Polypropylene Blend with Polyphenols through Dynamic Vulcanization: Mechanical, Rheological, Crystalline, Thermal, and UV Protective Property
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pre-Reacted Tannin and Lignin with Glyoxal
2.3. Spray Dried the Pre-Reacted Tannin and Lignin
2.4. Composites Preparation
2.5. Test Sample Preparation
2.6. Characterization
3. Results and Discussion
3.1. The Effects of Native Tannin/Lignin and Vulcanized Tannin/Lignin on PP Matrix
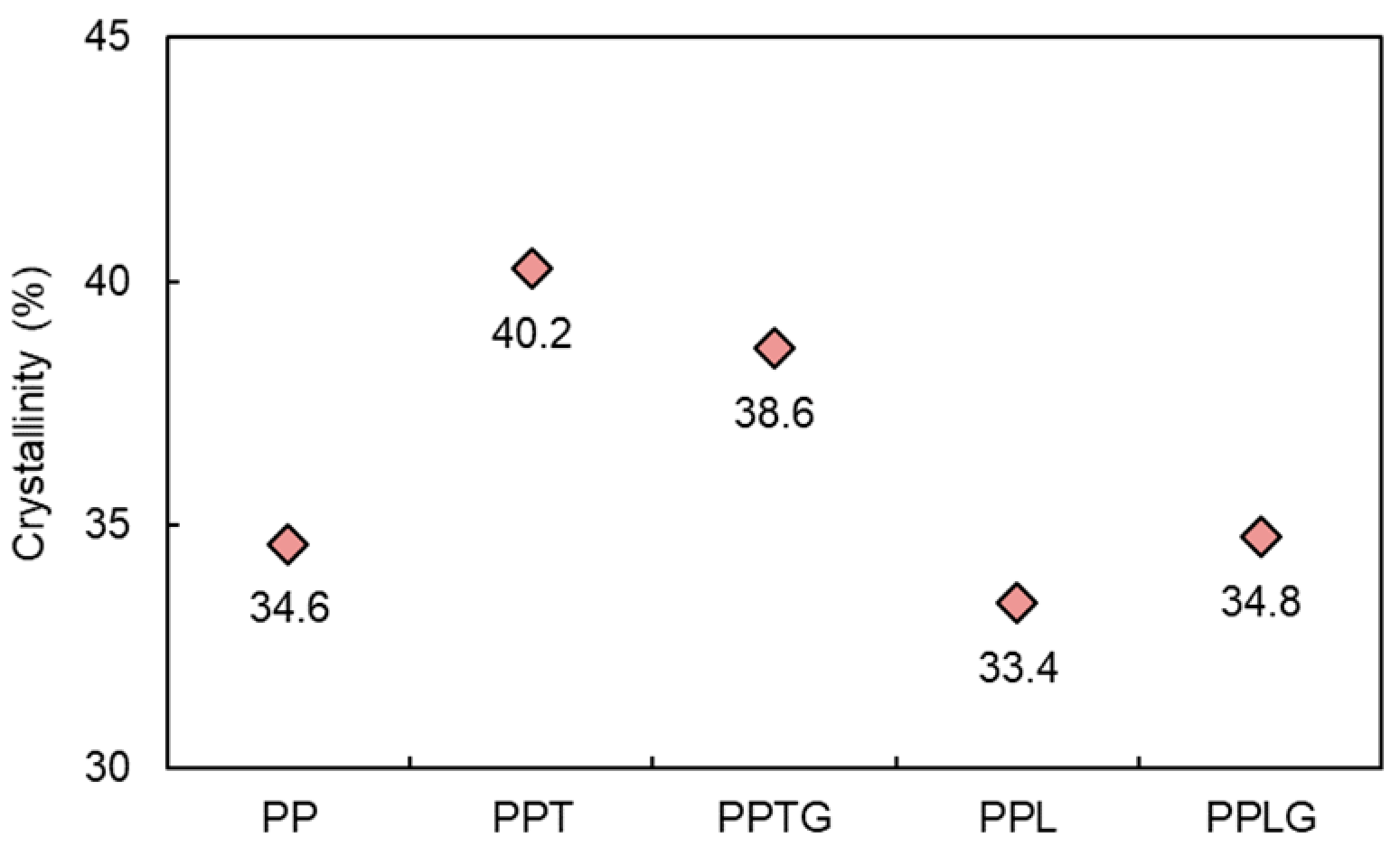
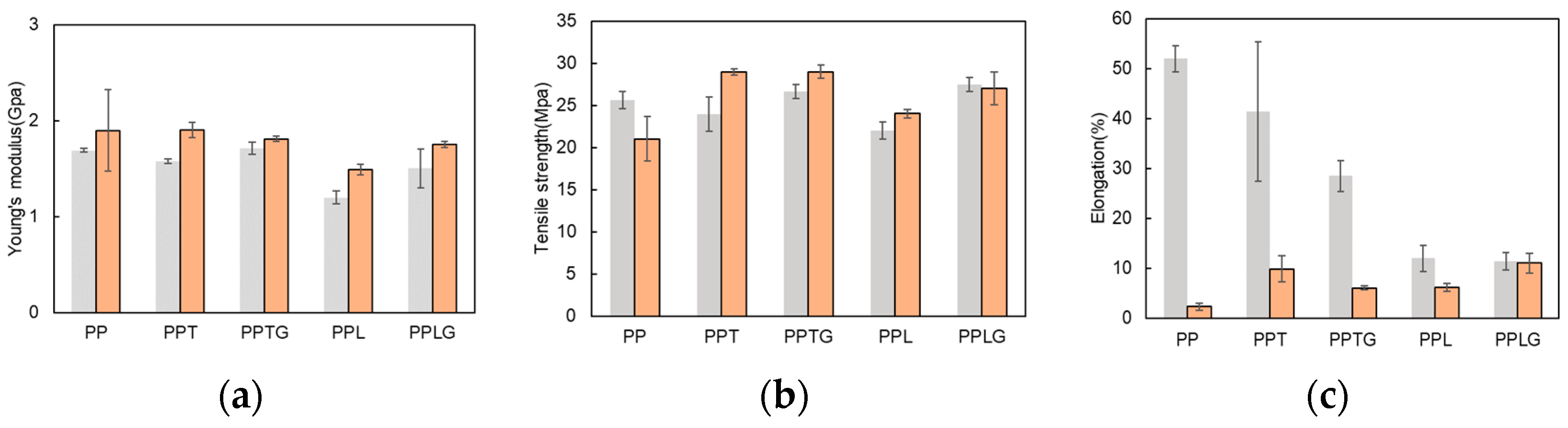
3.1.1. Mechanical, Crystalline, and Thermal Properties
3.1.2. Rheological Behavior
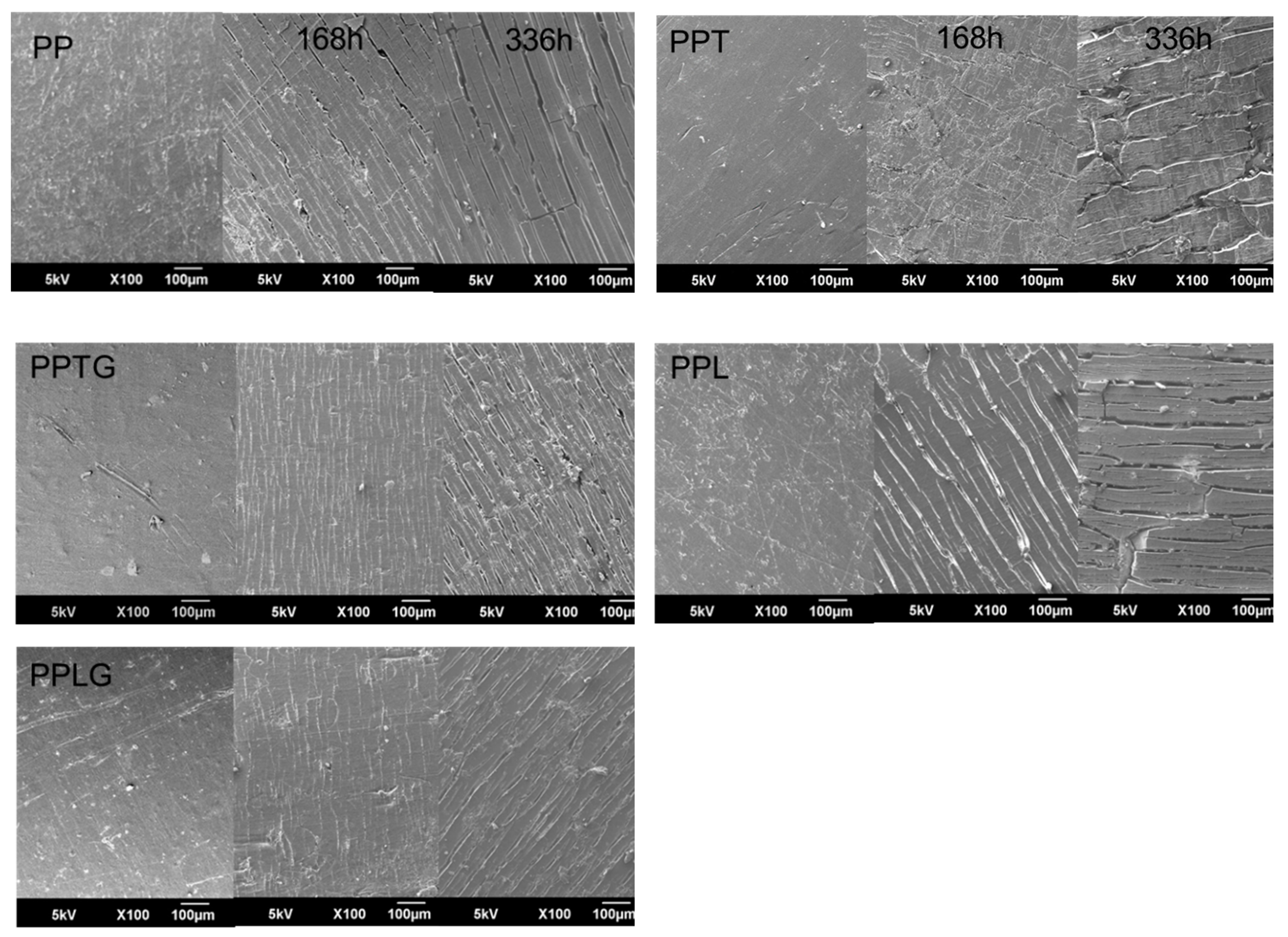
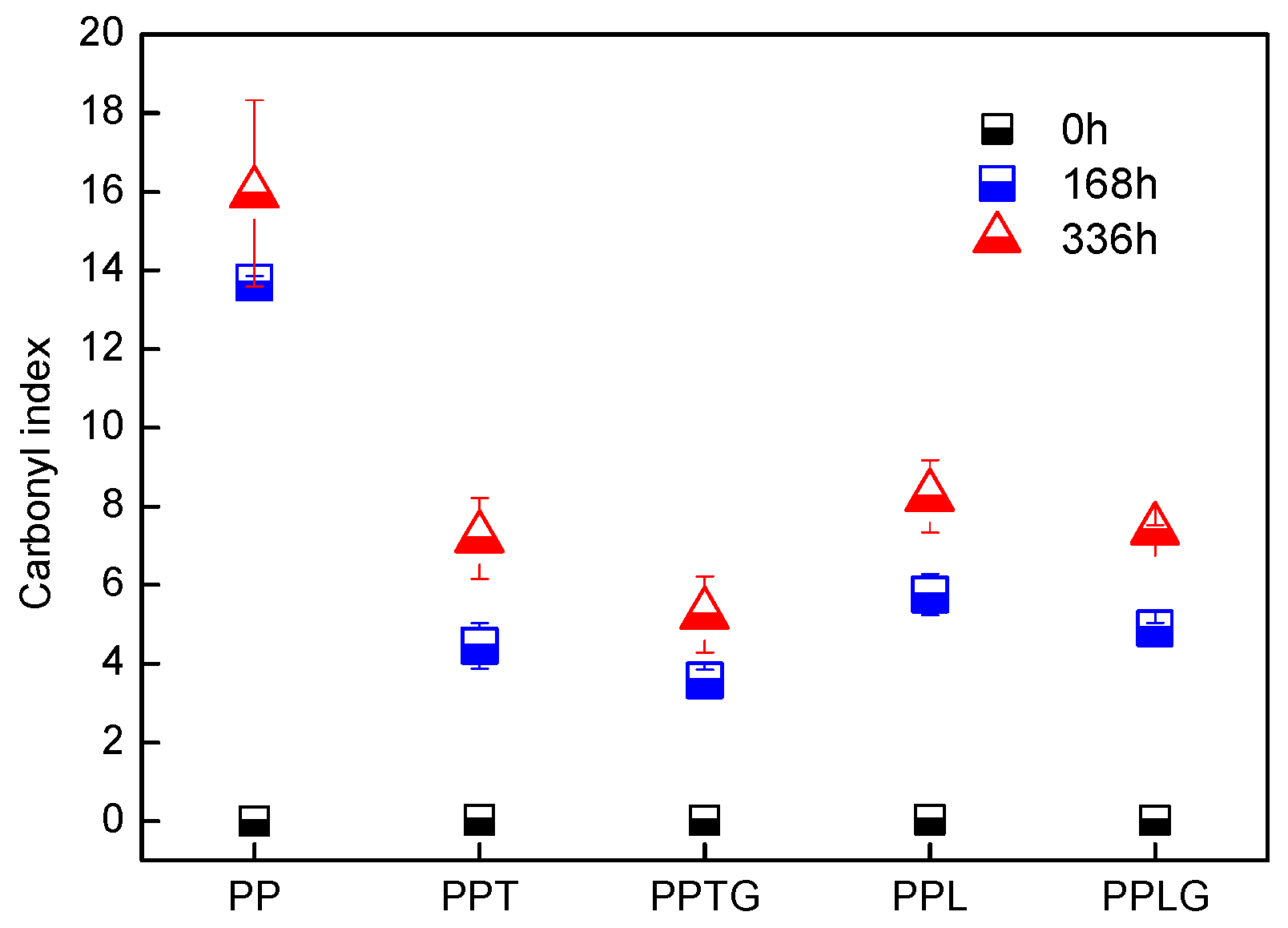
3.2. Characterization of UV Protection Property of Native Tannin/Lignin and Vulcanized Tannin/Lignin on PP Matrix
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hisham, M.H. Polypropylene as a promising plastic: A review. Am. J. Polym. Sci. 2016, 6, 1–11. [Google Scholar]
- Karian, H.G. (Ed.) Handbook of Polypropylene and Polypropylene Composites, 2nd ed.; Plastics Engineering; Rev. and Expanded.; Marcel Dekker: New York, NY, USA, 2003; ISBN 978-0-8247-4064-1. [Google Scholar]
- Visakh, P.M.; Poletto, M. (Eds.) Polypropylene-Based Biocomposites and Bionanocomposites; Scrivener Publishing, Wiley: Hoboken, NJ, USA, 2018; ISBN 978-1-119-28356-0. [Google Scholar]
- Kar, K.K.; Pandey, J.K.; Rana, S. (Eds.) Handbook of Polymer Nanocomposites. Processing, Performance and Application; Springer Berlin Heidelberg: Berlin, Heidelberg, 2015; ISBN 978-3-642-45228-4. [Google Scholar]
- Hassan, M.L.; Mathew, A.P.; Hassan, E.A.; Fadel, S.M.; Oksman, K. Improving cellulose/polypropylene nanocomposites properties with chemical modified bagasse nanofibers and maleated polypropylene. J. Reinf. Plast. Compos. 2014, 33, 26–36. [Google Scholar] [CrossRef]
- Serra, T.; Ortiz-Hernandez, M.; Engel, E.; Planell, J.A.; Navarro, M. Relevance of PEG in PLA-based blends for tissue engineering 3D-printed scaffolds. Mater. Sci. Eng. C 2014, 38, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Sonnier, R.; Taguet, A.; Ferry, L.; Lopez-Cuesta, J.-M. Towards Bio-based Flame Retardant Polymers; Biobased Polymers; Springer International Publishing: New York, NY, USA, 2018; ISBN 978-3-319-67082-9. [Google Scholar]
- García, D.; Glasser, W.; Pizzi, A.; Paczkowski, S.; Laborie, M.-P. Modification of condensed tannins: from polyphenol chemistry to materials engineering. New J. Chem. 2015, 40, 36–49. [Google Scholar] [CrossRef]
- Stewart, D. Lignin as a base material for materials applications: Chemistry, application and economics. Ind. Crop Prod. 2008, 27, 202–207. [Google Scholar] [CrossRef]
- Tolinski, M. Additives for Polyolefins: Getting the Most out of Polypropylene, Polyethylene and TPO, 2nd ed.; PDL handbook series; Elsevier, William Andrew is an imprint of Elsevier: Kidlington, Oxford, UK, 2015; ISBN 978-0-323-35884-2. [Google Scholar]
- Sorieul, M.; Dickson, A.; Hill, S.; Pearson, H. Plant Fibre: Molecular Structure and Biomechanical Properties, of a Complex Living Material, Influencing Its Deconstruction towards a Biobased Composite. Materials 2016, 9, 618. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, P.; Anguillesi, I.; Lazzeri, A. Green synthesis of flexible polyurethane foams from liquefied lignin. Eur. Polym. J. 2013, 49, 1174–1184. [Google Scholar] [CrossRef]
- Asada, C.; Basnet, S.; Otsuka, M.; Sasaki, C.; Nakamura, Y. Epoxy resin synthesis using low molecular weight lignin separated from various lignocellulosic materials. Int. J. Biol. Macromol. 2015, 74, 413–419. [Google Scholar] [CrossRef]
- Pizzi, A. Recent developments in eco-efficient bio-based adhesives for wood bonding: opportunities and issues. J. Adhes. Sci. Technol. 2006, 20, 829–846. [Google Scholar] [CrossRef]
- Sakai, H.; Kuroda, K.; Tsukegi, T.; Ogoshi, T.; Ninomiya, K.; Takahashi, K. Butylated lignin as a compatibilizing agent for polypropylene-based carbon fiber-reinforced plastics. Polym. J. 2018, 50, 997–1002. [Google Scholar] [CrossRef]
- Gadioli, R.; Waldman, W.R.; De Paoli, M.A. Lignin as a green primary antioxidant for polypropylene. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Pouteau, C.; Dole, P.; Cathala, B.; Averous, L.; Boquillon, N. Antioxidant properties of lignin in polypropylene. Polym. Degrad. Stab. 2003, 81, 9–18. [Google Scholar] [CrossRef]
- Gregorová, A.; Cibulková, Z.; Košíková, B.; Šimon, P. Stabilization effect of lignin in polypropylene and recycled polypropylene. Polym. Degrad. Stabil. 2005, 89, 553–558. [Google Scholar] [CrossRef]
- Blanco, I.; Cicala, G.; Latteri, A.; Saccullo, G.; El-Sabbagh, A.M.M.; Ziegmann, G. Thermal characterization of a series of lignin-based polypropylene blends. J. Therm. Anal. Calorim. 2017, 127, 147–153. [Google Scholar] [CrossRef]
- Alexy, P.; Košíková, B.; Podstránska, G. The effect of blending lignin with polyethylene and polypropylene on physical properties. Polymer 2000, 41, 4901–4908. [Google Scholar] [CrossRef]
- Kabir, A.; Yuan, Z.; Kuboki, T.; Xu, C. De-polymerization of industrial lignins to improve the thermo-oxidative stability of polyolefins. Ind. Crop Prod. 2018, 120, 238–249. [Google Scholar] [CrossRef]
- Wang, C.; Kelley, S.S.; Venditti, R.A. Lignin-Based Thermoplastic Materials. ChemSusChem 2016, 9, 770–783. [Google Scholar] [CrossRef]
- Pizzi, A. Tannin-Based Adhesives. J. Macromol. Sci. Part C 1980, 18, 247–315. [Google Scholar] [CrossRef]
- Becker, D.; Roeder, J.; Oliveira, R.V.B.; Soldi, V.; Pires, A.T.N. Blend of thermosetting polyurethane waste with polypropylene: influence of compatibilizing agent on interface domains and mechanical properties. Polym. Test. 2003, 22, 225–230. [Google Scholar] [CrossRef]
- Li, X.; Pizzi, A.; Lacoste, C.; Fierro, V.; Celzard, A. Physical Properties of Tannin/Furanic Resin Foamed With Different Blowing Agents. Bioresources 2012, 8, 743–752. [Google Scholar] [CrossRef]
- Lagel, M.C.; Zhang, J.; Pizzi, A. Cutting and grinding wheels for angle grinders with a bioresin matrix. Ind. Crops Prod. 2015, 67, 264–269. [Google Scholar] [CrossRef]
- Li, X.; Nicollin, A.; Pizzi, A.; Zhou, X.; Sauget, A.; Delmotte, L. Natural tannin–furanic thermosetting moulding plastics. RSC Adv. 2013, 3, 17732–17740. [Google Scholar] [CrossRef]
- Samper, M.D.; Fages, E.; Fenollar, O.; Boronat, T.; Balart, R. The potential of flavonoids as natural antioxidants and UV light stabilizers for polypropylene. J. Appl. Polym. Sci. 2013, 129, 1707–1716. [Google Scholar] [CrossRef]
- Ambrogi, V.; Cerruti, P.; Carfagna, C.; Malinconico, M.; Marturano, V.; Perrotti, M.; Persico, P. Natural antioxidants for polypropylene stabilization. Polym. Degrad. Stab. 2011, 96, 2152–2158. [Google Scholar] [CrossRef]
- Bridson, J.; Kaur, J.; Zhang, Z.; Donaldson, L.; Fernyhough, A. Polymeric flavonoids processed with co-polymers as UV and thermal stabilisers for polyethylene films. Polym. Degrad. Stabil. 2015, 122, 18–24. [Google Scholar] [CrossRef]
- Shnawa, H.; Khaleel, M.; Muhamed, F. Oxidation of HDPE in the Presence of PVC Grafted with Natural Polyphenols (Tannins) as Antioxidant. J. Polym. Chem. 2015, 05, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Y.; Wang, J.; Wang, H.; Song, T.; Hu, W.; Li, S. Preparation and Characterization of Antioxidative and UV-Protective Larch Bark Tannin/PVA Composite Membranes. Molecules 2018, 23, 2073. [Google Scholar] [CrossRef] [PubMed]
- Nicollin, A.; Zhou, X.; Pizzi, A.; Grigsby, W.; Rode, K.; Delmotte, L. MALDI-TOF and 13C NMR analysis of a renewable resource additive—Thermoplastic acetylated tannins. Ind. Crops Prod. 2013, 49, 851–857. [Google Scholar] [CrossRef]
- Grigsby, W.J.; Bridson, J.H.; Schrade, C. Modifying biodegradable plastics with additives based on condensed tannin esters. J. Appl. Polym. Sci. 2015, 132, 41626. [Google Scholar] [CrossRef]
- Grigsby, W.; Bridson, J.; Lomas, C.; Frey, H. Evaluating Modified Tannin Esters as Functional Additives in Polypropylene and Biodegradable Aliphatic Polyester. Macromol. Mater. Eng. 2014, 299, 1251–1258. [Google Scholar] [CrossRef]
- Grigsby, W.J.; Bridson, J.H.; Lomas, C.; Elliot, J.-A. Esterification of Condensed Tannins and Their Impact on the Properties of Poly(Lactic Acid). Polymers 2013, 5, 344–360. [Google Scholar] [CrossRef]
- Grigsby, W.; Kadla, J. Evaluating Poly(lactic acid) Fiber Reinforcement with Modified Tannins. Macromol. Mater. Eng. 2014, 299, 368–378. [Google Scholar] [CrossRef]
- Bridson, J.H.; Grigsby, W.J.; Main, L. One-pot solvent-free synthesis and characterisation of hydroxypropylated polyflavonoid compounds. Ind. Crop Prod. 2018, 111, 529–535. [Google Scholar] [CrossRef]
- Shnawa, H.A.; Jahani, Y.; Khalaf, M.N. Rheological properties of PVC stabilized with tannin based epoxy resin as non metallic thermal stabilizer. Polym. Bull. 2017, 74, 1077–1090. [Google Scholar] [CrossRef]
- George, S.; Ramamurthy, K.; Anand, J.S.; Groeninckx, G.; Varughese, K.T.; Thomas, S. Rheological behaviour of thermoplastic elastomers from polypropylene/acrylonitrile–butadiene rubber blends: effect of blend ratio, reactive compatibilization and dynamic vulcanization. Polymer 1999, 40, 4325–4344. [Google Scholar] [CrossRef]
- Nakason, C.; Worlee, A.; Salaeh, S. Effect of vulcanization systems on properties and recyclability of dynamically cured epoxidized natural rubber/polypropylene blends. Polym. Test. 2008, 27, 858–869. [Google Scholar] [CrossRef]
- Cui, L.; Wang, S.; Zhang, Y.; Zhang, Y. Dynamically cured polypropylene/Novolac blends compatibilized with maleic anhydride-g-polypropylene. J. Appl. Polym. Sci. 2007, 104, 3337–3346. [Google Scholar] [CrossRef]
- Chiang, W.-Y.; Wu, W.-C.; Pukánszky, B. Modification of polypropylene, blending with resole type phenol-formaldehyde resins. Eur. Polym. J. 1994, 30, 573–580. [Google Scholar] [CrossRef]
- Larsen Børve, K.; Kristian Kotlar, H. Preparation of high viscosity thermoplastic phenol formaldehyde polymers for application in reactive extrusion. Polymer 1998, 39, 6921–6927. [Google Scholar] [CrossRef]
- Liao, J.; Brosse, N.; Pizzi, A.; Hoppe, S. Dynamically Cross-Linked Tannin as a Reinforcement of Polypropylene and UV Protection Properties. Polymers 2019, 11, 102. [Google Scholar] [CrossRef]
- Pasch, H.; Pizzi, A.; Rode, K. MALDI–TOF mass spectrometry of polyflavonoid tannins. Polymer 2001, 42, 7531–7539. [Google Scholar] [CrossRef]
- Hu, Z.; Du, X.; Liu, J.; Chang, H.; Jameel, H. Structural Characterization of Pine Kraft Lignin: BioChoice Lignin vs Indulin AT. J. Wood Chem. Technol. 2016, 36, 432–446. [Google Scholar] [CrossRef]
- Chen, F.; Dai, H.; Dong, X.; Yang, J.; Zhong, M. Physical properties of lignin-based polypropylene blends. Polym. Compos. 2011, 32, 1019–1025. [Google Scholar] [CrossRef]
- Fu, S.-Y.; Feng, X.-Q.; Lauke, B.; Mai, Y.-W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos. Part B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Bozsódi, B.; Romhányi, V.; Pataki, P.; Kun, D.; Renner, K.; Pukánszky, B. Modification of interactions in polypropylene/lignosulfonate blends. Mater. Des. 2016, 103, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Toriz, G.; Denes, F.; Young, R.A. Lignin-polypropylene composites. Part 1: Composites from unmodified lignin and polypropylene. Polym. Compos. 2002, 23, 806–813. [Google Scholar] [CrossRef]
- Liang, J.-Z.; Du, Q.; Tsui, G.; Tang, C.-Y. Tensile properties of graphene nano-platelets reinforced polypropylene composites. Compos. Part B Eng. 2016, 95, 166–171. [Google Scholar] [CrossRef]
- Qiu, W.; Endo, T.; Hirotsu, T. Interfacial interaction, morphology, and tensile properties of a composite of highly crystalline cellulose and maleated polypropylene. J. Appl. Polym. Sci. 2006, 102, 3830–3841. [Google Scholar] [CrossRef]
- Srivabut, C.; Ratanawilai, T.; Hiziroglu, S. Effect of nanoclay, talcum, and calcium carbonate as filler on properties of composites manufactured from recycled polypropylene and rubberwood fiber. Constr. Build. Mater. 2018, 162, 450–458. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.; Zhang, Y. Crystallization behavior of dynamically cured polypropylene/epoxy blends. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 1181–1191. [Google Scholar] [CrossRef]
- Canetti, M.; Bertini, F.; Chirico, A.; Audisio, G. Thermal degradation behaviour of isotactic polypropylene blended with lignin. Polym. Degrad. Stabil. 2006, 91, 494–498. [Google Scholar] [CrossRef]
- Jiang, X.; Savithri, D.; Du, X.; Pawar, S.; Jameel, H.; Chang, H.; Zhou, X. Fractionation and Characterization of Kraft Lignin by Sequential Precipitation with Various Organic Solvents. ACS Sustain. Chem. Eng. 2017, 5, 835–842. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal degradation of lignin-a review. Cellul. Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- Zoukrami, F.; Haddaoui, N.; Sclavons, M.; Devaux, J.; Vanzeveren, C. Rheological properties and thermal stability of compatibilized polypropylene/untreated silica composites prepared by water injection extrusion process. Polym. Bull. 2018, 75, 5551–5566. [Google Scholar] [CrossRef]
- Jahani, Y. Comparison of the effect of mica and talc and chemical coupling on the rheology, morphology, and mechanical properties of polypropylene composites. Polym. Advan. Technol. 2011, 22, 942–950. [Google Scholar] [CrossRef]
- Bailly, M.; Kontopoulou, M. Preparation and characterization of thermoplastic olefin/nanosilica composites using a silane-grafted polypropylene matrix. Polymer 2009, 50, 2472–2480. [Google Scholar] [CrossRef]
- Hornsby, P.; Mthupha, A. Rheological characterization of polypropylene filled with magnesium hydroxide. J. Mater. Sci. 1994, 29, 5293–5301. [Google Scholar] [CrossRef]
- Badji, C.; Soccalingame, L.; Garay, H.; Bergeret, A.; Bénézet, J.-C. Influence of weathering on visual and surface aspect of wood plastic composites: Correlation approach with mechanical properties and microstructure. Polym. Degrad. Stabil. 2017, 137, 162–172. [Google Scholar] [CrossRef]
- Lv, Y.; Huang, Y.; Yang, J.; Kong, M.; Yang, H.; Zhao, J.; Li, G. Outdoor and accelerated laboratory weathering of polypropylene: A comparison and correlation study. Polym. Degrad. Stabil. 2015, 112, 145–159. [Google Scholar] [CrossRef]
- Soccalingame, L.; Perrin, D.; Bénézet, J.-C.; Mani, S.; Coiffier, F.; Richaud, E.; Bergeret, A. Reprocessing of artificial UV-weathered wood flour reinforced polypropylene composites. Polym. Degrad. Stab. 2015, 120, 313–327. [Google Scholar] [CrossRef]
- Beg, M.D.H.; Pickering, K.L. Accelerated weathering of unbleached and bleached Kraft wood fibre reinforced polypropylene composites. Polym. Degrad. Stab. 2008, 93, 1939–1946. [Google Scholar] [CrossRef]
- Stark, N.M.; Matuana, L.M. Surface chemistry and mechanical property changes of wood-flour/high-density-polyethylene composites after accelerated weathering. J. Appl. Polym. Sci. 2004, 94, 2263–2273. [Google Scholar] [CrossRef]
- Zaaba, N.; Ismail, H.; Jaafar, M. A study of the degradation of compatibilized and uncompatibilized peanut shell powder/recycled polypropylene composites due to natural weathering. J. Vinyl Addit. Technol. 2017, 23, 290–297. [Google Scholar] [CrossRef]







| Inlet Temperature | Aspirator | Pump Rate | Nozzle Cleaner | Feed Switch Valve | Flow Meter (mm) |
|---|---|---|---|---|---|
| 150 °C | 100% | 10% | 6 | 1 | 40 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, J.; Brosse, N.; Pizzi, A.; Hoppe, S.; Xi, X.; Zhou, X. Polypropylene Blend with Polyphenols through Dynamic Vulcanization: Mechanical, Rheological, Crystalline, Thermal, and UV Protective Property. Polymers 2019, 11, 1108. https://doi.org/10.3390/polym11071108
Liao J, Brosse N, Pizzi A, Hoppe S, Xi X, Zhou X. Polypropylene Blend with Polyphenols through Dynamic Vulcanization: Mechanical, Rheological, Crystalline, Thermal, and UV Protective Property. Polymers. 2019; 11(7):1108. https://doi.org/10.3390/polym11071108
Chicago/Turabian StyleLiao, Jingjing, Nicolas Brosse, Antonio Pizzi, Sandrine Hoppe, Xuedong Xi, and Xiaojian Zhou. 2019. "Polypropylene Blend with Polyphenols through Dynamic Vulcanization: Mechanical, Rheological, Crystalline, Thermal, and UV Protective Property" Polymers 11, no. 7: 1108. https://doi.org/10.3390/polym11071108








