Microbial Degradation of Synthetic Biopolymers Waste
Abstract
:1. Introduction
2. Microbial Degradation of Synthetic Biopolymer Wastes
3. Factors Affecting Degradation Process of Synthetic Biopolymer Wastes
3.1. Biodegradation in Liquid Environment
3.2. Biodegradation in Soil
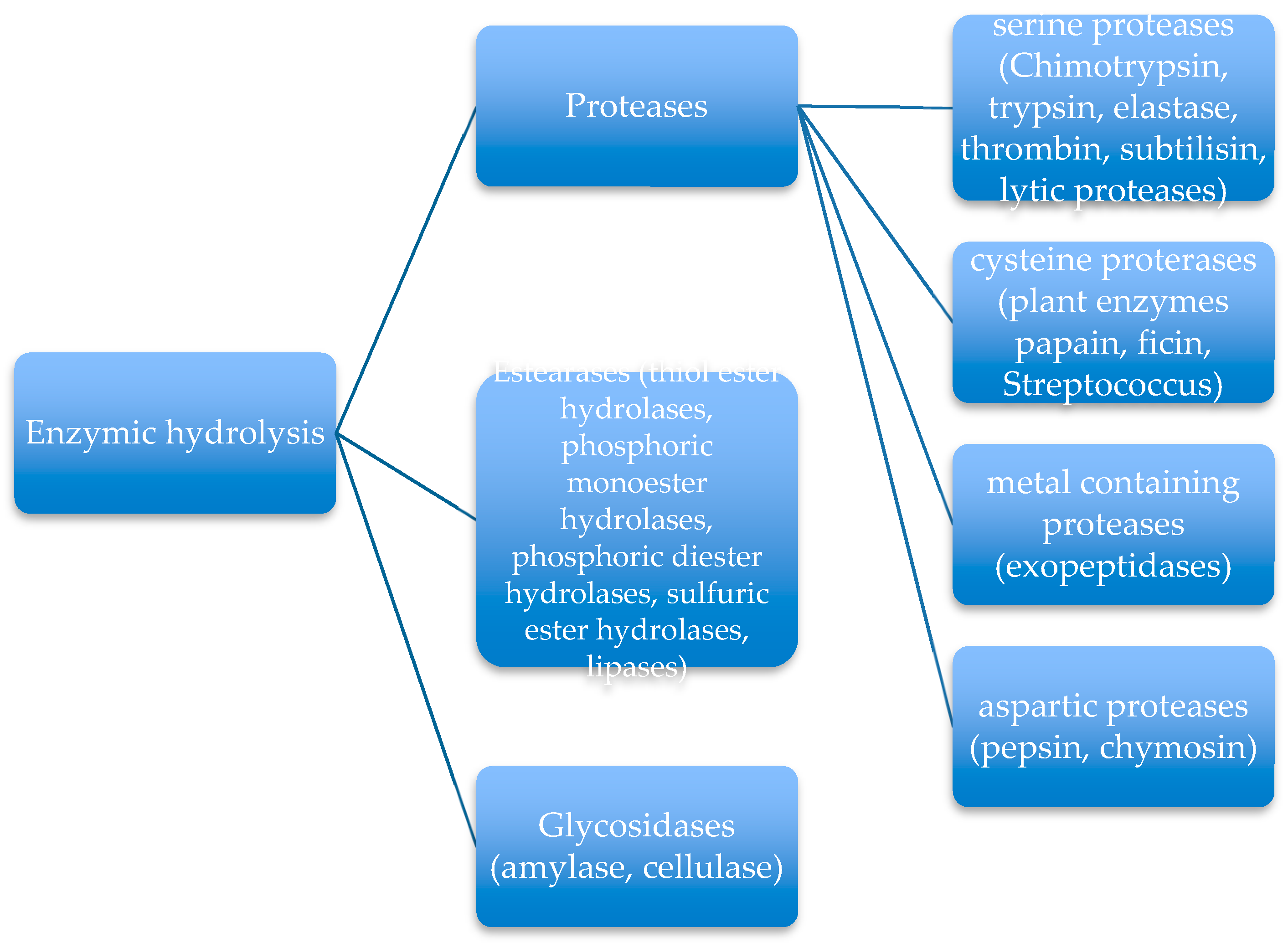
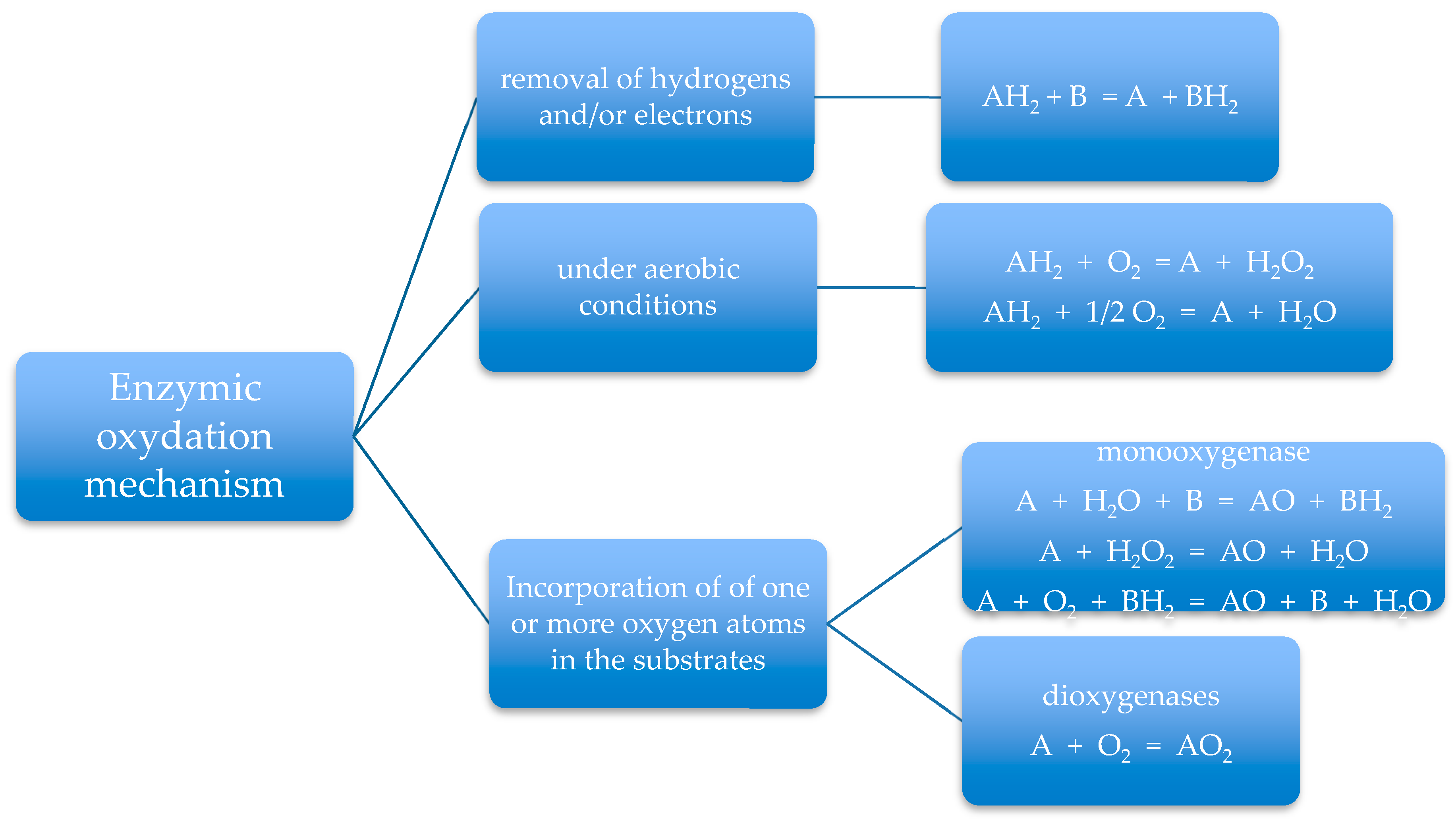
4. Involvement of Enzymes in Degradation Process of Synthetic Biopolymer Wastes
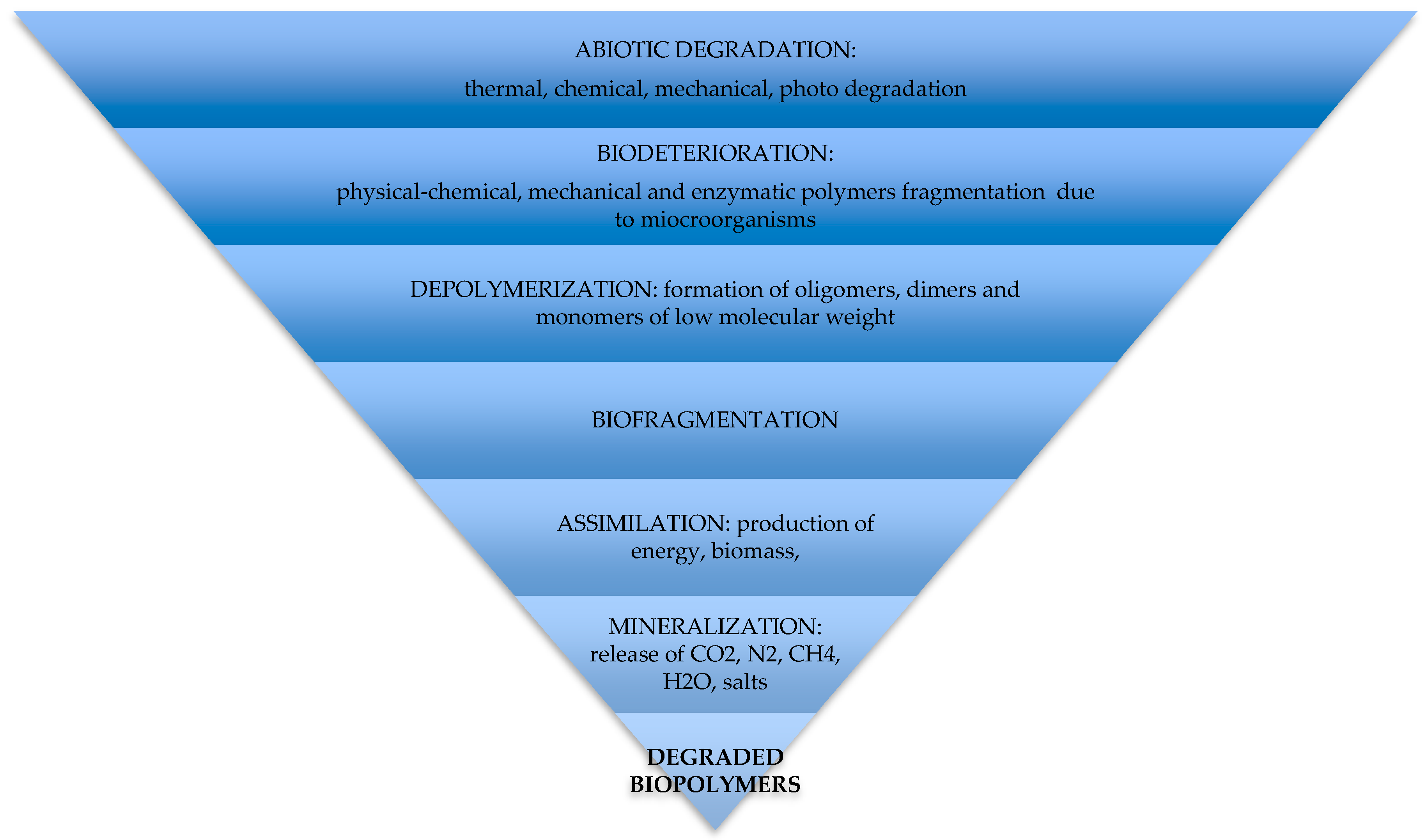
5. Mechanism and Pathways of Synthetic Biopolymer Degradation
5.1. Abiotic Degradation
5.2. Biodeterioration and Depolymerization
5.3. Biofragmentation
5.4. Assimilation and Mineralization
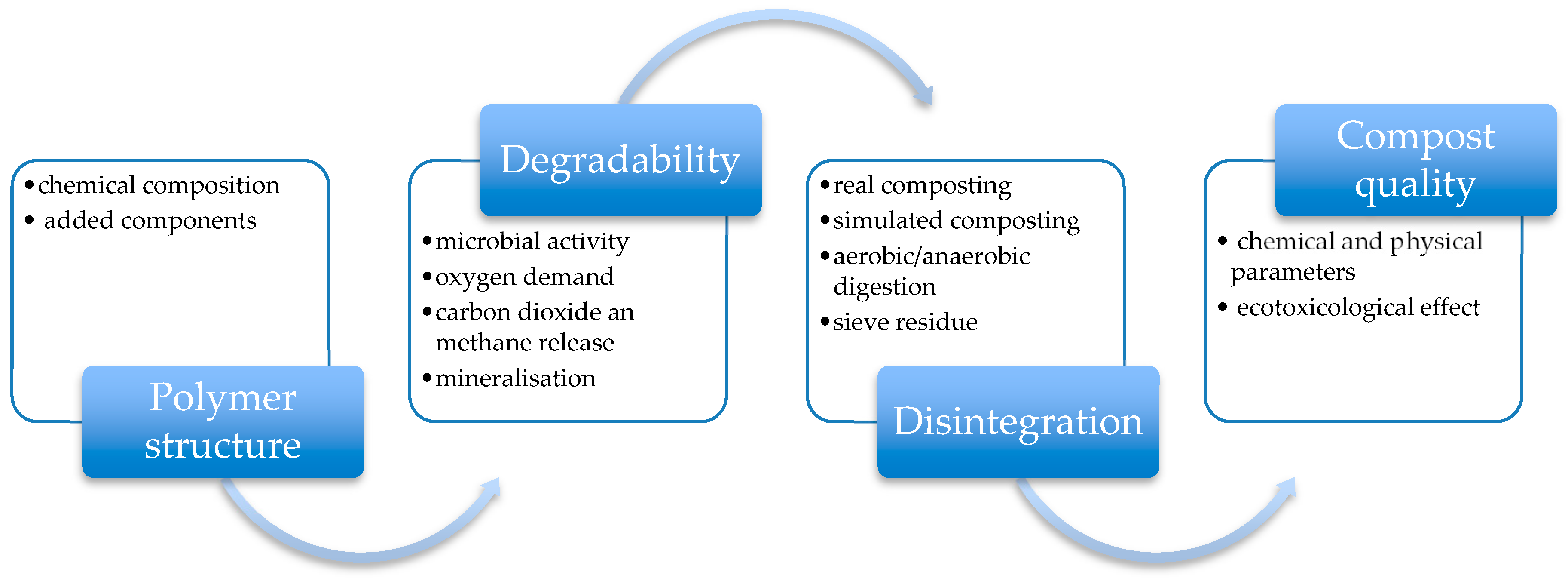
6. Microbial Toxicity of Synthetic Biopolymer Wastes and Degradation Products
7. Future Prospective
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biothechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Dalla Rosa, M. Biodegradable Polymers for food packaging: A review. Trends Food Sci. Technol. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Siracusa, V. Packaging Material in the Food Industry. In Antimicrobial Food Packaging; Barros-Velàzques, J., Ed.; Elsevier Academic Press: San Diego, CA, USA, 2016; pp. 95–106. [Google Scholar]
- Mensitieri, G.; Di Maio, E.; Buonocore, G.G.; Nedi, I.; Oliviero, M.; Sansone, L.; Iannace, S. Processing and shelf life issues of selected food packaging materials and structures from renewable resources. Trends Food Sci. Technol. 2011, 22, 72–80. [Google Scholar] [CrossRef]
- Vert, M. Aliphatic Polyesters: Great Degradable Polymers That Cannot do everything. Biomacromolecules 2005, 6, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Dijkstra, P.; Loos, K. The recent Developments in Biobased Polymers toward General and Engineering Applications: Polymers that are upgraded from Biodegradable Polymers, analogous to Petroleum-derived Polymers, and New Developed. Polymers 2017, 9, 523–549. [Google Scholar] [CrossRef] [PubMed]
- Garrison, T.F.; Murawski, A.; Quirino, R.L. Bio-Based Polymers with Potential for Biodegradability. Polymers 2016, 8, 262–283. [Google Scholar] [CrossRef] [PubMed]
- Kale, G.; Kijchavengkul, T.; Auras, R.; Ribino, M.; Selke, S.E.; Singh, S.P. Compostability of Bioplastic Packaging Materials: An Overview. Macromol. Biosci. 2007, 7, 255–277. [Google Scholar] [CrossRef]
- Bohlmann, G.M. General Characteristics, Processability, Industrial Applications and Market. Evolution of Biodegradable Polymers. In Handbook of Biodegradable Polymers; Bastioli, C., Ed.; RAPRA Technology Limited: Telford, UK, 2006; chapter 6. [Google Scholar]
- Huang, S.J. Poly(lactic acid) and Copolyesters. In Handbook of Biodegradable Polymers; Bastioli, C., Ed.; RAPRA Technology Limited: Telford, UK, 2006; chapter 9. [Google Scholar]
- Siracusa, V.; Dalla Rosa, M.; Iordanskii, A.L. Performance of Poly(lactic acid) Surface Modified Films for Food Packaging Application. Materials 2017, 10, 850–872. [Google Scholar] [CrossRef]
- Müller, R.J. Aliphatic-Aromatic Polyesters. In Handbook of Biodegradable Polymers; Bastioli, C., Ed.; RAPRA Technology Limited: Telford, UK, 2006; chapter 10. [Google Scholar]
- Van der Zee, M. Biodegradability of Polymers Mechanisms and Evaluation Methods. In Handbook of Biodegradable Polymers; Bastioli, C., Ed.; RAPRA Technology Limited: Telford, UK, 2006. [Google Scholar]
- Müller, R.J. Biodegradation Behavior of Polymers in Liquid Environments. In Handbook of Biodegradable Polymers; Bastioli, C., Ed.; RAPRA Technology Limited: Telford, UK, 2006; chapter 2. [Google Scholar]
- Degli Innocenti, F. Biodegradation Behavior of Polymers in the Soil. In Handbook of Biodegradable Polymers; Bastioli, C., Ed.; RAPRA Technology Limited: Telford, UK, 2006; chapter 3. [Google Scholar]
- Müller, R.J.; Marten, E.; Deckwer, W.D. Biorelated Polymers: Sustainable Polymer Science and Technology; Chiellini, E., Gil, H., Braunegg, G., Buchert, J., Gatenholm, P., Van der Zee, M., Eds.; Plenum Publishers: New York, NY, USA, 2001. [Google Scholar]
- Guidotti, G.; Soccio, M.; Siracusa, V.; Gazzano, M.; Salatelli, E.; Munari, A.; Lotti, N. Novel random PBS-based copolymers containing aliphatic side chains for sustainable flexible food packaging. Polymers 2017, 9, 724–740. [Google Scholar] [CrossRef]
- Genovese, L.; Lotti, N.; Gazzano, M.; Siracusa, V.; Dalla Rosa, M.; Munari, A. Novel biodegradable aliphatic copolyesters based on poly(butylene succinate) containing thioether-linkages for sustainable food packaging applications. Polym Degrd. Stab. 2016, 132, 191–201. [Google Scholar] [CrossRef]
- Genovese, L.; Soccio, M.; Lotti, N.; Gazzano, M.; Siracusa, V.; Salatelli, E.; Balestra, F.; Munari, A. Design of biobased PLLA triblock copolymers for sustainable food packaging: Thermo-mechanical properties, gas barrier ability and compostability. Eur. Polym J. 2017, 95, 289–303. [Google Scholar] [CrossRef]
- Lucas, N.; Bienaime, C.; Belloy, C.; Queneudec, M.; Silvestre, F.; Nava-Saucedo, J.-E. Polymer biodegradation: Mechanisms and estimation techniques. Chemosphere 2008, 73, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, I.; Yarahmadi, N.; Petersen, H. Evaluation of the rate of abiotic degradation of biodegradable polyethylene in various environments. Polym. Degrd. Stab. 2006, 91, 1556–1562. [Google Scholar] [CrossRef]
- Wyart, D. Les Polymers Biodegradable. Techniques de l’ingénieur. 2007. Available online: www.techniques-inginieur.fr (accessed on 1 March 2019).
- Iovino, R.; Zullo, R.; Rao, M.A.; Cassar, L.; Gianfreda, L. Biodegradation of poly(lactic acid)/starch/coir biocomposites under controlled composting conditions. Poly. Degrd. Stab. 2008, 93, 147–157. [Google Scholar] [CrossRef]
- El-Hadi, A.; Schnabel, R.; Straube, E.; Muller, G.; Henning, S. Correlation between degree of crystallinity, morphology, glass temperature, mechanical properties and biodegradation of poly(3-hydroxyalkanoate) PHAs and their blends. Polym. Test. 2002, 21, 665–674. [Google Scholar] [CrossRef]
- Gan, Z.; Kuwaabara, K.; Abe, H.; Iwata, T.; Doi, Y. The role of polymorphic crystal structure and morphology in enzymatic degradation of melt-crystallized poly(butylene adipate) films. Polym. Degrd. Stab. 2005, 87, 191–199. [Google Scholar] [CrossRef]
- Zhao, L.; Gan, Z. Effect of copolymerized butylene terephthalate chains on polymorphism and enzymatic degradation of poly(butylene adipate). Polym. Degrad. Stab. 2006, 91, 2429–2436. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, X.; Li, L.; Gan, Z. Structural analysis of poly(butylene adipate) banded spherulites from their biodegradation behavior. Polymer 2007, 48, 6152–6161. [Google Scholar] [CrossRef]
- Duval, C. Matériaux Degradable In Matières Plastiques et environment-Recyclage, Valorization, Biodégradabilité, écoconception; DUNOD: Paris, France, 2004. [Google Scholar]
- Briassoulis, D. The effect of tensile stress and agrochemicals Vapam on the ageing of low density polyethylene (LDPE) agricultural films. Part I. Mechanical behavior. Polym. Degrd. Stab. 2005, 86, 489–503. [Google Scholar] [CrossRef]
- Jung, J.H.; Ree, M.; Kim, H. Acid- and base-catalyzed hydrolyses of aliphatic polycarbonates and polyesters. Catal. Today 2006, 115, 283–287. [Google Scholar] [CrossRef]
- De Joung, S.J.; Arias, E.R.; Rijkers, D.T.S.; Van Nostrum, C.F.; Kettenes-Van Den Bosh, J.J.; Hennink, W.E. New insights into the hydrolytic degradation of poly(lactic acid): Participation of the alcohol terminus. Polymer 2001, 42, 2795–2802. [Google Scholar] [CrossRef]
- Nakamura, H.; Nakamura, T.; Noguchi, T.; Imagawa, K. Photodegradation of PEEK sheets under tensile stess. Polym Degrad. Stab. 2006, 9, 740–746. [Google Scholar] [CrossRef]
- Tsuji, H.; Echizen, Y.; Nishimura, Y. Photodegradation of biodegradable polyesters: A comprehensive study of poly(L-lactide) and poly(ε-caprolactone). Polym. Degrad. Stab. 2006, 91, 1128–1137. [Google Scholar] [CrossRef]
- Kijchavengkul, T.; Auras, R.; Rubino, M.; Ngouajio, M.; Fernandez, R.T. Assessment of aliphatic-aromatic copolyester biodegradable mulch films. Part II: Laboratory simulated conditions. Chemosphere 2008, 71, 1607–1616. [Google Scholar] [CrossRef]
- Hueck, H.J. The biodeterioration of materials: An appraisal. Int. Biodeteter. Biodegrad. 2001, 48, 5–11. [Google Scholar] [CrossRef]
- Walsh, J.H. Ecological considerations of biodeterioration. Int. Biodeteter. Biodegrad. 2001, 48, 16–25. [Google Scholar] [CrossRef]
- Capitelli, F.; Principi, P.; Sorlini, C. Biodeterioration of modern materials in contemporary collections: Can biotechnology help? Trends Biotechnol. 2006, 24, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Bonhomme, S.; Cuer, A.; Delort, A.M.; Lemaire, J.; Sancelme, M.; Scott, G. Environmental biodegradation of polyethylene. Polym Degrad. Stab. 2003, 81, 441–452. [Google Scholar] [CrossRef]
- Zanardini, E.; Abbruscato, P.; Ghedini, N.; Realini, M.; Sorlini, C. Influence of atmospheric pollutants on the biodeterioration of stone. Int. Biodeteter. Biodegrad. 2000, 45, 35–42. [Google Scholar] [CrossRef]
- Lugauskas, A.; Levinkaite, L.; Peciulyte, D. Micromycetes as deterioration agents of polymeric materials. Int. Biodeteter. Biodegrad. 2003, 52, 233–242. [Google Scholar] [CrossRef]
- Warscheid, T.; Braams, J. Biodeterioration of stone: A review. Int. Biodeteter. Biodegrad. 2000, 46, 343–368. [Google Scholar] [CrossRef]
- Pelmont, J. Enzymes. Catalyseurs du monde vivante; EDP Sciences: Les Ulis, Paris, France, 1995. [Google Scholar]
- Pelmont, J. Biodégradation et Metabolisms. Les Bactéries pour les Technologies de l’environment; EDP Sciences: Les Ulis, Paris, France, 2005. [Google Scholar]
- Müller, R.J.; Witt, U.; Rantze, E.; Deckwer, W.D. Architecture of biodegradable copolyesters containing aromatic constituents. Polym. Degrad. Stab. 1998, 59, 203–208. [Google Scholar] [CrossRef]
- DIN 54900, Part 1-4, Testing of the Compostability of Plastics; German Institute for Standardisation: Berlin, Germany, 1998.
- ASTM D6002-96, Standard Guide for Assessing the Compostability of Environmentally Degradable Plastics; ASTM INTERNATIONAL: West Conshohocken, PA, USA, 2002.
- EN 13432, Packaging–Requirements for Packaging Recoverable through Composting and Biodegradation – Test Scheme and Evaluation Criteria for the Final Acceptance of Packaging. 2000. Available online: http://www.bread4pla-life.eu/detalle_normativa.php?no_id=14 (accessed on 19 June 2019).
- Frits, J. Ecotoxicological Aspects in the Biodegradation Process of Polymers. In Handbook of Biodegradable Polymers; Bastioli, C., Ed.; RAPRA Technology Limited: Telford, UK, 2006; chapter 4. [Google Scholar]
- Salehpour, S.; Jonoobi, M.; Ahmadzadeh, M.; Siracusa, V.; Rafieian, F.; Oksman, K. Biodegradation and ecotoxicological impact of celluose nanocomposites in municipal solid waste composting. Int. J. Biol. Macromol. 2018, 111, 264–270. [Google Scholar] [CrossRef]
- Calow, P. Handbook of Ecotoxicology; Blackwell Science Ltd.: Oxford, UK, 1993. [Google Scholar]
- Fent, K. Ecotoxicology–Environmental Chemistry, Toxicology, Ecology (Ökotoxikologie–Umweltchemie, Toxikologie, Ökologie); Georg Thieme Verlag, Ed.; ACADEMIA: Stuttgard, Germany, 1998; chapter 6. [Google Scholar]
- Forbes, V.E.; Forbes, T.L. Ecotoxicology in Theory and Practice; Chapman & Hall: London, UK, 1994. [Google Scholar]
- Landis, W.G.; Yu, M.H. Introduction to Environmental Toxicology: Impacts of Chemicals Upon Ecological Systems; Lewis Publishers: Boca Raton, FL, USA, 1999. [Google Scholar]
- Scott, G. Polymers and the Environments; The Royal Society of Chemistry: Cambridge, UK, 1999. [Google Scholar]
- Müller, R.J. Mechanistic Studies on the Biodegradation of Polyester; Sand, W., Ed.; DECHEMA Monographs: Hamburg, Germany, 1996; Volume 133. [Google Scholar]
- Suffet, I.H.; MacCarthy, P. Aquatic Humic Substances: Influence on Fate and Treatment of Pollutants; Suffet, I.H., MacCarthy, P., Eds.; American Chemical Society: Washington DC, USA, 1989. [Google Scholar]
- Coleman, D.C.; Crossley, D.A. Fundamentals of Soil Ecology; Academic Press Limited: London, UK, 1996. [Google Scholar]
- Rizzarelli, P.; Puglisi, C.; Montaudo, G. Soil burial and enzymatic degradation in solution of aliphatic co-polyester. Polym. Degrad. Stab. 2004, 85, 855–863. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H.J.; Lee, J.W.; Choi, I.G. Biodegradability of bio-flour filled biodegradable poly(butylene succinate) bio-composites in natural and compost soil. Polym. Degrad. Stab. 2006, 91, 1117–1127. [Google Scholar] [CrossRef]
- Wang, Y.W.; Mo, W.; Yao, H.; Wu, Q.; Chen, J.; Chen, G.Q. Biodegradation studies of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate). Polym. Degrad. Stab. 2004, 85, 815–821. [Google Scholar] [CrossRef]
- Pagga, U.; Schafer, A.; Muller, R.J.; Pantke, M. Determination of the aerobic biodegradability of polymeric material in aquatic batch test. Chemosphere 2001, 42, 319–331. [Google Scholar] [CrossRef]


© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siracusa, V. Microbial Degradation of Synthetic Biopolymers Waste. Polymers 2019, 11, 1066. https://doi.org/10.3390/polym11061066
Siracusa V. Microbial Degradation of Synthetic Biopolymers Waste. Polymers. 2019; 11(6):1066. https://doi.org/10.3390/polym11061066
Chicago/Turabian StyleSiracusa, Valentina. 2019. "Microbial Degradation of Synthetic Biopolymers Waste" Polymers 11, no. 6: 1066. https://doi.org/10.3390/polym11061066
APA StyleSiracusa, V. (2019). Microbial Degradation of Synthetic Biopolymers Waste. Polymers, 11(6), 1066. https://doi.org/10.3390/polym11061066




