Adsorption, Antibacterial and Antioxidant Properties of Tannic Acid on Silk Fiber
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Adsorption Experiments
2.2.1. Effect of pH and Temperature on the Adsorption of Tannic Acid
2.2.2. Adsorption Kinetics and Adsorption Isotherms of Tannic Acid
2.2.3. Building-Up Property of Tannic Acid
2.3. Measurements
2.3.1. Uptake of Tannic Acid on Silk
2.3.2. Whiteness and Yellowness Indexes
2.3.3. Antioxidant Activity
2.3.4. Antibacterial Activity
2.3.5. Washing Durability
3. Results and Discussion
3.1. Adsorption Properties of Tannic Acid on Silk
3.1.1. pH and Temperature Dependence of Tannic Acid Adsorption
3.1.2. Adsorption Kinetics of Tannic Acid
3.1.3. Equilibrium Adsorption Isotherms of Tannic Acid
3.1.4. Building-Up Property
3.2. Whiteness and Yellowness Indexes of the Tannic Acid Treated Silk
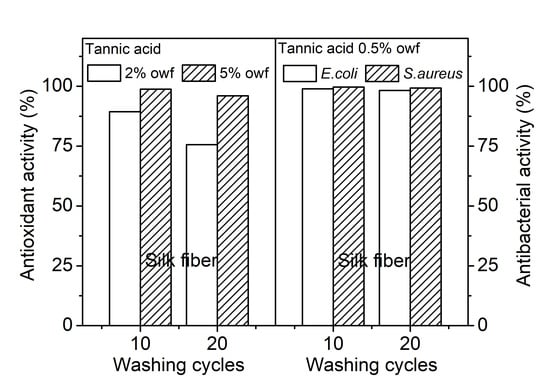
3.3. Antioxidant Property of the Tannic Acid Treated Silk
3.4. Antibacterial Property of the Tannic Acid Treated Silk
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shahid, M.; Mohammad, F. Perspectives for natural product based agents derived from industrial plants in textile applications—A review. J. Clean. Prod. 2013, 57, 2–18. [Google Scholar] [CrossRef]
- Shahid, M.; Mohammad, F. Recent advancements in natural dye applications: A review. J. Clean. Prod. 2013, 53, 310–331. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, R.-C. Natural flavonoid-functionalized silk fiber presenting antibacterial, antioxidant, and UV protection performance. ACS Sustain. Chem. Eng. 2017, 5, 10518–10526. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.R.; Varma, A.J. Functional nanoparticles obtained from cellulose: Engineering the shape and size of 6-carboxycellulose. Chem. Commun. 2013, 49, 8818–8820. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.R.; Kamble, S.; Sarkar, D.; Anand, A.; Varma, A.J. Shape and size engineered cellulosic nanomaterials as broadspectrum anti-microbial compounds. Int. J. Biol. Macromol. 2016, 87, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Son, K.; Yoo, D.I.; Shin, Y. Fixation of vitamin E microcapsules on dyed cotton fabrics. Chem. Eng. J. 2014, 239, 284–289. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Z.-Y.; Tang, R.-C. Bioactive and UV protective silk materials containing baicalin—The multifunctional plant extract from Scutellaria baicalensis Georgi. Mater. Sci. Eng. C 2016, 67, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Punrattanasin, N.; Nakpathom, M.; Somboon, B.; Narumol, N.; Rungruangkitkrai, N.; Mongkholrattanasit, R. Silk fabric dyeing with natural dye from mangrove bark (Rhizophora apiculata Blume) extract. Ind. Crops Prod. 2013, 49, 122–129. [Google Scholar] [CrossRef]
- ShahmoradiGhaheh, F.; Mortazavi, S.M.; Alihosseini, F.; Fassihi, A.; Shams Nateri, A.; Abedi, D. Assessment of antibacterial activity of wool fabrics dyed with natural dyes. J. Clean. Prod. 2014, 72, 139–145. [Google Scholar] [CrossRef]
- Koh, L.-D.; Cheng, Y.; Teng, C.-P.; Khin, Y.-W.; Loh, X.-J.; Tee, S.-Y.; Low, M.; Ye, E.; Yu, H.-D.; Zhang, Y.-W.; et al. Structures, mechanical properties and applications of silk fibroin materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Li, G.; Liu, H.; Li, T.D.; Wang, J. Surface modification and functionalization of silk fibroin fibers/fabric toward high performance applications. Mater. Sci. Eng. C 2012, 32, 627–636. [Google Scholar] [CrossRef]
- Koller, D.Y.; Halmerbauer, G.; Böck, A.; Engstler, G. Action of a silk fabric treated with AEGISTM in children with atopic dermatitis: A 3-month trial. Pedi-atr. Allergy Immunol. 2007, 18, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tang, R.-C. Facile and eco-friendly fabrication of colored and bioactive silk materials using silver nanoparticles synthesized by two flavonoids. Polymers 2018, 10, 404. [Google Scholar] [CrossRef] [PubMed]
- Periolatto, M.; Ferrero, F.; Vineis, C. Antimicrobial chitosan finish of cotton and silk fabrics by UV-curing with 2-hydroxy-2-methylphenylpropane-1-one. Carbohyd. Polym. 2012, 88, 201–205. [Google Scholar] [CrossRef]
- Zhang, W.; Dai, X.; Zhou, J.; Zhu, G. Antibacterial Bombyx Mori silk fabric modified with reactive chitosan quaternary ammonium salt and its laundering durability. Fibers Polym. 2017, 18, 290–295. [Google Scholar] [CrossRef]
- Baliarsingh, S.; Jena, J.; Das, T.; Das, N.B. Role of cationic and anionic surfactants in textile dyeing with natural dyes extracted from waste plant materials and their potential antimicrobial properties. Ind. Crops Prod. 2013, 50, 618–624. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.; Tang, R.-C.; Zhang, J. Simultaneous dyeing and functionalization of silk with three natural yellow dyes. Ind. Crops Prod. 2015, 64, 224–232. [Google Scholar] [CrossRef]
- Shahid, M.; Zhou, Y.; Tang, R.-C.; Chen, G.; Wani, W.A. Colorful and antioxidant silk with chlorogenic acid-process development and optimization by central composite design. Dyes Pigment. 2017, 138, 30–38. [Google Scholar] [CrossRef]
- Alonso, C.; Martí, M.; Martínez, V.; Rubio, L.; Parra, J.L.; Coderch, L. Antioxidant cosmeto-textiles: Skin assessment. Eur. J. Pharm. Biopharm. 2013, 84, 192–199. [Google Scholar] [CrossRef]
- Mocanu, G.; Nichifor, M.; Mihai, D.; Oproiu, L.C. Bioactive cotton fabrics containing chitosan and biologically active substances extracted from plants. Mater. Sci. Eng. C 2013, 33, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, K.J.; Wong, H.-K.; Oddos, T.; Southall, M.; Frei, B.; Kaur, S. A purified feverfew extract protects from oxidative damage by inducing DNA repair in skin cells via a PI3-kinase-dependent Nrf2/ARE pathway. J. Dermatol. Sci. 2013, 72, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ.; Huyut, Z.; Elmastaş, M.; Aboul-Enein, H.Y. Radical scavenging and antioxidant activity of tannic acid. Arab. J. Chem. 2010, 3, 43–53. [Google Scholar] [CrossRef]
- King, A.; Young, G. Characteristics and occurrence of phenolic phytochemicals. J. Am. Diet. Assoc. 1999, 99, 213–218. [Google Scholar] [CrossRef]
- Lopes, G.K.B.; Schulman, H.M.; Hermes-Lima, M. Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions. BBActa—Gen. Subj. 1999, 1472, 142–152. [Google Scholar] [CrossRef]
- Zengin, A.C.A.; Çolak, S.M.; Zengin, G.; Kiliç, E. Eco-friendly soaking process using tannic acid as an alternative bactericide. Arch. Environ. Protect. 2014, 40, 3–12. [Google Scholar] [CrossRef][Green Version]
- Chung, K.T.; Lu, Z.; Chou, M.W. Mechanism of inhibition of tannic acid and related compounds on the growth of intestinal bacteria. Food Chem. Toxicol. 1998, 36, 1053–1060. [Google Scholar] [CrossRef]
- Darvin, P.; Baeg, S.J.; Joung, Y.H.; Sp, N.; Kang, D.Y.; Byun, H.J.; Park, J.U.; Yang, Y.M. Tannic acid inhibits the Jak2/STAT3 pathway and induces G1/S arrest and mitochondrial apoptosis in YD-38 gingival cancer cells. Int. J. Oncol. 2015, 47, 1111–1120. [Google Scholar] [CrossRef]
- Higazy, A.; Hashem, M.; Elshafei, A.; Shaker, N.; Hady, M.A. Development of anti-microbial jute fabrics via in situ formation of cellulose-tannic acid-metal ion complex. Carbohyd. Polym. 2010, 79, 890–897. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Textiles—Evaluation for Antibacterial Activity—Part 3: Shake Flask Method; GB/T 20944.3–2008; China’s General Administration of Quality Supervision, Inspection and Quarantine and Standardization Administration of China: Beijing, China, 2008.
- Ho, Y.-S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Rather, L.J.; Khan, M.A.; Mohammad, F. Adsorption and kinetic studies of Adhatodavasica, natural dye onto woolen yarn with evaluations of colorimetric and fluorescence characteristics. J. Environ. Chem. Eng. 2016, 4, 1780–1796. [Google Scholar] [CrossRef]
- Chiou, M.S.; Li, H.Y. Adsorption behavior of reactive dye in aqueous solution on chemical cross-linked chitosan beads. Chemosphere 2003, 50, 1095–1105. [Google Scholar] [CrossRef]
- Chiou, M.S.; Ho, P.Y.; Li, H.Y. Adsorption of anionic dyes in acid solutions using chemically cross-linked chitosan beads. Dyes Pigment. 2004, 60, 69–84. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, C.; Ding, S.; Ma, H.; Ji, Y. Behaviors and mechanisms of tannic acid adsorption on an amino-functionalized magnetic nanoadsorbent. Desalination 2011, 273, 285–291. [Google Scholar] [CrossRef]
- Lin, J.; Zhan, Y.; Zhu, Z.; Xing, Y. Adsorption of tannic acid from aqueous solution onto surfactant-modified zeolite. J. Hazard. Mater. 2011, 193, 102–111. [Google Scholar] [CrossRef]
- Harbourne, N.; Jacquier, J.C.; O’Riordan, D. Effects of addition of phenolic compounds on the acid gelation of milk. Int. Dairy J. 2011, 21, 185–191. [Google Scholar] [CrossRef]
- Ng, T.B.; Liu, F.; Wang, Z.T. Antioxidative activity of natural products from plants. Life Sci. 2000, 66, 709–723. [Google Scholar] [CrossRef]










| Temperature (°C) | ri (mg/(g·min)) | t1/2(min) | K (g/(mg·min)) | C∞ (mg/g) | R2 |
|---|---|---|---|---|---|
| 70 | 23.42 | 3.92 | 0.00278 | 91.74 | 0.9994 |
| 80 | 58.14 | 1.45 | 0.00823 | 84.03 | 0.9993 |
| 90 | 125.00 | 0.62 | 0.02113 | 76.92 | 0.9995 |
| Temperature (°C) | Langumuir | Freundlich | ||
|---|---|---|---|---|
| KL (L/g) | S (mg/g) | R2 | R2 | |
| 70 | 0.84 | 0.55 | 0.9891 | 0.9054 |
| 80 | 0.64 | 0.46 | 0.9957 | 0.9558 |
| 90 | 0.45 | 0.36 | 0.9994 | 0.9738 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Yang, Z.-Y.; Cheng, X.-W.; Tang, R.-C.; Qiao, Y.-F. Adsorption, Antibacterial and Antioxidant Properties of Tannic Acid on Silk Fiber. Polymers 2019, 11, 970. https://doi.org/10.3390/polym11060970
Zhang W, Yang Z-Y, Cheng X-W, Tang R-C, Qiao Y-F. Adsorption, Antibacterial and Antioxidant Properties of Tannic Acid on Silk Fiber. Polymers. 2019; 11(6):970. https://doi.org/10.3390/polym11060970
Chicago/Turabian StyleZhang, Wen, Zhi-Yi Yang, Xian-Wei Cheng, Ren-Cheng Tang, and Yi-Fan Qiao. 2019. "Adsorption, Antibacterial and Antioxidant Properties of Tannic Acid on Silk Fiber" Polymers 11, no. 6: 970. https://doi.org/10.3390/polym11060970
APA StyleZhang, W., Yang, Z.-Y., Cheng, X.-W., Tang, R.-C., & Qiao, Y.-F. (2019). Adsorption, Antibacterial and Antioxidant Properties of Tannic Acid on Silk Fiber. Polymers, 11(6), 970. https://doi.org/10.3390/polym11060970