In this work, hybrid gels composed of iPS and [Fe(II) (4-octadecyl-1,2,4-triazole)3(ClO4)2]n metallo-organic polymer were prepared and the effect of each component concentration in the gel formation was analyzed. For this study, two sets of hybrid gels were prepared: (i) Hybrid gels with a fixed iPS concentration (4%, g/mL) and variable metallo-organic polymer concentration and (ii) hybrid gels with fixed metallo-organic polymer concentration (1.4%, g/mL) and variable iPS content. The fixed concentrations of 4% for iPS and 1.4% for metallo-organic polymer were selected, taking into account that this is the critical concentration above which each polymer is capable to form gel independently. In order to understand the formation and the properties of the obtained hybrid gels, we studied the thermal, viscoelastic, and morphological properties of the hybrid gels.
3.1. Thermal Properties of Hybrid iPS/Metallo-Organic Polymer Gels
Figure 1a shows the thermogram corresponding to pure iPS gel in cis-decaline (4%, g/mL). As observed from the plot, the iPS gel exhibits two endothermic peaks at 18 and 43 °C upon heating and a single exothermic peak at 6 °C when cooling (See
Table 1). The temperature at which this first endothermic peak occurs is independent of the iPS concentration, and corresponds to the monotectic transformation derived from the phase separation effect involved in the gelation phenomena [
18,
19,
20,
21]. The second peak corresponds to the iPS gel melting temperature, which varies with iPS concentration. As it is known, the iPS/cis-decalin is a “non-equilibrium” system. In this type of system, the speed at which a polymer solution is cooled down in a given solvent governs the formation of a determined molecular structure, with a greater or lower degree of organization at the molecular level. Rapid sub-cooling leads to the formation of the gel, while sufficiently slow cooling results in the formation of the crystalline state. Between these two extremes, there are two crystalline phases: Phase s and phase p. Except for the crystalline state, the remaining phases contain intercalated solvent molecules. The gel state has an order of nematic type, while the phase s presents characteristics of a smectic arrangement, in which the folding of polymer chains begins to occur. Phase p is less solvated and can be defined as a peritectic system [
18,
22,
23].
The DSC curve of the pure metallo-organic polymer gel (0.9%, g/mL) in cis-decaline is presented in
Figure 1b. The thermogram exhibits an exothermic peak at 11 °C when cooling, which is related to gel formation and an endothermic peak of gel melting at 28 °C upon heating.
In
Figure 1c,d, thermograms corresponding to iPS /hybrid gels are shown.
Figure 1c corresponds to a representative hybrid gel prepared from a fixed iPS concentration (4%, g/mL) with variable concentration of metallo-organic polymer (in this case, 5.1%, g/mL). As observed from the figure, the hybrid gel exhibits an endothermic peak at 20 °C and a wide endotherm where two peaks are vaguely noticeable upon heating (at approximately 30 and 43 °C). The temperature of the first peak, 20 °C, is similar to that observed in pure iPS gel thermogram (
Figure 1a); therefore, this peak corresponds to the monotectic transformation of iPS gel. Regarding the two peaks subtly observed in the wide endotherm, the peak appearing at 30 °C coincides with that observed in
Figure 1b corresponding to the melting of the metallo-organic polymer. Similar results are observed in
Figure 1d, where the thermograms corresponding to an example of hybrid gel prepared from a fixed metallo-organic polymer concentration (1.4%, g/mL) and variable content of iPS (5.4%, g/mL, for this representative graph) are shown. Three endothermic peaks are depicted in the graph upon heating: 16, 28, and 38 °C (See
Table 1), which may correspond to the monotectic transformation of iPS gel, the melting temperature of the metallo-organic polymer gel, and melting temperature of the iPS gel, respectively.
In order to better understand the effect of the composition in the formation of the hybrid gel, in
Figure 2a,b, partial phase diagrams are shown. In particular, in
Figure 2a, the partial phase diagram of the hybrid gel with a fixed iPS concentration (4%, g/mL) and the pure metallo-organic polymer gel are shown, both as a function of metallo-organic polymer content. As it can be seen, the melting temperature of the pure metallo-organic polymer gel increases slightly with concentration. As previously studied, this is due to an increase in the crosslink density as the concentration of the polymer increases [
15,
24]. Additionally, it was determined that the physical gel formation was induced by the crystallization of the metallo-organic polymer chains. For the hybrid gel (with a fixed iPS content of 4 g/mL), two different sets of points are represented, each set corresponding to the first and third endothermic peak observed in the thermograms (
Figure 1c). The temperature at which the first peak appears remains constant with the increase of the metallo-organic polymer content, indicating that this first peak corresponds to the monotectic transformation of iPS gel.
The temperature of the third endothermic peak corresponding to the hybrid gel decreases with respect to the peak related to the pure iPS gel at low metallo-organic polymer concentrations. This result is related to the fact that the metallo-organic polymer is not capable of forming a gel on its own at low concentrations, besides hindering the gelation of iPS. Then, the melting temperature stabilizes for intermediate concentrations of metallo-organic polymer (up to 2.6%, g/mL). At such concentrations, the metallo-organic polymer is capable of forming a gel, however the melting temperatures of the iPS in the hybrid gel are still lower than those of the pure iPS gel. Although the hybrid gel is formed, the metallo-organic polymer forms a “weak” gel at this intermediate concentration, and, therefore, iPS dilution phenomena continue to occur.
When the metallo-organic polymer concentration is high enough to form a gel, and the gelation of the iPS is not hampered, the melting temperature of the iPS in the hybrid gel is higher than the melting temperature of the pure iPS gel. At this point, phase separation arises and separate gelation of the two systems occur.
Similar analysis is performed for the results collected in
Figure 2b. In this case, partial phase diagrams as a function of iPS content are shown for pure iPS polymer gel and for hybrid gels with a fixed metallo-organic polymer concentration (1.4%, g/mL). In the case of pure iPS gel, it is observed that both the temperature where the monotectic transition peak appears and the temperature at which the melting peak occurs remain constant with iPS concentration. Regarding the hybrid gel, two series of points corresponding to the first and third peak of the thermograms (
Figure 2b) are plotted. From the graph, it is observed that: i) The temperature of the first peak remains constant with the concentration of iPS and ii) the third peak, which also remains constant, only appears above iPS concentrations of 3.5% (g/mL).
As mentioned before, the third peak observed upon heating in the thermograms corresponds to the melting of the iPS gel. The fact that melting temperature of the hybrid gel occurs at temperatures lower than those of iPS gel responds to a dilution phenomenon derived from the presence of the metal-organic polymer in the mixture. However, the temperature at which this peak appears hardly varies with the concentration of iPS, which could indicate the slight influence of the metallo-organic polymer on the gelation of the hybrid system.
Therefore, the obtained results indicate that the hybrid gels are formed by the independent gelation of both systems: iPS/cis-decalin and metallo-organic polymer/cis-decalin. Thus, the limiting parameter for the formation of the hybrid gel is the critical concentration of each polymer independently. Therefore, the hybrid gel will be formed as long as the two polymers are in concentrations above the critical gelation concentrations, which increase in relation to the pure components.
3.2. Viscoelastic Properties of the Hybrid iPS/Metallo-Organic Polymer Gels—Effect of iPS and Metallo-Organic Polymer Concentrations
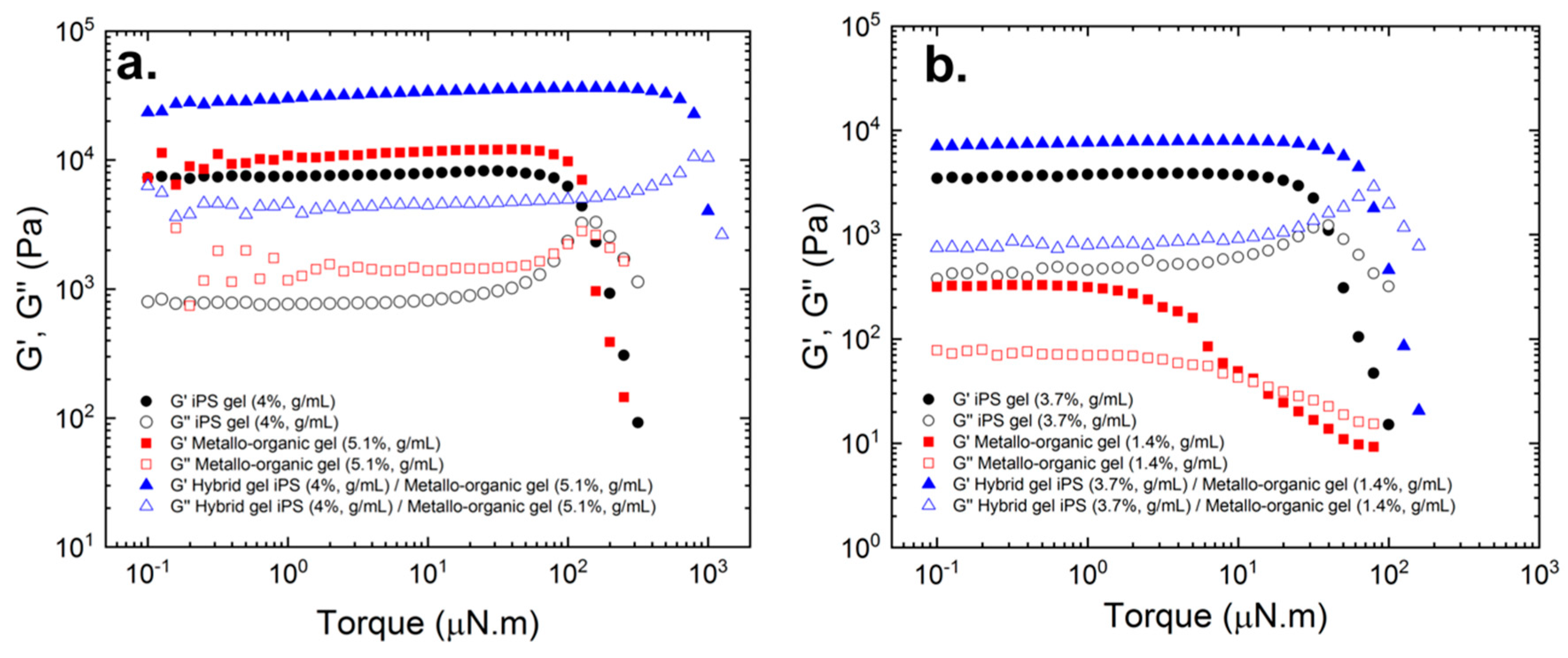
In
Figure 3a,b, the evolution of storage G’ and loss modulus G’’ with the oscillation torque is described for pure iPS gels, pure metallo-organic polymer gels, and for hybrid iPS/metallo-organic polymer gels. The three gels describe a common rheological comportment showing G’ higher than G’’, which is indicative of a solid-like behavior. G’ and G’’ show a plateau up to a critical torque value where both moduli decrease abruptly and crossover modulus (G’ = G’’). The decreasing and crossover of both moduli entails the transition from a solid-like to liquid-like behavior. From these graphs, we could also determine the linear viscoelastic regime (LVR) where the values of G’ and G’’ are independent of the applied torque [
16]. As observed from
Figure 3a,b, the values of the storage modulus and the critical torque of the hybrid gels are greater than those of the pure polymers separately. This suggests the interaction or interpenetration of the two networks.
In
Figure 4a,b, the evolution of storage and loss modulus with temperature for the pure iPS gel and hybrid iPS/metallo-organic polymer gels is represented. If we focus on the temperature dependence of iPS (
Figure 4a,b), two pseudo-equilibrium plateaus can be observed, where G’ is independent of the temperature. Between the two plateaus there is a transition, detected by the presence of a maximum in the loss tangent (tan δ) at 18 °C. At higher temperatures, the drop of the modulus and subsequent crossover occurs, which marks the melting of the gel (53 °C). The first transition could correspond to the monotectic transition of the iPS gel, also observed in the thermogram of the pure iPS gel (
Figure 1a). The temperature at which this transition occurs is different depending on the experimental technique used to determine it. Temperatures determined by rheometry and DSC do not coincide, since the speeds at which the heating scans have been carried out are not the same. In DSC, a speed of 5 °C/min has been used, while in rheometry, the speed has been 2.5 °C/min. From the analysis performed on the hybrid gel with a fixed concentration of iPS (4%, g/mL) and variable concentration of metallo-organic polymer (5.1%, g/mL), two plateaus defining two pseudo-equilibrium storage moduli are also observed. In this case, this first transition between both plateaus, also determined by a maximum of the loss tangent, occurs at 25 °C. The crossover of the modulus determining the melting of the hybrid gel occurs at the same temperature as pure iPS gel, 52 °C. Similar conclusions are obtained when comparing pure iPS (4%, g/mL) with the hybrid gel with a fixed concentration of metallo-organic polymer (1.4%, g/mL) and variable concentration of iPS (3.7%, g/mL) (
Figure 4b). A difference is only found in the temperature at which the melting takes place: In the hybrid gel, melting occurs at 33 °C, being significantly lower than the melting temperature of the pure iPS gel (53 °C).
In
Figure 5a, we have represented the equilibrium storage modulus G’ as a function of metallo-organic polymer concentration for hybrid gels with fixed iPS concentration (4%, g/mL) and for pure metallo-organic polymer gels. As it can be observed, the equilibrium storage modulus of pure metal-organic polymer gels increase with concentration; besides, the value of storage modulus appears to be above the equilibrium storage modulus of the pure iPS gel of 4% (g/mL) at a concentration of metal-organic polymer close to 3.6% (g/mL). In the case of the hybrid gel (with fixed iPS concentration), the equilibrium storage modulus decreases up to a concentration of 0.5% (g/mL), from which it begins to increase, ending at a value that is higher than the one shown by pure iPS gel (4%, g/mL) above a metallo-organic polymer concentrations of 1% (g/mL). The equilibrium storage modulus of the hybrid gels is always higher than those of the pure metal-organic polymer gels with the same metallo-organic polymer concentration. However, the equilibrium storage modulus of the hybrid gels with metallo-organic polymer concentrations below 1% (g/mL) are lower than the equilibrium value of the pure iPS gel of 4% (g/mL). At such low concentrations (>1%, g/mL), the metallo-organic polymer is not able to form gel on its own, furthermore preventing the gelation of the iPS. This could be explained by a hindrance of the rigidification of the iPS chains or a process of dilution of the iPS in the mixture because of the presence of metallo-organic polymer. When the concentration of metallo-organic polymer in the hybrid gel is enough for the its gelation to take place, then the equilibrium storage modulus increases. This augment could be due to the formation of an interpenetrated network of iPS and metallo-organic polymer gels.
We also analyzed the effect of iPS concentration in the equilibrium storage modulus of hybrid iPS/metallo-organic polymer gel with fixed metallo-organic polymer content (1.4%, g/mL). As observed from
Figure 5b, the equilibrium storage modulus of pure iPS gels increases with the concentration of iPS. In the case of the hybrid gels, those with an iPS concentration below 1.2% (g/mL) have lower equilibrium storage modulus values than the pure metal-organic polymer gel. At iPS concentrations below 1.2% (g/mL), the iPS is not capable of gelifying and it could probably inhibit the gelation of the metallo-organic polymer. However, above 1.2% iPS concentration, hybrid gels present equilibrium storage modulus higher than the storage modulus of the pure metallo-organic polymer gel. Moreover, above iPS concentration of 3.5%, g/mL, the equilibrium storage modulus of both pure iPS gel and hybrid gel become similar. This could be due to the fact that the value of the equilibrium storage modulus of the pure metal-organic polymer gel is too low to contribute significantly to the value of the equilibrium modulus of the hybrid gel.
In
Figure 6, the double logarithmic plots of the equilibrium storages modulus as a function of metallo-organic polymer concentration (
Figure 6a) and iPS concentration (
Figure 6b) are represented for both hybrid gels and pure metallo-organic polymer and iPS gels, respectively. The obtained plots have been analyzed using a theoretical model based on de Gennes scaling law, in which the moduli are related to the concentration as follows: G α C
n [
25]; n being an exponent that depends on the conformation of the polymer chains linking the connection points.
As it has already been indicated in our previous works, these correlations were developed for chemical gels but they were already applied for physical gels [
15]. Since the storage modulus of the studied gels describe two pseudo-equilibrium moduli where G ≠ G(T), it is possible to indicate that the obtained gels are formed by networks presenting enthalpic elasticity. For this type of network, there is an approximation that correlates the equilibrium storage modulus with the concentration through the fractal dimension (υ-1) of the objects linking the connection points: G α C
(3υ + 1)/(3υ − 1) [
26]. We have applied this approximation to pure metallo-organic polymer (
Figure 6a) and pure iPS gels (
Figure 6b) as well as to the hybrid gels with fixed iPS polymer concentration 4%, g/mL (
Figure 6a) and fixed metallo-organic polymer concentration 1.4%, g/mL (
Figure 6b) and obtained the fractal dimensions shown in
Table 2.
In the case of pure iPS gel, the obtained value is 1.5, which would correspond to polymer fibers swollen by the solvent. If we focus on the fractal dimensions of the hybrid gels, the obtained values (1.4 for hybrid gels with fixed iPS concentration and 1.5 for hybrid gels with fixed metallo-organic polymer concentration) are similar to the fractal dimension of pure iPS gel, and far from the fractal dimension of the pure metallo-organic polymer gel (2.5) [
15]. This would indicate that the iPS determines the structure and the viscoelastic properties of the hybrid gel, even though the two networks are formed independently.
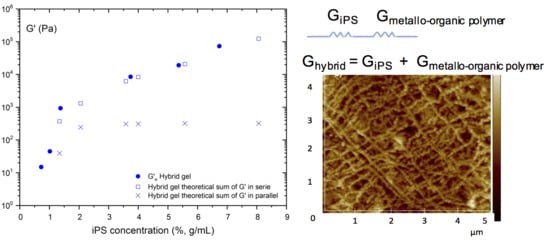
When a material is deformed, its elastic behavior can be outlined simply by the Hook’s law. However, if the material is multicomponent, as it is the case for the hybrid gel that is composed of two polymeric gels (iPS and metallo-organic polymer), there is more than one force constant responsible for the final module of the system. Therefore, it is necessary to find a model that could consider these constants so that the resulting force constant describes the elastic behavior of the hybrid gel. Therefore, in order to determine the contribution that each individual gel (iPS gel and metallo-organic polymer gel) has in the storage modulus of the hybrid gel, we have suggested the following two theoretical models. (i) Sum of the individual gel modulus in series: The force constants will act independently against an external force, initially deforming the less rigid spring, and later, the one with the highest rigidity (see the corresponding scheme in
Figure 7). (ii) Sum of the modules of the individual gels in parallel: The force constants of the individual gels are interdependent, in such a way that the elastic response of the material is limited by the system whose force constant is higher (see the corresponding scheme in
Figure 7).
In
Figure 7a,b, experimentally obtained equilibrium storage moduli of the hybrid gel are compared with the values determined through the application the two theoretical models from the experimental data. As concluded from both graphs, the theoretical model that best describes the experimental elastic behavior of the hybrid gel is the one that considers the elastic behavior of the hybrid gel as the sum of individual gel’s storage modulus in series. That is, the hybrid gel would consist of two networks of pure polymers independent of each other.
For the sake of comparison, in
Figure 8a, we have collected the melting temperatures (Tm) obtained for pure metallo-organic polymer gels and iPS/metallo-organic polymer hybrid gels with a fixed iPS concentration of 4%, g/mL and represented as a function of metallo-organic polymer concentration (%, g/mL). From the figure, we observe how the melting temperature values of the metallo-organic polymer gels increase with the concentration in the studied range of concentrations. In contrast, the melting temperatures of the hybrid gels decreases up to a concentration of metallo-organic polymer of 0.4%, g/mL; beyond this concentration, the melting temperatures increase to values slightly above the pure iPS gel of 4% (g/mL). The melting temperatures of the hybrid gels are lower than those of the pure iPS gel (4%, g/mL) in almost the entire range of metallo-organic polymer concentrations. Therefore, at low metallo-organic polymer concentrations, dilution phenomena may occur, preventing the gelation of the iPS, which causes the melting temperature of the hybrid gel to decrease. This confirms the idea of the formation of the hybrid gels, from the gelation of both iPS and metallo-organic polymers independently, as long as the two polymers are in concentrations above the critical gelation concentrations.
Figure 8b shows the melting temperatures of pure iPS gels and hybrid iPS/metallo-organic polymer gel with a fixed metallo-organic polymer concentration (1.4%, g/mL) as a function of iPS concentration. As observed, melting temperature values of pure iPS gels increase with the concentration of iPS. This increase seems to stabilize at concentrations of 4%, g/mL, above which Tm keeps constant. Similar to what occurs for pure iPS gels, the melting temperatures of the hybrid gels also increase with iPS concentration until it reaches a concentration (close to 4%, g/mL) at which it remains constant. Besides, hybrid gels present melting temperatures higher than the pure metallo-organic polymer gel of 1.4%, (g/mL) (18 °C) but significantly lower than the melting temperature values of the pure iPS gels at each iPS concentration.
When the concentration of iPS in the hybrid gel is not enough for the polymer to undergo gelation on its own, what we are really observing is the melting of the metallo-organic polymer. But, with enough concentration of iPS to gelate on its own, the melting temperature trend observed for the hybrid gels is the same as that of the pure iPS gels. The main difference is that the melting temperature values of hybrid gels are much lower than those of pure iPS gels. It is probably that the observed melting may correspond to the iPS gel, but the temperatures at which this melting occurs are lower due to a dilution effects in the blend.