Removal of Linear and Branched Alkylphenols with the Combined Use of Polyphenol Oxidase and Chitosan
Abstract
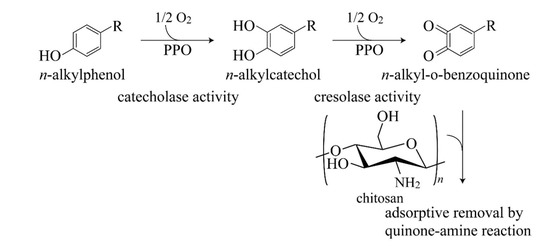
:1. Introduction
2. Experimental
2.1. Materials and Chemicals
2.2. Removal of Alkylphenols in the Heterogeneous Reaction
2.3. Quantitative Assay of Alkylphenols
2.4. Removal of Alkylphenols in the Homogeneous System
3. Results and Discussion
3.1. Polyphenol Oxidase (PPO) Concentration Dependence of Quinone Oxidation
3.2. PPO-Catalyzed Quinone Oxidation and Subsequent Quinone Adsorption on Chitosan Beads
3.3. PPO-Catalyzed Quinone Oxidation and Subsequent Quinone Adsorption on Chitosan Powder
3.4. Removal with PPO and Chitosan in the Homogeneous System
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Coster, S.; van Larebeke, N. Endocrine-disrupting chemicals: Associated disorders and mechanisms of action. J. Environ. Public Health 2012, 2012. [Google Scholar] [CrossRef]
- Kerdivel, G.; Habauzit, D.; Pakdel, F. Assessment and molecular actions of endocrine-disrupting chemicals that interfere with estrogen receptor pathways. Int. J. Endocrinol. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Guenther, K.; Heinke, V.; Thiele, B.; Kleist, E.; Prast, H.; Raecker, T. Endocrine disrupting nonylphenols are ubiquitous in food. Environ. Sci. Technol. 2002, 36, 1676–1680. [Google Scholar] [CrossRef] [PubMed]
- Lagurdia, M.J.; Hale, R.C.; Harvey, E.; Mainor, T.M. Alkylphenol ethoxylate degradation products in land-applied sewage sludge (biosolids). Environ. Sci. Technol. 2001, 35, 4798–4804. [Google Scholar] [CrossRef]
- Kochukov, M.Y.; Jeng, Y.J.; Watson, C.S. Alkylphenol xenoestrogens with varying carbon chain lengths differentially and potently activate signaling and functional responses in GH3/B6/F10 Somatomammotropes. Environ. Health Perspect. 2009, 117, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Nishikawa, J.; Kanayama, T.; Dakeyama, F.; Saito, K.; Imagawa, M.; Takatori, S.; Kitagawa, Y.; Hori, S.; Utsumi, H. Estrogenic activities of 517 chemicals by yeast two-hybrid assay. J. Health Sci. 2000, 46, 282–298. [Google Scholar]
- De Leon-Condes, C.; Barrera-Diaz, C.; Barrios, J.; Becerri, E.; Reyes-Perez, H. A coupled ozonation-electrooxidation treatment for removal of bisphenol A, nonylphenol and triclosan from wastewater. Int. J. Environ. Sci. Technol. 2017, 14, 707–716. [Google Scholar] [CrossRef]
- Xing, R.; Wu, L.; Fei, Z. Photodegradation of 4-methylphenol on palladium phthalocyaninesulfonate functionalized mesopolymer under visible light irradiation. Acta Chim. Slov. 2014, 61, 406–413. [Google Scholar]
- Wu, Y.L.; Shi, J.; Chen, H.C.; Zhao, J.F.; Dong, W.B. Aqueous photodegradation of 4-tert-butylphenol: By-products, degradation pathway and theoretical calculation assessment. Sci. Total Environ. 2016, 566, 86–92. [Google Scholar] [CrossRef]
- Ardelean, R.; Davidescu, C.M.; Pora, A.; Ilia, G. Removal of phenol, 2,3-dimethlphenol, 2,6-dimethlphenol and 2,4,6-trimethlphenol from aqueous solutions by polymeric sorbents having olefin groups. Rev. Chim. 2012, 63, 1065–1068. [Google Scholar]
- Hidalgo, A.M.; León, G.; Gómez, M.; Murcia, M.D.; Gómez, E.; Penalva, P. Removal of 4-chloro-2-methylphenol from aueous solutions by nanofiltration and reverse osmosis. Desalin. Water Treat. 2015, 53, 1499–1505. [Google Scholar] [CrossRef]
- Aboussaoud, W.; Manero, M.H.; Pic, J.S.; Debellefontaine, H. Combined ozonation using alumino-silica materials for the removal of 2,4-dimethylphenol from water. Ozone Sci. Eng. 2014, 36, 221–228. [Google Scholar] [CrossRef]
- Romero, A.; Santos, A.; Vicente, F. Chemical oxidation of 2,4-dimethylphenol in soil by heterogeneous Fenton process. J. Hazard. Mater. 2009, 162, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Ogata, Y.; Toyama, T.; Yu, N.; Wang, X.; Sei, K.; Ike, M. Occurrence of 4-tert-butyl (4-t-BP) biodegradation in an aquatic sample caused by the presence of Sperodela polyrrhiza and isolation of 4-t-BP-utilizaing bacterium. Biodegradation 2013, 24, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, Y.; Dai, Y.; Xie, S. Anaerobic biodegradation of nonylphenol in river sediment under nitrate- or sulfate-reducing conditions and associated bacterial community. J. Hazard. Mater. 2015, 289, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Różalska, S.; Soboń, A.; Pawłowska, J.; Wrzosek, M.; Długoński, J. Biodegradation of nonylphenol by a novel entomopathogenic Metarhizium robertsii strain. Bioresour. Technol. 2015, 191, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, N.; Yamada, K.; Shibuya, T.; Kashiwada, A.; Matsuda, K.; Hirata, M. Removal of linear and branched alkylphenols from aqueous solutions with horseradish peroxidase. Biosci. Biotechnol. Biochem. 2008, 72, 1368–1371. [Google Scholar] [CrossRef]
- Yamada, K.; Ikeda, N.; Takano, Y.; Kashiwada, A.; Matsuda, K.; Hirata, M. Determination of optimum process parameters for peroxidase-catalyzed treatment of bisphenol A and application to the removal of bisphenol derivatives. Environ. Technol. 2010, 31, 243–256. [Google Scholar] [CrossRef]
- Watanabe, C.; Kashiwada, A.; Matsuda, K.; Yamada, K. Soybean peroxidase-catalyzed treatment and removal of BPA and bisphenol derivatives from aqueous solutions. Environ. Prog. Sustain. Energy 2011, 30, 81–91. [Google Scholar] [CrossRef]
- Yamada, K.; Akiba, Y.; Shibuya, T.; Kashiwada, A.; Matsuda, K.; Hirata, M. Water purification through bioconversion of phenol compounds by tyrosinase and chemical adsorption by chitosan beads. Biotechnol. Prog. 2005, 21, 823–829. [Google Scholar] [CrossRef]
- Tamura, A.; Satoh, E.; Kashiwada, A.; Matsuda, K.; Yamada, K. Removal of alkylphenols by the combined use of tyrosinase immobilized on ion-exchange resins and chitosan beads. J. Appl. Polym. Sci. 2010, 115, 137–145. [Google Scholar] [CrossRef]
- Suzuki, M.; Sugiyama, T.; Musashi, E.; Kobiyama, Y.; Kashiwada, A.; Matsuda, K.; Yamada, K. Use of chitosan for removal of bisphenol A and bisphenol derivatives through tyrosinase-catalyzed quinone oxidation. J. Appl. Polym. Sci. 2009, 118, 721–732. [Google Scholar] [CrossRef]
- Yamada, K.; Tamura, T.; Azaki, Y.; Kashiwada, A.; Hata, Y.; Higashida, K.; Nakamura, Y. Removal of linear and branched p-alkylphenols from aqueous solution by combined use of melB tyrosinase and chitosan beads. J. Polym. Environ. 2009, 17, 95–102. [Google Scholar] [CrossRef]
- Agarwal, P.; Gupta, R.; Agarwal, N. A review on enzymatic treatment of phenols in wastewater. J. Biotechnol. Biomater. 2016, 6, 249. [Google Scholar] [CrossRef]
- Wada, S.; Ichikawa, H.; Tatsumi, K. Removal of phenols and aromatic amines from wastewater by a combination treatment with tyrosinase and a coagulant. Biotechnol. Bioeng. 1995, 45, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.Q.; Payne, G.F. Tyrosinase-containing chitosan gels: A combined catalyst and sorbent for selective phenol removal. Biotechnol. Bioeng. 1996, 51, 79–86. [Google Scholar] [CrossRef]
- Jiménez, M.; García-Carmona, F. Hydrogen peroxide-dependent 4-t-butylphenol hydroxylation by tyrosinase—A new catalytic activity. Biochim. Biophys. Acta 1996, 1297, 33–39. [Google Scholar] [CrossRef]
- Garcia-Molina, M.M.; Muñoz-Muñoz, J.L.; Berna, J.; Rodríguez-López, J.N.; Varón, R.; García-Cánovas, F. Hydrogen peroxide helps in the identification of monophenols as possible substrates of tyrosinase. Biosci. Biotechnol. Biochem. 2013, 77, 2383–2388. [Google Scholar] [CrossRef]
- Atlow, S.C.; Bonadonna-Aparo, L.; Klibanov, A.M. Dephenolization of industrial wastewaters catalyzed by polyphenol oxidase. Biotechnol. Bioeng. 1984, 26, 599–603. [Google Scholar] [CrossRef]
- Burton, S.G.; Boshoff, A.; Edwards, W.; Rose, P.D. Biotransformation of phenols using immoblised polyphenol oxidase. J. Mol. Catal. B Enzym. 1998, 5, 411–416. [Google Scholar] [CrossRef]
- Edwards, W.; Bownes, R.; Leukes, W.D.; Jacobs, E.P.; Sanderson, R.; Rose, P.D.; Burton, S.G. A capillary membrane bioreactor using immobilized polyphenol oxidase for the removal of phenols from industrial effluents. Enzyme Microb. Technol. 1999, 24, 209–217. [Google Scholar] [CrossRef]
- Mukherjee, S.; Basak, B.; Bhunia, B.; Dey, A.; Mondal, B. Potential use of polyphenol oxidase (PPO) in the bioremediation of phenolic contaminants containing industrial wastewater. Rev. Environ. Sci. Biotechnol. 2013, 12, 61–73. [Google Scholar] [CrossRef]
- Kimura, Y.; Yamamoto, M.; Shimazaki, R.; Kashiwada, A.; Matsuda, K.; Yamada, K. Use of chitosan for removal of bisphenol A from aqueous solutions through quinone oxidation by polyphenol oxidation. J. Appl. Polym. Sci. 2012, 124, 796–804. [Google Scholar] [CrossRef]
- Kimura, Y.; Takahashi, A.; Kashiwada, A.; Yamada, K. Removal of bisphenol derivatives through quinone oxidation by polyphenol oxidase and subsequent quinone adsorption on chitosan in the heterogeneous system. Environ. Technol. 2015, 36, 2265–2277. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, H.W.; Coleman, J.E. Physicochemical and kinetic properties of mushroom tyrosinase. J. Biolog. Chem. 1970, 245, 1613–1625. [Google Scholar]
- Sugumaran, M.; Lipke, M. Quinone methide formation from 4-alkylcatechols: A novel reaction catalyzed by cuticular polyphenol oxidase. FEBS Lett. 1983, 155, 65–68. [Google Scholar] [CrossRef]
- Lončar, N.; Božić, N.; Anđelković, I.; Milovanović, A.; Dojnov, B.; Vujčić, M.; Roglić, G.; Vujčić, Z. Removal of aqueous phenol and phenol derivatives by immobilized potato polyphenol oxidase. J. Seb. Chem. Soc. 2011, 76, 513–522. [Google Scholar] [CrossRef]










| Alkyl Chain, R | Abbreviation | Initial Concentration (mM) | Solvent Composition (1) |
|---|---|---|---|
| –CH3 | p-cresol | 0.30 | buffer |
| –CH2CH3 | 4EP | 0.30 | buffer |
| –(CH2)2CH3 | 4NProP | 0.30 | buffer |
| –CH(CH3)CH3 | 4IProP | 0.30 | buffer |
| –(CH2)3CH3 | 4NBP | 0.30 | buffer |
| –CH(CH3)CH2CH3 | 4SBP | 0.30 | buffer |
| –C(CH3)3 | 4TBP | 0.30 | buffer |
| -(CH2)4CH3 | 4NPenP | 0.30 | buffer |
| –C(CH2CH3)(CH3)2 | 4TPenP | 0.30 | buffer |
| –(CH2)5CH3 | 4NHexP | 0.20 | buffer |
| –(CH2)6CH3 | 4NHepP | 0.10 | 11.25 vol% |
| –(CH2)7CH3 | 4NOP | 0.10 | 26.66 vol% |
| –C(CH3)2CH2C(CH3)3 | 4TOP | 0.10 | 7.5 vol% |
| –(CH2)8CH3 | 4NNP | 0.10 | 31.5 vol% |
| Alkylphenol | [PPO] (U/cm3) | Absence of Chitosan Beads | Presence of Chitosan Beads | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Max Abs. | Time | Conversion | Time | Min Abs. | Time | Conversion | Time | ||
| (min) | (%) | (min) | (min) | (%) | (min) | ||||
| p-cresol | 8 | 0.278 | 50 | 96.5 | 360 | 0.02 | 120 | 100 | 300 |
| 4EP | 8 | 0.289 | 30 | 100 | 180 | 0.03 | 120 | 99.3 | 180 |
| 4NProP | 5 | 0.254 | 60 | 100 | 180 | 0.03 | 180 | 100 | 300 |
| 4IProP | 10 | 0.339 | 90 | 100 | 180 | 0.04 | 180 | 100 | 180 |
| 4NBP | 4 | 0.285 | 40 | 100 | 180 | 0.02 | 180 | 100 | 180 |
| 4SBP | 10 | 0.315 | 120 | 99.3 | 240 | 0.02 | 180 | 99.3 | 240 |
| 4TBP | 30 | 0.327 | 240 | 99.7 | 300 | 0.03 | 240 | 100 | 180 |
| 4NPenP | 4 | 0.309 | 30 | 100 | 180 | 0 | 120 | 99.3 | 180 |
| 4TPenP | 50 | 0.335 | 180 | 100 | 240 | 0 | 120 | 100 | 90 |
| 4NHexP | 3 | 0.183 | 30 | 99.1 | 180 | 0 | 60 | 100 | 180 |
| 4NHepP | 2 | 0.076 | 60 | 97.5 | 240 | 0 | 120 | 98.4 | 180 |
| 4NOP | 2 | 0.112 | 180 | 97.3 | 180 | 0 | - | 97.0 | 180 |
| 4TOP | 40 | 0.10 | 240 | 100 | 360 | 0 | 120 | 100 | 240 |
| 4NNP | 2 | 0.08 | 240 | 93.3 | 240 | 0 | - | 97.3 | 180 |
| Alkylphenol | [PPO] (U/cm3) | Type of Chitosan | Size (μm) | Reaction Time (min) | Min Abs. | Reaction Time (min) | Maximum Conversion |
|---|---|---|---|---|---|---|---|
| 4NBP | 3.0 | chitosan beads | 50 | <0.01 | 240 | 94.0 | |
| 10–42 | 20 | <0.01 | 240 | 92.7 | |||
| chitosan powder | 42–74 | 30 | 0.01 | 240 | 91.7 | ||
| 74–100 | 60 | 0.01 | 360 | 92.3 | |||
| 4SBP | 8.0 | chitosan beads | 60 | 0.02 | 300 | 97.8 | |
| 10–42 | 50 | 0.01 | 120 | 97.8 | |||
| chitosan powder | 42–74 | 120 | 0.03 | 180 | 93.0 | ||
| 74–100 | 120 | 0.03 | 300 | 88.0 | |||
| 4TBP | 20.0 | chitosan beads | 180 | 0.03 | 300 | 94.0 | |
| 10–42 | 180 | 0.06 | 180 | 100.0 | |||
| chitosan powder | 42–74 | 360 | 0.05 | 240 | 98.2 | ||
| 74–100 | 360 | 0.03 | 240 | 95.2 | |||
| 4NPenP | 20.0 | chitosan beads | 20 | 0 | 300 | 94.8 | |
| 10–42 | 20 | <0.01 | 90 | 97.6 | |||
| chitosan powder | 42–74 | 40 | <0.01 | 120 | 93.4 | ||
| 74-100 | 60 | <0.01 | 300 | 85.0 | |||
| 4TPenP | 20.0 | chitosan beads | 240 | 0 | 240 | 100 | |
| 10–42 | 120 | 0 | 180 | 100 | |||
| chitosan powder | 42–74 | 180 | 0 | 180 | 100 | ||
| 74–100 | 180 | 0 | 90 | 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsushima, M.; Kimura, Y.; Kashiwada, A.; Yamada, K. Removal of Linear and Branched Alkylphenols with the Combined Use of Polyphenol Oxidase and Chitosan. Polymers 2019, 11, 931. https://doi.org/10.3390/polym11060931
Tsushima M, Kimura Y, Kashiwada A, Yamada K. Removal of Linear and Branched Alkylphenols with the Combined Use of Polyphenol Oxidase and Chitosan. Polymers. 2019; 11(6):931. https://doi.org/10.3390/polym11060931
Chicago/Turabian StyleTsushima, Mitsuki, Yuji Kimura, Ayumi Kashiwada, and Kazunori Yamada. 2019. "Removal of Linear and Branched Alkylphenols with the Combined Use of Polyphenol Oxidase and Chitosan" Polymers 11, no. 6: 931. https://doi.org/10.3390/polym11060931
APA StyleTsushima, M., Kimura, Y., Kashiwada, A., & Yamada, K. (2019). Removal of Linear and Branched Alkylphenols with the Combined Use of Polyphenol Oxidase and Chitosan. Polymers, 11(6), 931. https://doi.org/10.3390/polym11060931