Thermotropic Liquid Crystalline Polymers with Various Alkoxy Side Groups: Thermal Properties and Molecular Dynamics
Abstract
:1. Introduction
2. Experimental
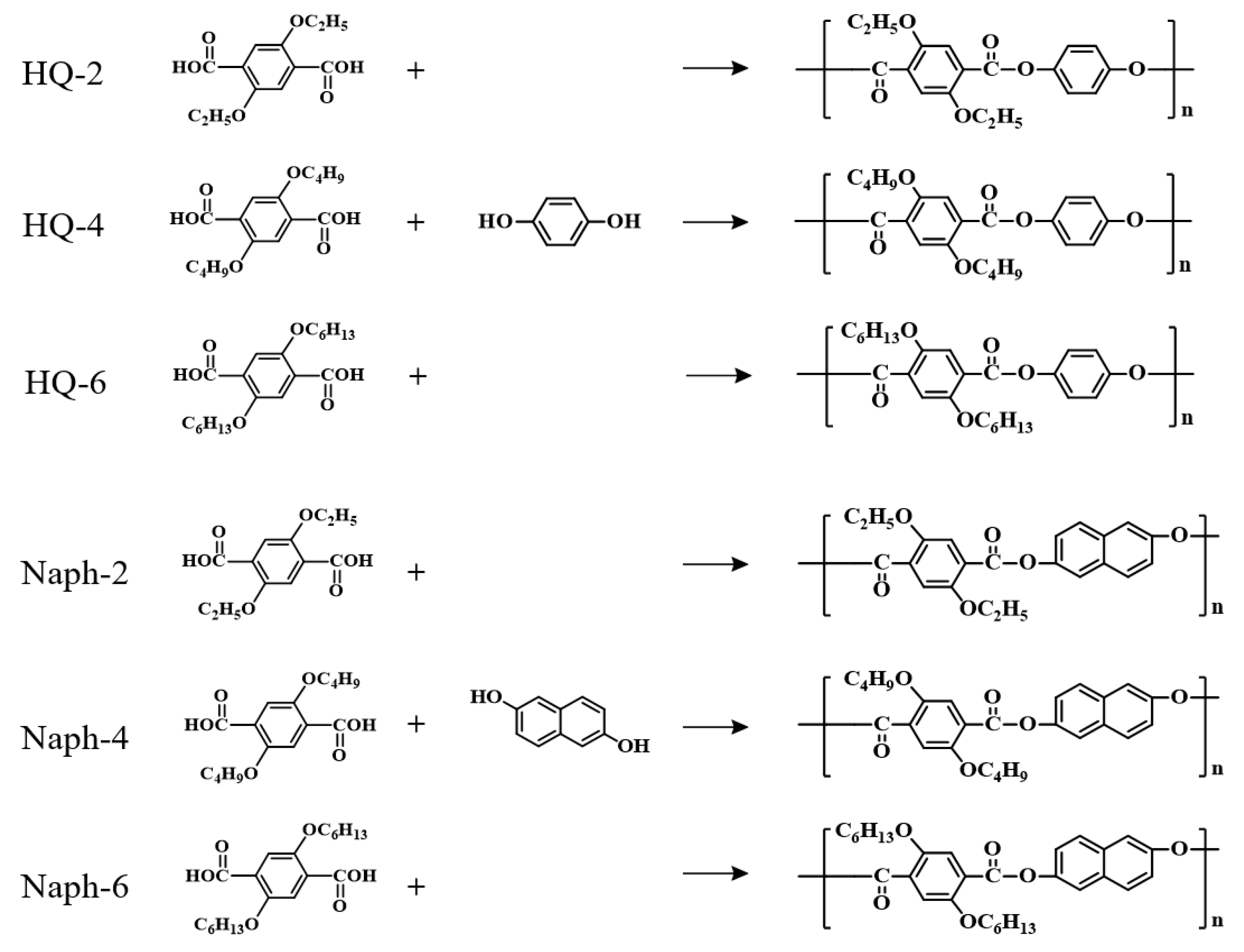
2.1. Synthesis of 2,5-Dialkoxylterephthaloyl Chloride
2.2. Synthesis of TLCPs
2.3. Characterization
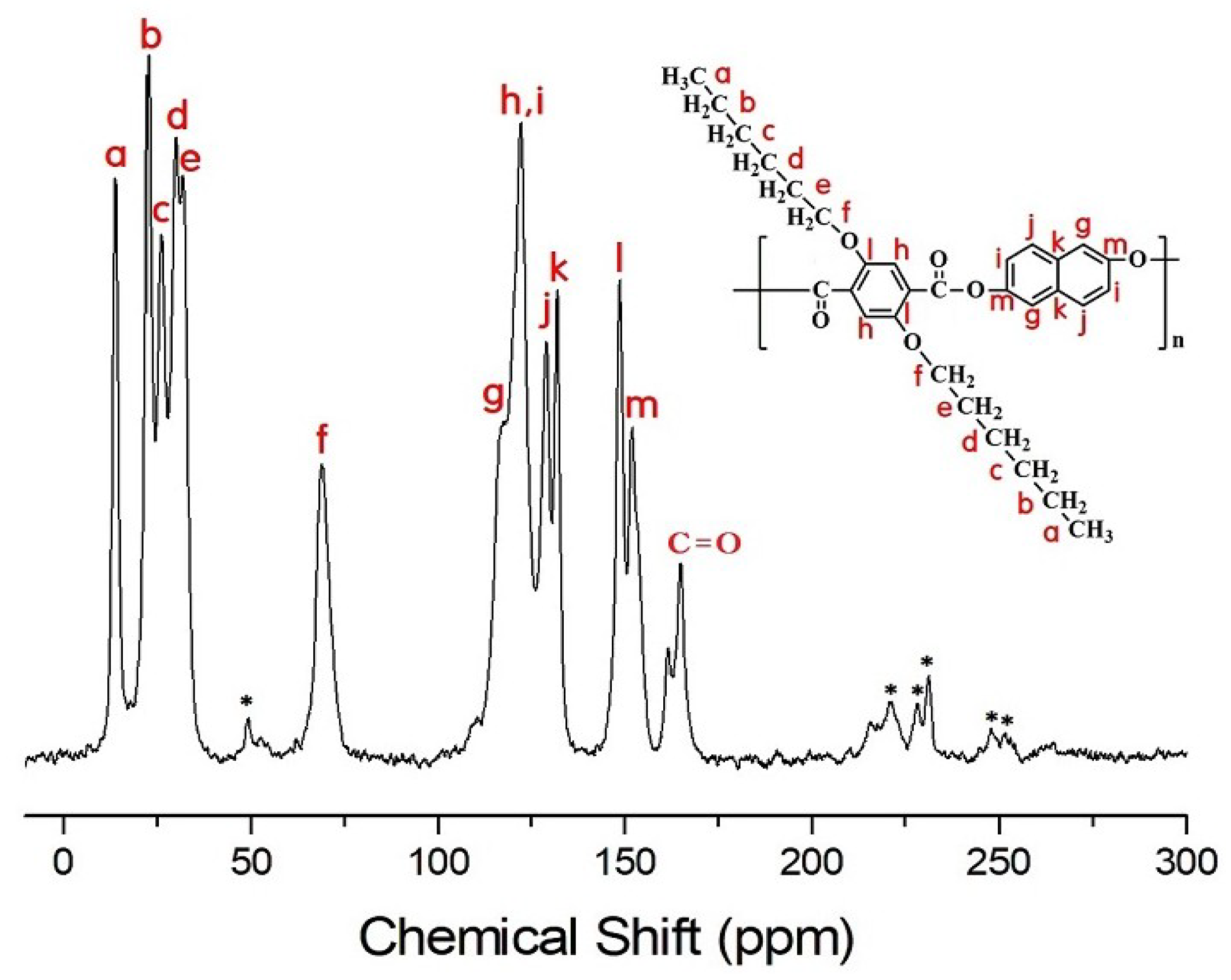
2.4. NMR Spectroscopy
3. Results and Discussion
3.1. Thermal Properties of the TLCPs
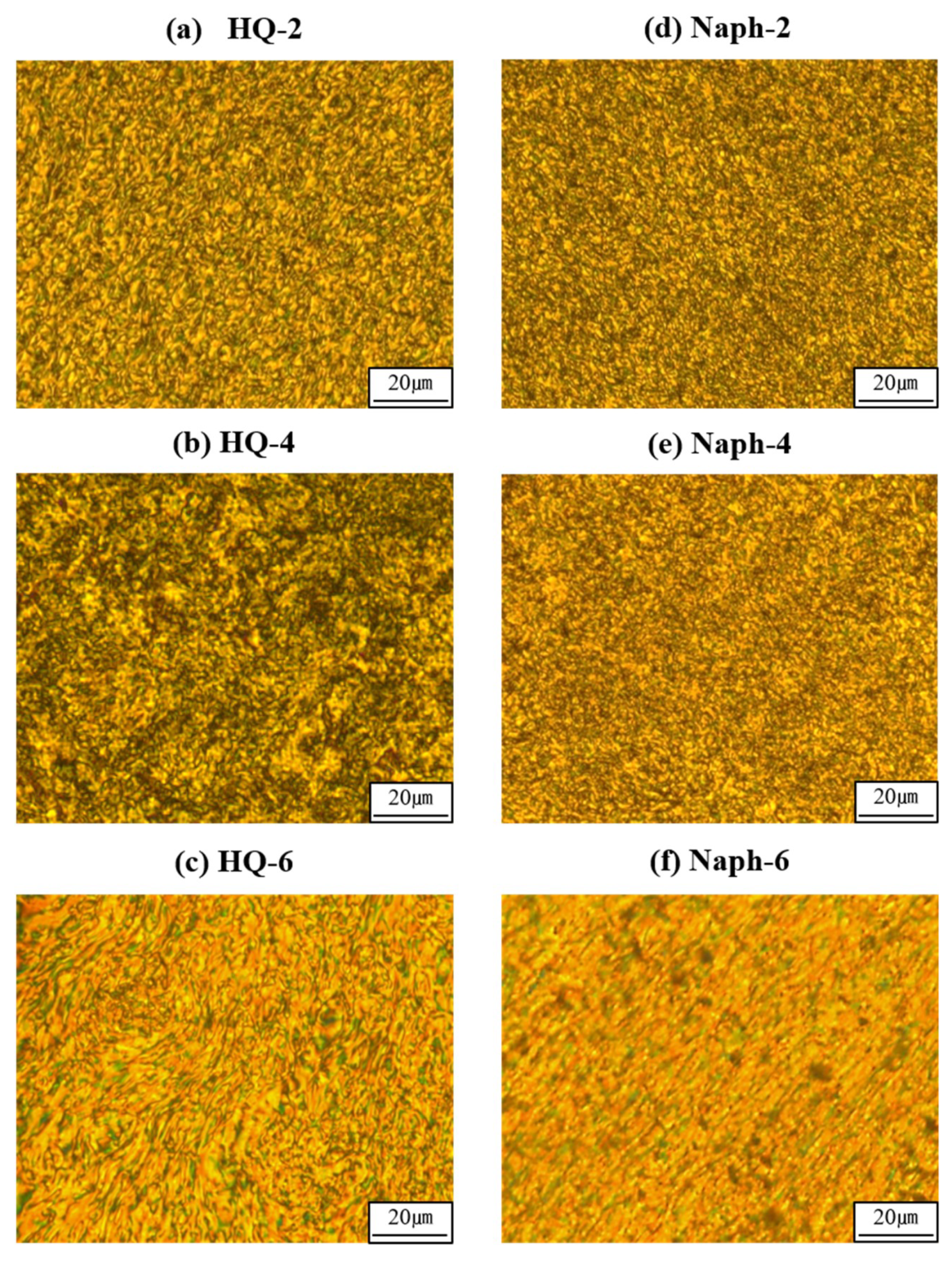
3.2. LC Mesophases
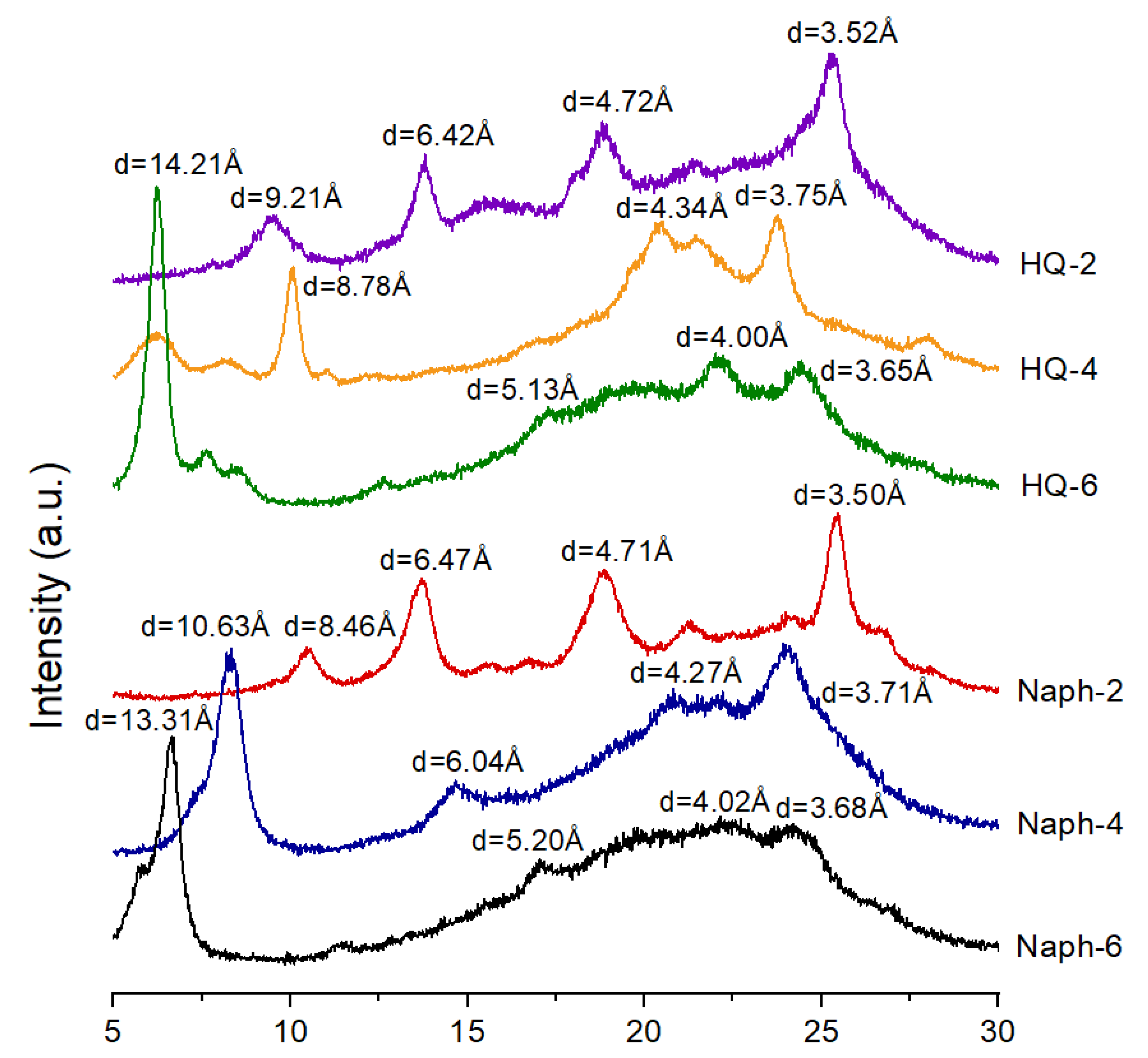
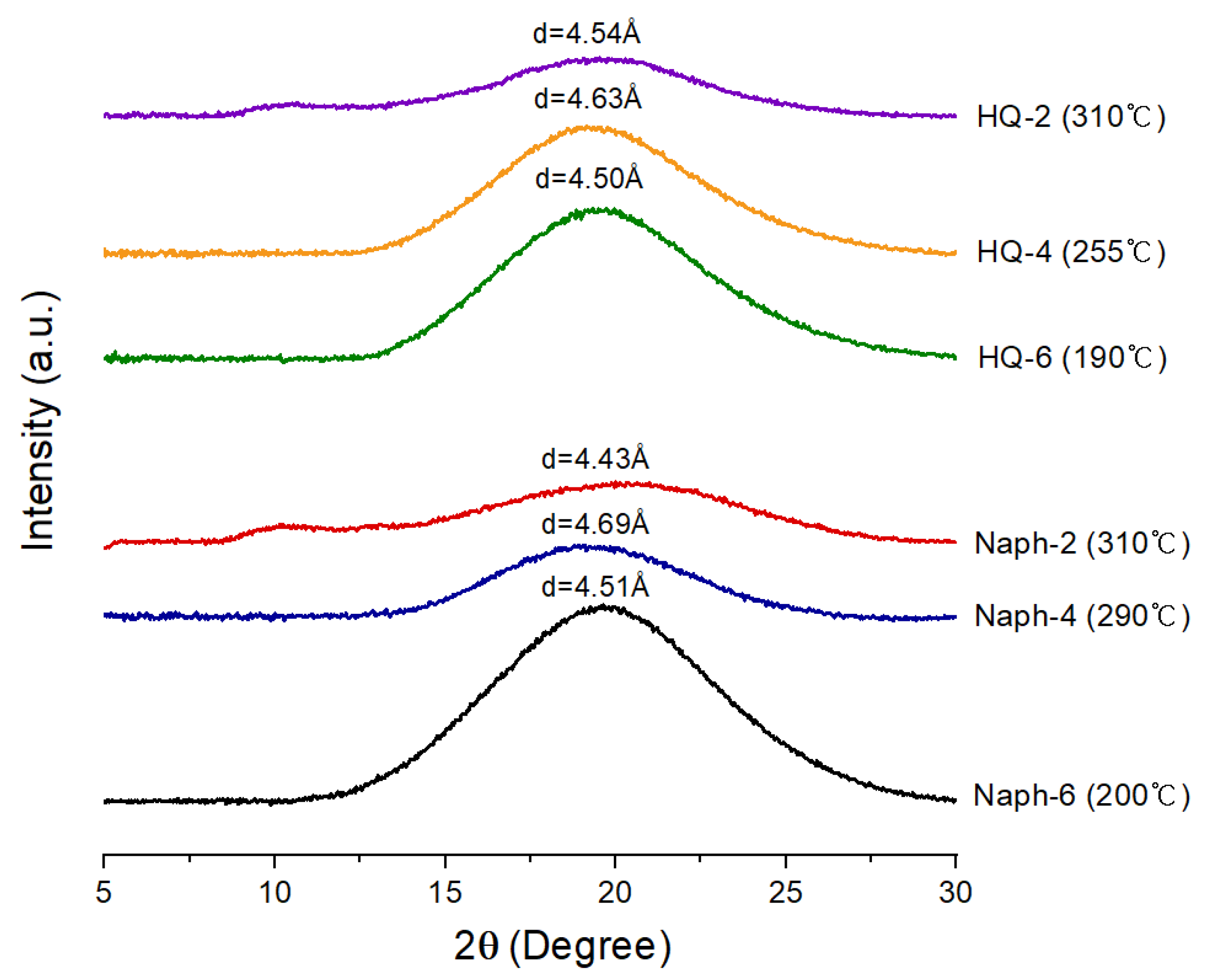
3.3. WAXD Analyses
3.4. 13C Chemical Shifts and Relaxation Times
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, S. Liquid Crystals: Experimental Study of Physical Properties and Phase Transitions; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Somma, E.; Nobile, M.R. Rheology of a semi-rigid thermotropic liquid crystalline polymer. Macromol. Symp. 2005, 228, 71–79. [Google Scholar] [CrossRef]
- Lenz, R.W. Synthesis and properties of thermotropic liquid crystal polymers with main chain mesogenic units. Polym. J. 1985, 17, 105–115. [Google Scholar] [CrossRef]
- Zeng, L.; Li, R.; Chen, P.; Xu, J.; Liu, P. Synthesis and characterization of thermotropic liquid crystalline polyarylate with ether ether ketone segments in the main chain. J. Appl. Polym. Sci. 2016, 133, 43800. [Google Scholar] [CrossRef]
- Beers, D.E.; Ramirez, J.E. Vectran high-performance fibre. J. Text. Inst. 1990, 81, 561–574. [Google Scholar] [CrossRef]
- Gong, X.; Chen, X.; Zhou, Y. Advanced weaving technologies for high-performance fabrics. In High-Performance Apparel; McLoughlin, J., Sabir, T., Eds.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Duxford, UK, 2018; Chapter 5; pp. 75–112. [Google Scholar]
- Wolfe, J.F.; Loo, B.H.; Arnold, F.E. Rigid-rod polymers. 2. Synthesis and thermal properties of para-aromatic polymers with 2,6-benzobisthiazole units in the main chain. Macromolecules 1981, 14, 915–920. [Google Scholar] [CrossRef]
- Wilsens, C.H.R.M.; Noordover, B.A.J.; Rastogi, S. Aromatic thermotropic polyester based on 2,5-furandicarboxylic acid and vanillic acid. Polymer 2014, 55, 2432–2439. [Google Scholar] [CrossRef]
- Seo, B.-S.; Chang, J.-H. 4-Synthesis, characterization, and applications of liquid crystalline polymer-based microfibrillar and nanofibrillar composites. In Micro and Nano Fibrillar Composites (MFCs and NFCs) from Polymer Blends; Mishra, R.K., Thomas, S., Kalarikkal, N., Eds.; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Duxford, UK, 2017; Chapter 4; pp. 73–95. [Google Scholar]
- Komatsu, S.; Wetzel, B.; Friedrich, K. Novel liquid crystal polymers with tailored chemical structure for high barrier, mechanical and tribological performance. In Liquid Crystalline Polymers: Volume 2—Processing and Applications; Thakur, V.K., Kessler, M.R., Eds.; Springer International Publishing: Berlin, Germany, 2015; pp. 15–39. [Google Scholar]
- Wilsens, C.H.R.M.; Deshmukh, Y.S.; Liu, W.; Noordover, B.A.J.; Yao, Y.; Meijer, H.E.H.; Rastogi, S. Processing and performance of aromatic-aliphatic thermotropic polyesters based on vanillic acid. Polymer 2015, 60, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Schaller, R.; Peijs, T.; Tervoort, T.A. High-performance liquid-crystalline polymer films for monolithic composites. Compos. Part A Appl. Sci. Manuf. 2016, 81, 296–304. [Google Scholar] [CrossRef]
- Bertolini, G.; Montani, E.; Pedretti, U.; Sortino, G.; Scaffaro, R.; La Mantia, F.P. Synthesis and characterization of a new fully aromatic LCP. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 1996, 290, 77–85. [Google Scholar] [CrossRef]
- Sloan, F. 5-Liquid crystal aromatic polyester-arylate (LCP) fibers: Structure, properties, and applications. Struct. Prop. High-Perform. Fibers 2017, 2017, 113–140. [Google Scholar]
- Jackson, W.J. Liquid crystalline polymers. 5. Liquid crystalline polyesters containing naphthalene rings. Macromolecules 1983, 16, 1027–1033. [Google Scholar] [CrossRef]
- Hirano, H.; Kadota, J.; Agari, Y.; Harada, T.; Tanaka, M.; Hasegawa, K. Linear polymers with sulfur in the main chain. IV. Synthesis of thermotropic liquid-crystalline polythioesters based on 4,4′-biphenyldithiol with excellent adhesive properties. Polym. Eng. Sci. 2007, 47, 262–269. [Google Scholar] [CrossRef]
- Antoun, S.; Lenz, R.W.; Jin, J.-I. Liquid crystal polymers. IV. Thermotropic polyesters with flexible spacers in the main chain. J. Polym. Sci. Part A Polym. Chem. 1981, 19, 1901–1920. [Google Scholar] [CrossRef]
- Jackson, W.J.; Kuhfuss, H.F. Liquid crystal polymers. I. Preparation and properties of p-hydroxybenzoic acid copolyesters. J. Polym. Sci. Part A Polym. Chem. 1976, 14, 2043–2058. [Google Scholar] [CrossRef]
- Ophir, Z.; Ide, Y. Injection molding of thermotropic liquid crystal polymers. Polym. Eng. Sci. 1983, 23, 792–796. [Google Scholar] [CrossRef]
- Uzman, M.; Kuhnpast, K.; Springer, J. Orientational behavior of polymer blends containing a liquid crystalline component. Die Makromol. Chem. 1989, 190, 3185–3194. [Google Scholar] [CrossRef]
- Martínez-Gómez, A.; Encinar, M.; Fernández-Blázquez, J.P.; Rubio, R.G.; Pérez, E. Relationship between composition, structure and dynamics of main-chain liquid crystalline polymers with biphenyl mesogens. In Liquid Crystalline Polymers: Volume 1—Structure and Chemistry; Thakur, V.K., Kessler, M.R., Eds.; Springer International Publishing: New York, NY, USA, 2016; pp. 453–476. [Google Scholar]
- Damman, S.B.; Mercx, F.P.M.; Kootwijk-Damman, C.M. Liquid-crystalline main-chain polymers with a poly(p-phenylene terephthalate) backbone: 1. Synthesis, characterization and rheology of polyesters with alkoxy side chains. Polymer 1993, 34, 1891–1897. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.-H.; Jo, B.-W. Blends of PBT with rigid thermotropic LCP having flexible side groups: Comparison of their tensile properties with semirigid main-chain TLCP blends. J. Appl. Polym. Sci. 1996, 60, 939–946. [Google Scholar] [CrossRef]
- Griffin, A.C.; Samulski, E.T. Dimer vs. polymer liquid crystals: Alkyl chain flexibility via deuterium NMR. J. Am. Chem. Soc. 1985, 107, 2975–2976. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Berl, M.; Scharnagl, N. Poly(lactones). 9. Polymerization mechanism of metal alkoxide initiated polymerizations of lactide and various lactones. Macromolecules 1988, 21, 286–293. [Google Scholar] [CrossRef]
- Wang, T.; Li, X.; Dong, Z.; Huang, S.; Yu, H. Vertical orientation of nanocylinders in liquid -crystalline block copolymers directed by light. ACS Appl. Mater. Interfaces 2017, 9, 24864–24872. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, S.; Wang, T.; Dong, Z.; Yu, H. Confined self-assembly enables stabilization and patterning of nanostructures in liquid-crystalline block copolymers. Macromolecules 2019, 52, 1892–1898. [Google Scholar] [CrossRef]
- Huang, S.; Chen, Y.; Ma, S.; Yu, H. Hierarchical self-assembly in liquid -crystalline block copolymers enabled by chirality transfer. Angew. Chem. Int. Ed. 2018, 57, 12524–12528. [Google Scholar] [CrossRef] [PubMed]
- Koenig, J.L. Spectroscopy of Polymers; Elsevier: New York, NY, USA, 1999; Chapter 6; pp. 255–313. [Google Scholar]
- Lim, A.R. Effect on local structure and phase transition of perovskite-type [N(CH3)4]2 Zn1−xCuxBr4 (x = 0, 0.5, 0.7, and 1). Sci. Rep. 2018, 8, 14095–14114. [Google Scholar] [CrossRef] [PubMed]
- Ballauff, M. Rigid rod polymers having flexible side chains, 1. Thermotropic poly(1,4-phenylene 2,5-dialkoxyterephthalate)s. Die Makromol. Chem. Rapid Commun. 1986, 7, 407–414. [Google Scholar] [CrossRef]
- Majnusz, J.; Lenz, R.W. Liquid crystal polymers-22. Synthesis and properties of monomers for the preparation of poly(2-alkyl-1,4-phenylene terephthalates). Eur. Polym. J. 1985, 21, 565–568. [Google Scholar] [CrossRef]
- Barclay, G.G.; Ober, C.K. Liquid crystalline and rigid-rod networks. Prog. Polym. Sci. 1993, 18, 899–945. [Google Scholar] [CrossRef]
- Jin, J.-I.; Kang, C.-S. Thermotropic main chain polyesters. Prog. Polym. Sci. 1997, 22, 937–973. [Google Scholar] [CrossRef]
- Song, J.-Y.; Yun, Y.-K.; Jin, J.-I. Synthesis and characterization of a series of wholly aromatic copolyesters containing 2-(α-phenylisopropyl)hydroquinone moiety. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 881–889. [Google Scholar] [CrossRef]
- Rubin, S.F.; Kannan, R.M.; Kornfield, J.A.; Boeffel, C. Effect of mesophase order and molecular weight on the dynamics of nematic and smectic side-group liquid-crystalline polymers. Macromolecules 1995, 28, 3521–3530. [Google Scholar] [CrossRef]
- Cai, R.; Preston, J.; Samulski, E.T. Liquid crystalline aromatic polyesters derived from 2,5-thiophene. Macromolecules 1992, 25, 563–568. [Google Scholar] [CrossRef]
- Khan, N.; Price, D.M.; Bashir, Z. Synthesis and mesophase characterization of liquid crystalline polyesters with bulky, rigid, lateral substituents. J. Polym. Sci. Part B Polym. Phys. 1994, 32, 2509–2518. [Google Scholar] [CrossRef]
- Elias, F.; Clarke, S.M.; Peck, R.; Terentjev, E.M. Equilibrium textures in main-chain liquid crystalline polymers. Europhys. Lett. 1999, 47, 442–448. [Google Scholar] [CrossRef] [Green Version]
- Elias, F.; Clarke, S.M.; Peck, R.; Terentjev, E.M. Nematic order drives phase separation in polydispersed liquid crystalline polymers. Macromolecules 2000, 33, 2060–2068. [Google Scholar] [CrossRef]
- Kavesh, S.; Schultz, J.M. Meaning and measurement of crystallinity in polymers: A Review. Polym. Eng. Sci. 1969, 9, 331–338. [Google Scholar] [CrossRef]
- Abragam, A. The Principles of Nuclear Magnetism; Oxford University Press: Oxford, UK, 1961; Chapter IX; pp. 354–423. [Google Scholar]
- Lim, A.R.; Joo, Y.L. Cation dynamics by 1H and 13C MAS NMR in hybrid organic-inorganic (CH3CH2NH3)2 CuCl4. RSC Adv. 2018, 8, 34110–34115. [Google Scholar] [CrossRef]





| TLCP | IV 1 | Tg (°C) | Tm (°C) | Ti (°C) | TDi 2 (°C) | wtR600 (%) 3 | LC Phase | DC 4 (%) |
|---|---|---|---|---|---|---|---|---|
| HQ-2 | 0.99 | 71 | 279 | 325 | 357 | 27 | Nematic | 25 |
| HQ-4 | 0.83 | 34 | 249 | 269 | 343 | 21 | Nematic | 19 |
| HQ-6 | Insol. 5 | 11 | 164 | 200 | 342 | 20 | Nematic | 18 |
| Naph-2 | 0.95 | 76 | 282 | 329 | 365 | 48 | Nematic | 30 |
| Naph-4 | Insol. | 54 | 258 | 312 | 362 | 34 | Nematic | 24 |
| Naph-6 | Insol. | 38 | 164 | 227 | 348 | 26 | Nematic | 20 |
| TLCP | Distance (Å) | |||
|---|---|---|---|---|
| HQ-2 | 9.21 (9.6) 1 | 6.42 (13.8) | 4.72 (18.8) | 3.52 (25.28) |
| HQ-4 | 14.21 (6.22) | 8.78 (10.08) | 4.34 (20.48) | 3.75 (23.74) |
| HQ-6 | 14.21 (6.22) | 5.13 (17.28) | 4.00 (22.2) | 3.65 (24.38) |
| Naph-2 | 8.46 (10.46) | 6.47 (13.68) | 4.71 (18.86) | 3.50 (25.46) |
| Naph-4 | 10.63 (8.32) | 6.04 (14.66) | 4.27 (20.82) | 3.71 (23.98) |
| Naph-6 | 13.31 (6.64) | 5.20 (17.04) | 4.02 (22.14) | 3.68 (24.20) |
| TLCP | HQ-2 | HQ-4 | HQ-6 | Naph-2 | Naph-4 | Naph-6 |
|---|---|---|---|---|---|---|
| CH3-a | 30.7 | 70.2 | 96.8 | 25.1 | 53.3 | 50.0 |
| CH2-b | 3.1 | 13.4 | 24.5 | 1.9 | 9.9 | 10.6 |
| CH2-c | 7.9 | 12.8 | 7.1 | 5.8 | ||
| CH2-d | 3.3 | 6.8 | 3.2 | 4.6 | ||
| CH2-e | 5.3 | 3.5 | ||||
| CH2-f | 3.8 | 2.4 | ||||
| g | 8.7 | 8.0 | 10.7 | 19.1 | 26.7 | 6.6 |
| h | 45.2 | 44.5 | 47.2 | 51.4 | 35.8 | 20.5 |
| i | 107.9 | 147.3 | 124.8 | |||
| j | 105.1 | 115.2 | 108.5 | 120.2 | 106.1 | 149.9 |
| k | 51.2 | 38.8 | 96.6 | |||
| l | 158.4 | 140.5 | 96.1 | |||
| m | 102.6 | 125.8 | 69.1 | |||
| C=O | 118.5 | 163.3 | 144.7 | 179.3 | 193.4 | 104.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, G.T.; Chang, J.-H.; Lim, A.R. Thermotropic Liquid Crystalline Polymers with Various Alkoxy Side Groups: Thermal Properties and Molecular Dynamics. Polymers 2019, 11, 992. https://doi.org/10.3390/polym11060992
Park GT, Chang J-H, Lim AR. Thermotropic Liquid Crystalline Polymers with Various Alkoxy Side Groups: Thermal Properties and Molecular Dynamics. Polymers. 2019; 11(6):992. https://doi.org/10.3390/polym11060992
Chicago/Turabian StylePark, Gi Tae, Jin-Hae Chang, and Ae Ran Lim. 2019. "Thermotropic Liquid Crystalline Polymers with Various Alkoxy Side Groups: Thermal Properties and Molecular Dynamics" Polymers 11, no. 6: 992. https://doi.org/10.3390/polym11060992







