Preparation of PolyHIPE Scaffolds for 3D Cell Culture and the Application in Cytotoxicity Evaluation of Cigarette Smoke
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
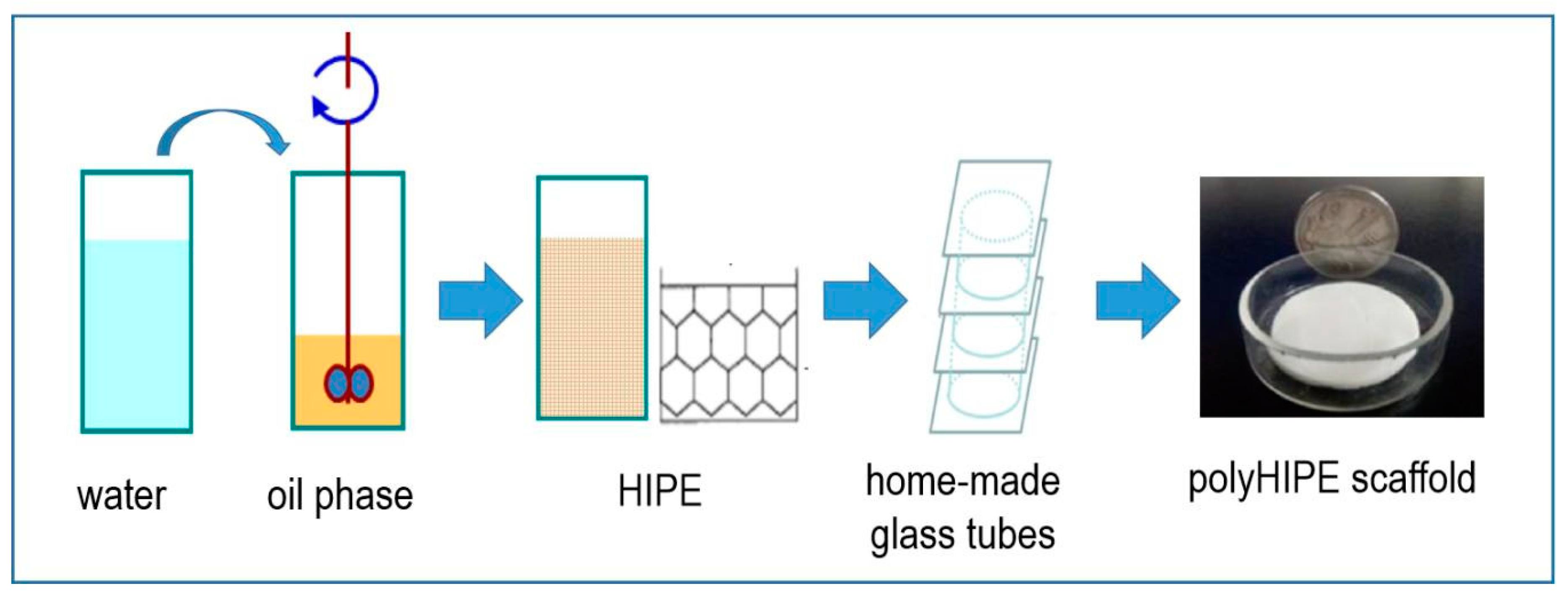
2.2. Preparation of the PolyHIPE Scaffolds
2.3. Characterization of PolyHIPE Scaffolds
2.4. Cell Culture and Cell Proliferation
2.5. Cytotoxicity Evaluation of the Cigarette Smoke
2.5.1. Cigarette Smoke TPM Collection
2.5.2. Cytotoxicity Evaluation of Cigarette Smoke
3. Results and Discussion
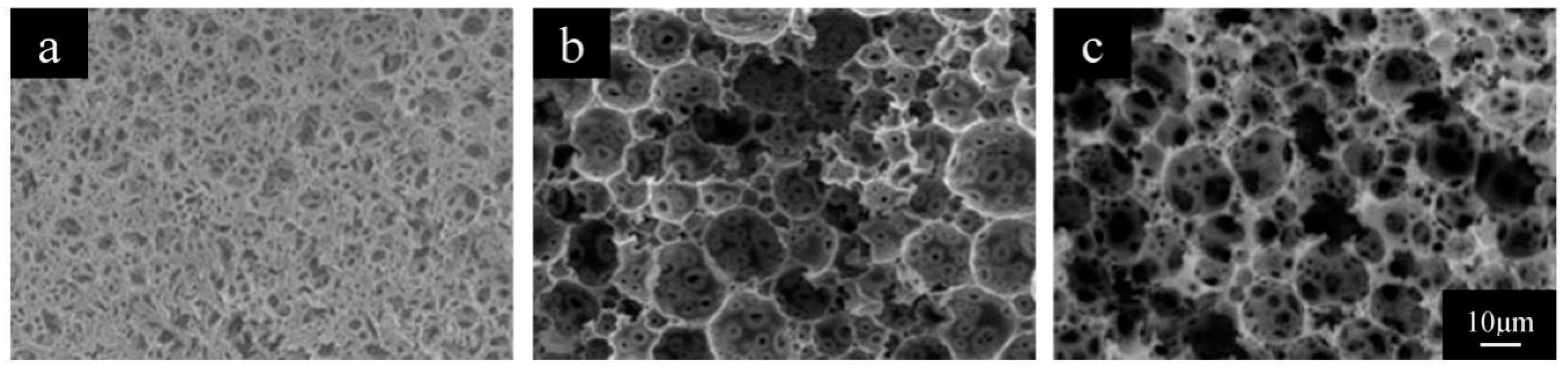
3.1. Preparation of the PolyHIPE Materials
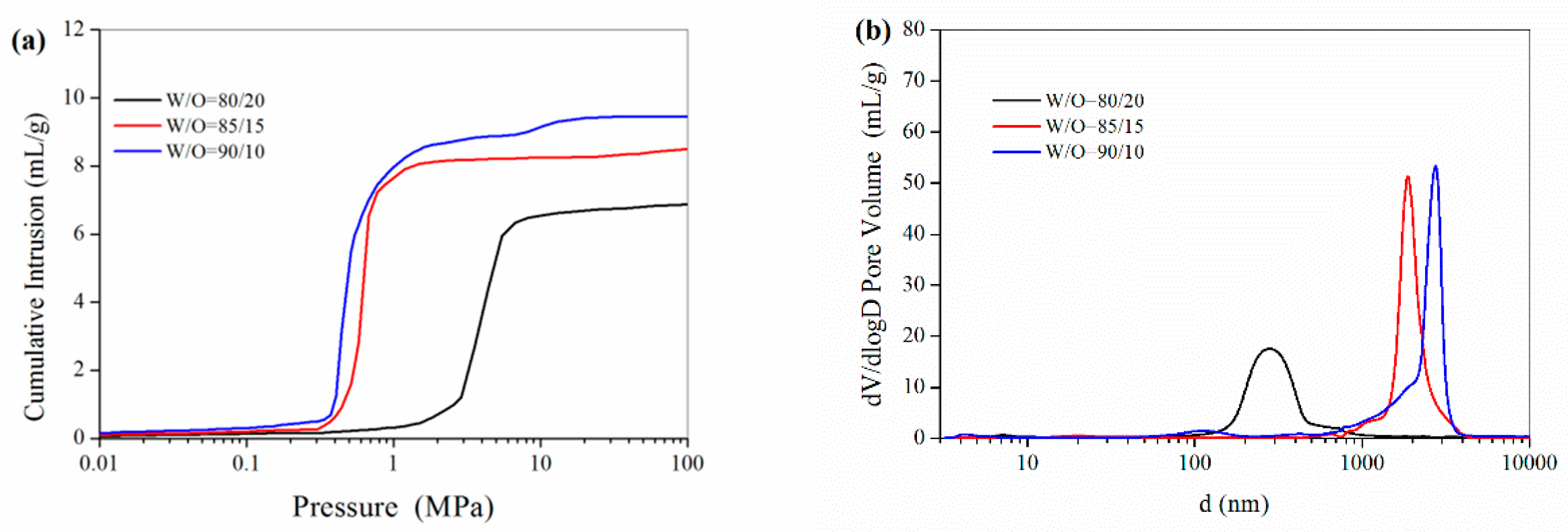
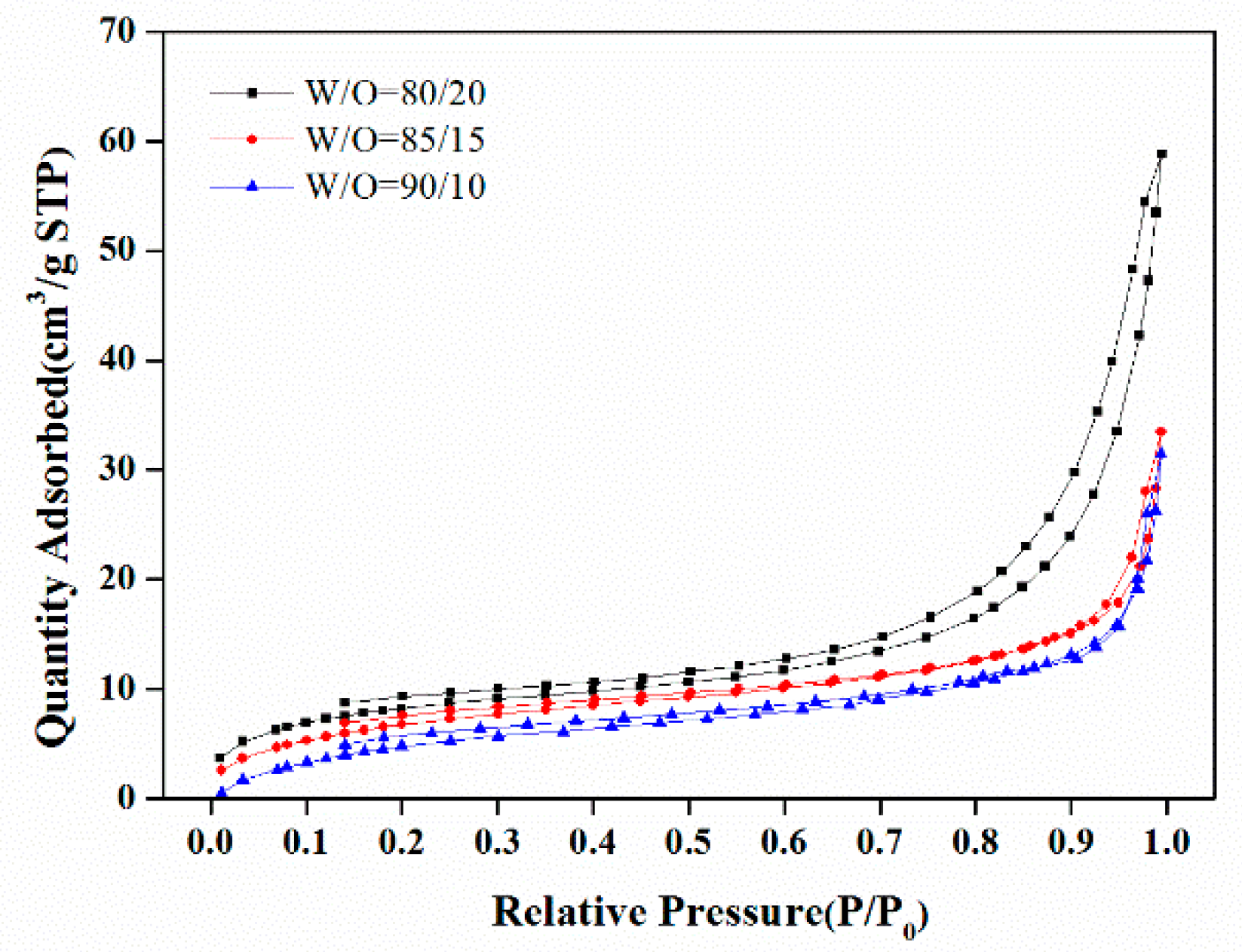
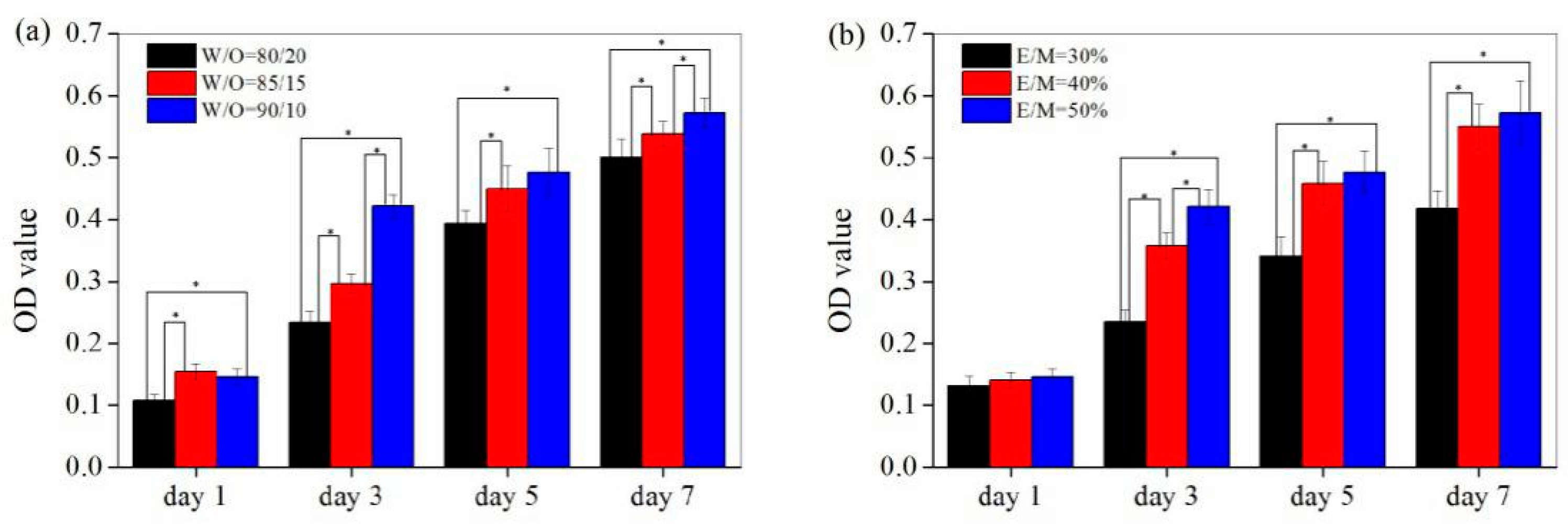
3.1.1. The W/O Ratio
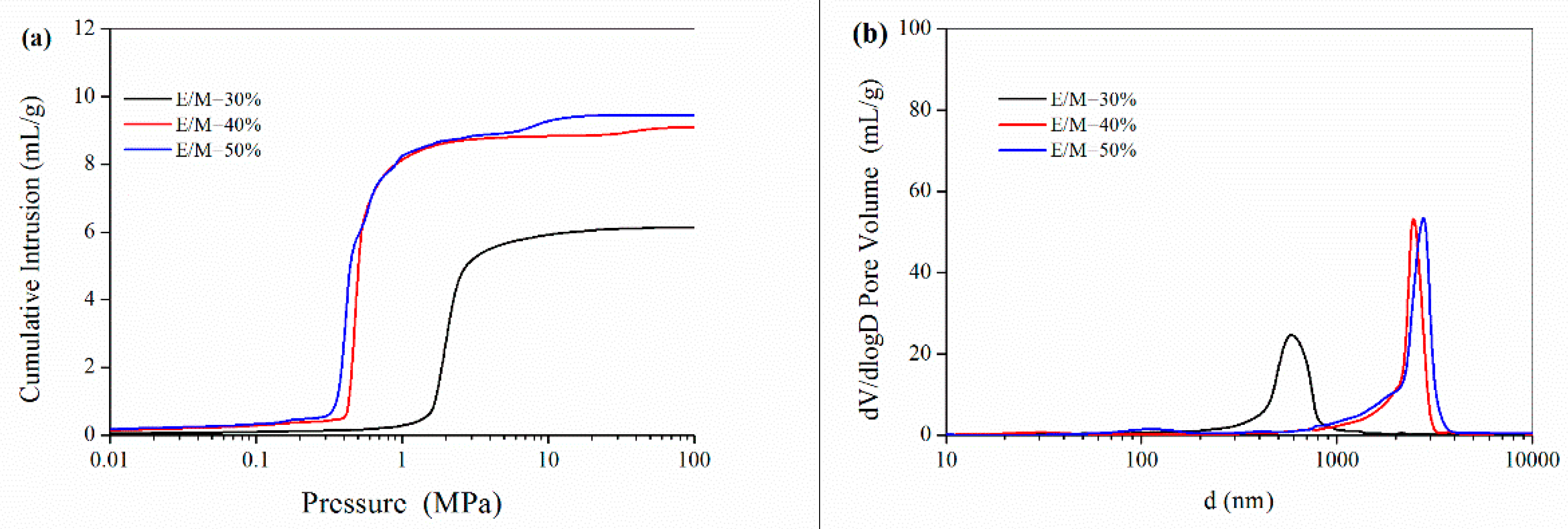
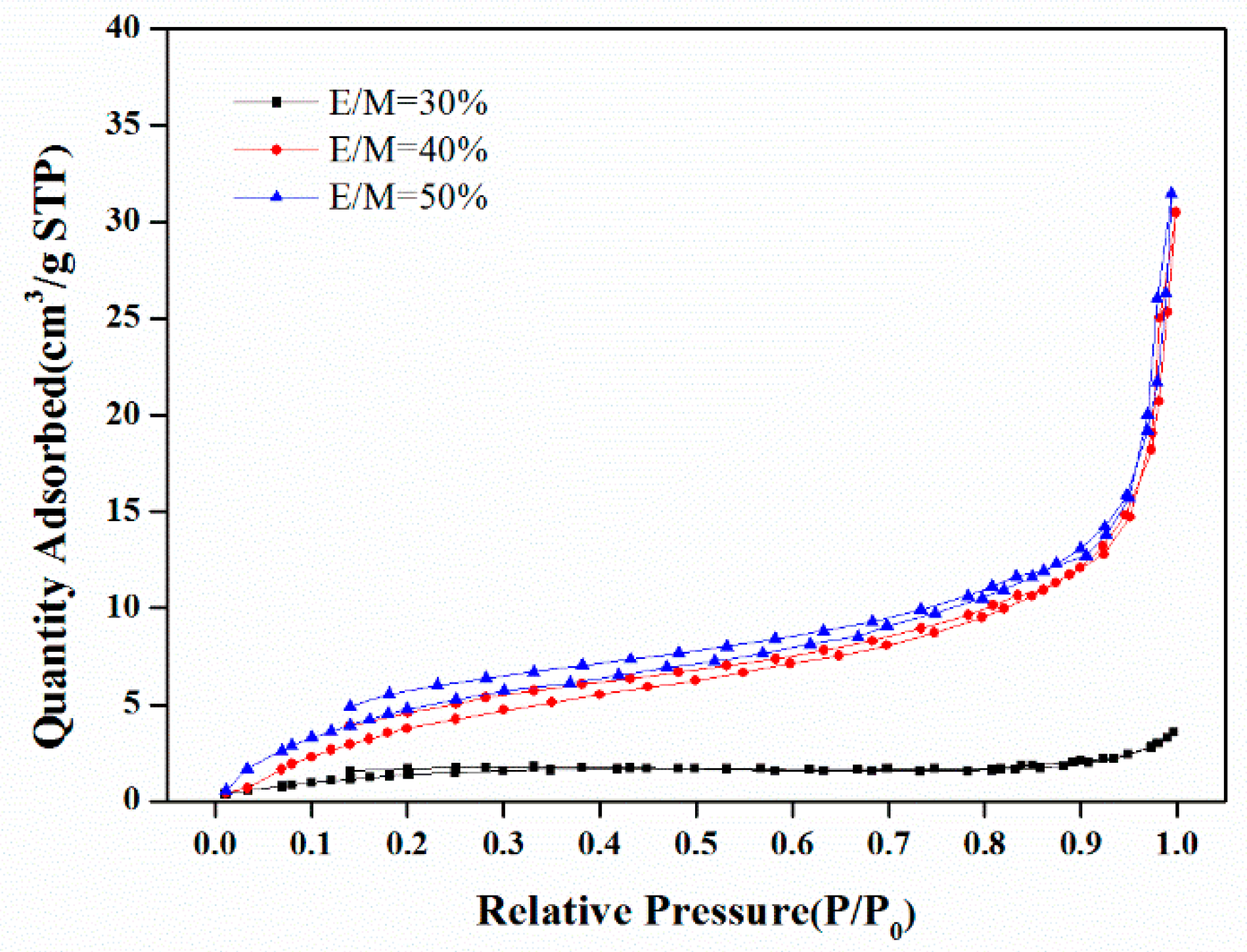
3.1.2. The Emulsifier Amount
3.2. Cell Proliferation on the PolyHIPE Scaffolds
3.3. Application of the PolyHIPE Scaffolds in Cytotoxicity Evaluation of Cigarette Smoke
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rodgman, A.; Perfetti, T.A. The Chemical Components of Tobacco and Tobacco Smoke; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2008; pp. 1–928. [Google Scholar]
- Sun, P.; Yang, S.; Sun, X.; Wang, Y.; Pan, L.; Wang, H.; Wang, X.; Guo, J.; Nie, C. Functional porous carboxymethyl cellulose/cellulose acetate composite microspheres: Preparation, characterization, and application in the effective removal of HCN from cigarette smoke. Polymers 2019, 11, 181. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, C.; Gigante, D.; Nunziata, A. A review of in vitro methods to assess the biological activity of tobacco smoke with the aim of reducing the toxicity of smoke. Toxicol. In Vitro 2003, 17, 587–594. [Google Scholar] [CrossRef]
- Chen, H.; Cui, L.; Jiang, X.Y.; Pang, Y.Q.; Tang, G.L.; Hou, H.W.; Jiang, J.H.; Hu, Q.Y. Evaluation of the cytotoxicity of cigarette smoke condensate by a cellular impedance biosensor. Food Chem. Toxicol. 2012, 50, 612–618. [Google Scholar] [CrossRef]
- Zhang, L.; Ning, M.; Xu, Y.; Wang, C.; Zhao, G.; Cao, Q.; Zhang, J. Predicting the cytotoxic potency of cigarette smoke by assessing the thioredoxin reductase inhibitory capacity of cigarette smoke extract. Int. J. Environ. Res. Public Health 2016, 13, 348. [Google Scholar] [CrossRef] [PubMed]
- Combes, R.; Scott, K.; Crooks, I.; Dillon, D.; Meredith, C.; McAdam, K.; Proctor, C. The in vitro cytotoxicity and genotoxicity of cigarette smoke particulate matter with reduced toxicant yields. Toxicol. In Vitro 2013, 27, 1533–1541. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Nie, C.; Shang, P.; Xie, F.; Liu, H.; Xie, J. Evaluation method for the cytotoxicity of cigarette smoke by in vitro whole smoke exposure. Exp. Toxicol. Pathol. 2014, 66, 27–33. [Google Scholar] [CrossRef]
- Xin, X.; Yang, H.; Zhang, F.; Yang, S. 3D cell coculture tumor model: A promising approach for future cancer drug discovery. Process Biochem. 2019, 78, 148–160. [Google Scholar] [CrossRef]
- Griffith, L.G.; Swartz, M.A. Capturing complex 3D tissue physiology in vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211. [Google Scholar] [CrossRef] [PubMed]
- Czekala, L.; Simms, L.; Stevenson, M.; Tschierske, N.; Maione, A.G.; Walele, T. Toxicological comparison of cigarette smoke and e-cigarette aerosol using a 3D in vitro human respiratory model. Regul. Toxicol. Pharm. 2019, 103, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839. [Google Scholar] [CrossRef] [PubMed]
- Thoms, S.; Ali, A.I.; Jonczyk, R.; Scheper, T.; Blume, C. Tacrolimus inhibits angiogenesis and induces disaggregation of endothelial cells in spheroids–toxicity testing in a 3D cell culture approach. Toxicol. In Vitro 2018, 53, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, M.; Piccinini, F.; Arienti, C.; Zamagni, A.; Santi, S.; Polico, R.; Bevilacqua, A.; Tesei, A. 3D tumor spheroid models for in vitro therapeutic screening: A systematic approach to enhance the biological relevance of data obtained. Sci. Rep. 2016, 6, 19103. [Google Scholar] [CrossRef]
- Lee, G.Y.; Kenny, A.P.; Lee, A.E.; Bissell, M.J. Three-dimensional culture models of normal and malignant breast epithelial cells. Nat. Methods 2007, 4, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Bazou, D. Biochemical properties of encapsulated high-density 3-D HepG2 aggregates formed in an ultrasound trap for application in hepatotoxicity studies: Biochemical responses of encapsulated 3-D HepG2 aggregates. Cell Biol. Toxicol. 2010, 26, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Paulsolomon, F.D. 3D Cell Culture Systems: Advantages and Applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef]
- Cukierman, E.; Pankov, R.; Stevens, D.R.; Yamada, K.M. Taking cell-matrix adhesions to the third dimension. Science 2001, 29, 1708–1712. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Mokhtari, H.; Kharaziha, M.; Karimzadeh, F.; Tavakoli, S. An injectable mechanically robust hydrogel of Kappa-carrageenan-dopamine functionalized graphene oxide for promoting cell growth. Carbohy. Polym. 2019, 214, 234–249. [Google Scholar] [CrossRef]
- Suo, A.; Xu, W.; Wang, Y.; Sun, T.; Ji, L.; Qian, J. Dual-degradable and injectable hyaluronic acid hydrogel mimicking extracellular matrix for 3D culture of breast cancer MCF-7 cells. Carbohy. Polym. 2019, 211, 336–348. [Google Scholar] [CrossRef]
- Chia, H.; Wu, B. Recent advances in 3D printing of biomaterials. J. Boil. Eng. 2015, 9, 4. [Google Scholar] [CrossRef]
- Park, T.Y.; Yang, Y.J.; Ha, D.H.; Cho, D.W.; Cha, H.J. Marine-derived natural polymer-based bioprinting ink for biocompatible, durable, and controllable 3D constructs. Biofabrication 2019, 11, 35001. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Shi, X.D.; Gan, Z.H.; Wang, F.S. Modification of porous PLGA microspheres by poly-L-lysine for use as tissue engineering scaffolds. Colloids Surf. B Biointerfaces 2018, 161, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Cui, L.; Sun, H.; Jiang, N.; Heng, L.; Zhuang, X.; Gan, Z.; Chen, X. Promoting cell growth on porous PLA microspheres through simple degradation methods. Polym. Degrad. Stab. 2019, 161, 319–325. [Google Scholar] [CrossRef]
- Sill, T.J.; von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Sherborne, C.; Owen, R.; Reilly, G.C.; Claeyssens, F. Light-based additive manufacturing of PolyHIPEs: Controlling the surface porosity for 3D cell culture applications. Mater. Des. 2018, 156, 494–503. [Google Scholar] [CrossRef]
- Richardson, S.A.; Rawlings, T.M.; Muter, J.; Walker, M.; Brosens, J.J.; Cameron, N.R.; Eissa, A.M. Covalent attachment of fibronectin onto emulsion-templated porous polymer scaffolds enhances human endometrial stromal cell adhesion, infiltration, and function. Macromol. Biosci. 2019, 19, 1800351. [Google Scholar] [CrossRef]
- Lee, A.; Langford, C.R.; Rodriguez-Lorenzo, L.M.; Thissen, H.; Cameron, N.R. Bioceramic nanocomposite thiol-acrylate polyHIPE scaffolds for enhanced osteoblastic cell culture in 3D. Biomater. Sci. 2017, 5, 2035–2047. [Google Scholar] [CrossRef]
- Naranda, J.; Sušec, M.; Maver, U.; Gradišnik, L.; Gorenjak, M.; Vukasović, A.; Ivković, A.; Rupnik, M.S.; Vogrin, M.; Krajnc, P. Polyester type polyHIPE scaffolds with an interconnected porous structure for cartilage regeneration. Sci. Rep. 2016, 6, 28695. [Google Scholar] [CrossRef]
- Sušec, M.; Liska, R.; Russmüller, G.; Kotek, J.; Krajnc, P. Microcellular open porous monoliths for cell growth by thiol-ene polymerization of low-toxicity monomers in high internal phase emulsions. Macromol. Biosci. 2015, 15, 253–261. [Google Scholar] [CrossRef]
- Mikos, A.G.; Sarakinos, G.; Leite, S.M.; Vacant, J.P.; Langer, R. Laminated three-dimensional biodegradable foams for use in tissue engineering. Biomaterials 1993, 14, 323–330. [Google Scholar] [CrossRef]
- Tan, H.; Wei, J.; Sun, G.; Mu, C.; Lin, W.; Ngai, T. Interconnected macroporous 3D scaffolds templated from gelatin nanoparticle-stabilized high internal phase emulsions for biomedical applications. Soft Matter 2017, 13, 3871–3878. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Gu, X.; Yang, Y.; Huang, J.; Hu, M.; Chen, W.; Tong, Z.; Wang, C. Facile fabrication of poly (L-lactic acid)-grafted hydroxyapatite/poly (lactic-co-glycolic acid) scaffolds by pickering high internal phase emulsion templates. ACS Appl. Mater. Interfaces 2014, 6, 17166–17175. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Han, W.; Chen, Y.; Zou, R.; Ouyang, Y.; Zhou, W.; Yang, Z.; Wang, C. One-pot fabrication of poly (ε-caprolactone)-incorporated bovine serum albumin/calcium alginate/hydroxyapatite nanocomposite scaffolds by high internal phase emulsion templates. Macromol. Mater. Eng. 2017, 302, 1600367. [Google Scholar] [CrossRef]
- Barbetta, A.; Dentini, M.; Zannoni, E.M.; De Stefano, M.E. Tailoring the porosity and morphology of gelatin-methacrylate polyHIPE scaffolds for tissue engineering applications. Langmuir 2005, 21, 12333–12341. [Google Scholar] [CrossRef] [PubMed]
- Hayward, A.S.; Sano, N.; Przyborski, S.A.; Cameron, N.R. Acrylic-acid-functionalized PolyHIPE scaffolds for use in 3D cell culture. Macromol. Rapid Comm. 2013, 34, 1844–1849. [Google Scholar] [CrossRef] [PubMed]
- Schutte, M.; Fox, B.; Baradez, M.O.; Devonshire, A.; Minguez, J.; Bokhari, M.; Przyborski, S.; Marshall, D. Rat primary hepatocytes show enhanced performance and sensitivity to acetaminophen during three-dimensional culture on a polystyrene scaffold designed for routine use. Assay Drug Dev. Technol. 2011, 9, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Cameron, N.R.; Sherrington, D.C.; Albiston, L.; Gregory, D.P. Study of the formation of the open-cellular morphology of poly (styrene/divinylbenzene) polyHIPE materials by cryo-SEM. Colloid Polym. Sci. 1996, 274, 592–595. [Google Scholar] [CrossRef]
- Yang, S.; Zeng, L.; Wang, Y.; Sun, X.; Sun, P.; Liu, H.; Nie, C.; Liu, H. Facile approach to glycidyl methacrylate-based polyHIPE monoliths with high epoxy-group content. Colloid Polym. Sci. 2014, 292, 2563–2570. [Google Scholar] [CrossRef]
- Jin, Q.; Jin, P.; Tan, K.; Jin, L.; Wang, R.; Li, F.; Zeng, L.; Luo, Z. Using 3-D Model to Study Cigarette Smoke Extract (CSE) Interaction with A549 Cells. Genom. Appl. Biol. (Chin.) 2017, 36, 3482–3491. [Google Scholar]
- Luo, Z.; Yue, Y.; Zhang, Y.; Yuan, X.; Gong, J.; Wang, L.; He, B.; Liu, Z.; Sun, Y.; Liu, J.; et al. Designer D-form self-assembling peptide nanofiber scaffolds for 3-dimensional cell cultures. Biomaterials 2013, 34, 4902–4913. [Google Scholar] [CrossRef]


| Formulation No. | St (mL) | DVB (mL) | Span80 (mL) | AIBN (g) | H2O (mL) | W/O a | E/M b |
|---|---|---|---|---|---|---|---|
| 1 | 5.00 | 0.80 | 2.90 | 0.25 | 34.8 | 80/20 | 50% |
| 2 | 5.00 | 0.80 | 2.90 | 0.25 | 49.3 | 85/15 | 50% |
| 3 | 5.00 | 0.80 | 2.90 | 0.25 | 78.3 | 90/10 | 50% |
| 4 | 5.00 | 0.80 | 2.30 | 0.25 | 72.9 | 90/10 | 40% |
| 5 | 5.00 | 0.80 | 1.70 | 0.25 | 68.0 | 90/10 | 30% |
| Formulation No. | BET Surface Area (m2/g) | Pore Volume (mL) a | Porosity (%) a | D (μm) b | D (μm) c | Dw (nm) d |
|---|---|---|---|---|---|---|
| 1 | 30.5 | 7.02 | 89.8 | 6.5 | 0.31 | 15.2 |
| 2 | 26.5 | 8.49 | 94.8 | 11.2 | 2.00 | 19.0 |
| 3 | 24.6 | 9.61 | 95.3 | 12.0 | 2.61 | 21.5 |
| 4 | 23.7 | 9.08 | 86.4 | 15.8 | 2.56 | 23.2 |
| 5 | 6.3 | 6.15 | 87.9 | 23.7 | 0.60 | 58.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, P.; Yang, S.; Sun, X.; Wang, Y.; Jia, Y.; Shang, P.; Tian, H.; Li, G.; Li, R.; Zhang, X.; et al. Preparation of PolyHIPE Scaffolds for 3D Cell Culture and the Application in Cytotoxicity Evaluation of Cigarette Smoke. Polymers 2019, 11, 959. https://doi.org/10.3390/polym11060959
Sun P, Yang S, Sun X, Wang Y, Jia Y, Shang P, Tian H, Li G, Li R, Zhang X, et al. Preparation of PolyHIPE Scaffolds for 3D Cell Culture and the Application in Cytotoxicity Evaluation of Cigarette Smoke. Polymers. 2019; 11(6):959. https://doi.org/10.3390/polym11060959
Chicago/Turabian StyleSun, Peijian, Song Yang, Xuehui Sun, Yipeng Wang, Yunzhen Jia, Pingping Shang, Haiying Tian, Guozheng Li, Ruyang Li, Xiaobing Zhang, and et al. 2019. "Preparation of PolyHIPE Scaffolds for 3D Cell Culture and the Application in Cytotoxicity Evaluation of Cigarette Smoke" Polymers 11, no. 6: 959. https://doi.org/10.3390/polym11060959
APA StyleSun, P., Yang, S., Sun, X., Wang, Y., Jia, Y., Shang, P., Tian, H., Li, G., Li, R., Zhang, X., & Nie, C. (2019). Preparation of PolyHIPE Scaffolds for 3D Cell Culture and the Application in Cytotoxicity Evaluation of Cigarette Smoke. Polymers, 11(6), 959. https://doi.org/10.3390/polym11060959





