed reepithelialization and basement membrane regeneration after wounding in mice lacking cxcr3. Wound Repair Regen. 2009, 17, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Green, H.; Easley, K.; Iuchi, S. Marker succession during the development of keratinocytes from cultured human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2003, 100, 15625–15630. [Google Scholar] [CrossRef] [PubMed]
- Chao, S.C.; Chen, Y.J.; Huang, K.H.; Kuo, K.L.; Yang, T.H.; Huang, K.Y.; Wang, C.C.; Tang, C.H.; Yang, R.S.; Liu, S.H. Induction of sirtuin-1 signaling by resveratrol induces human chondrosarcoma cell apoptosis and exhibits antitumor activity. Sci. Rep. 2017, 7, 3180. [Google Scholar] [CrossRef]
- Qiang, L.; Sample, A.; Liu, H.; Wu, X.; He, Y.Y. Epidermal sirt1 regulates inflammation, cell migration, and wound healing. Sci. Rep. 2017, 7, 14110. [Google Scholar] [CrossRef]
- Lima, F.M.; Pinto, F.C.; Andrade-da-Costa, B.L.; Silva, J.G.; Campos Junior, O.; Aguiar, J.L. Biocompatible bacterial cellulose membrane in dural defect repair of rat. J. Mater. Sci. Mater. Med. 2017, 28, 37. [Google Scholar] [CrossRef]
- El Masry, M.S.; Chaffee, S.; Das Ghatak, P.; Mathew-Steiner, S.S.; Das, A.; Higuita-Castro, N.; Roy, S.; Anani, R.A.; Sen, C.K. Stabilized collagen matrix dressing improves wound macrophage function and epithelialization. FASEB J. 2019, 33, 2144–2155. [Google Scholar] [CrossRef]
- Das, A.; Ghatak, S.; Sinha, M.; Chaffee, S.; Ahmed, N.S.; Parinandi, N.L.; Wohleb, E.S.; Sheridan, J.F.; Sen, C.K.; Roy, S. Correction of mfg-e8 resolves inflammation and promotes cutaneous wound healing in diabetes. J. Immunol. 2016, 196, 5089–5100. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, E.; Chen, C.-L.; Liu, C.-C.; Liu, C.-C.; Chang, S.-J.; Cherng, J.-H.; Wang, H.-H.; Wu, S.-T. Bioapplications of Bacterial Cellulose Polymers Conjugated with Resveratrol for Epithelial Defect Regeneration. Polymers 2019, 11, 1048. https://doi.org/10.3390/polym11061048
Meng E, Chen C-L, Liu C-C, Liu C-C, Chang S-J, Cherng J-H, Wang H-H, Wu S-T. Bioapplications of Bacterial Cellulose Polymers Conjugated with Resveratrol for Epithelial Defect Regeneration. Polymers. 2019; 11(6):1048. https://doi.org/10.3390/polym11061048
Chicago/Turabian StyleMeng, En, Chin-Li Chen, Chuan-Chieh Liu, Cheng-Che Liu, Shu-Jen Chang, Juin-Hong Cherng, Hsiao-Hsien Wang, and Sheng-Tang Wu. 2019. "Bioapplications of Bacterial Cellulose Polymers Conjugated with Resveratrol for Epithelial Defect Regeneration" Polymers 11, no. 6: 1048. https://doi.org/10.3390/polym11061048
APA StyleMeng, E., Chen, C.-L., Liu, C.-C., Liu, C.-C., Chang, S.-J., Cherng, J.-H., Wang, H.-H., & Wu, S.-T. (2019). Bioapplications of Bacterial Cellulose Polymers Conjugated with Resveratrol for Epithelial Defect Regeneration. Polymers, 11(6), 1048. https://doi.org/10.3390/polym11061048






