Synthesis and Characterization of Room Temperature Vulcanized Silicone Rubber Using Methoxyl-Capped MQ Silicone Resin as Self-Reinforced Cross-Linker
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
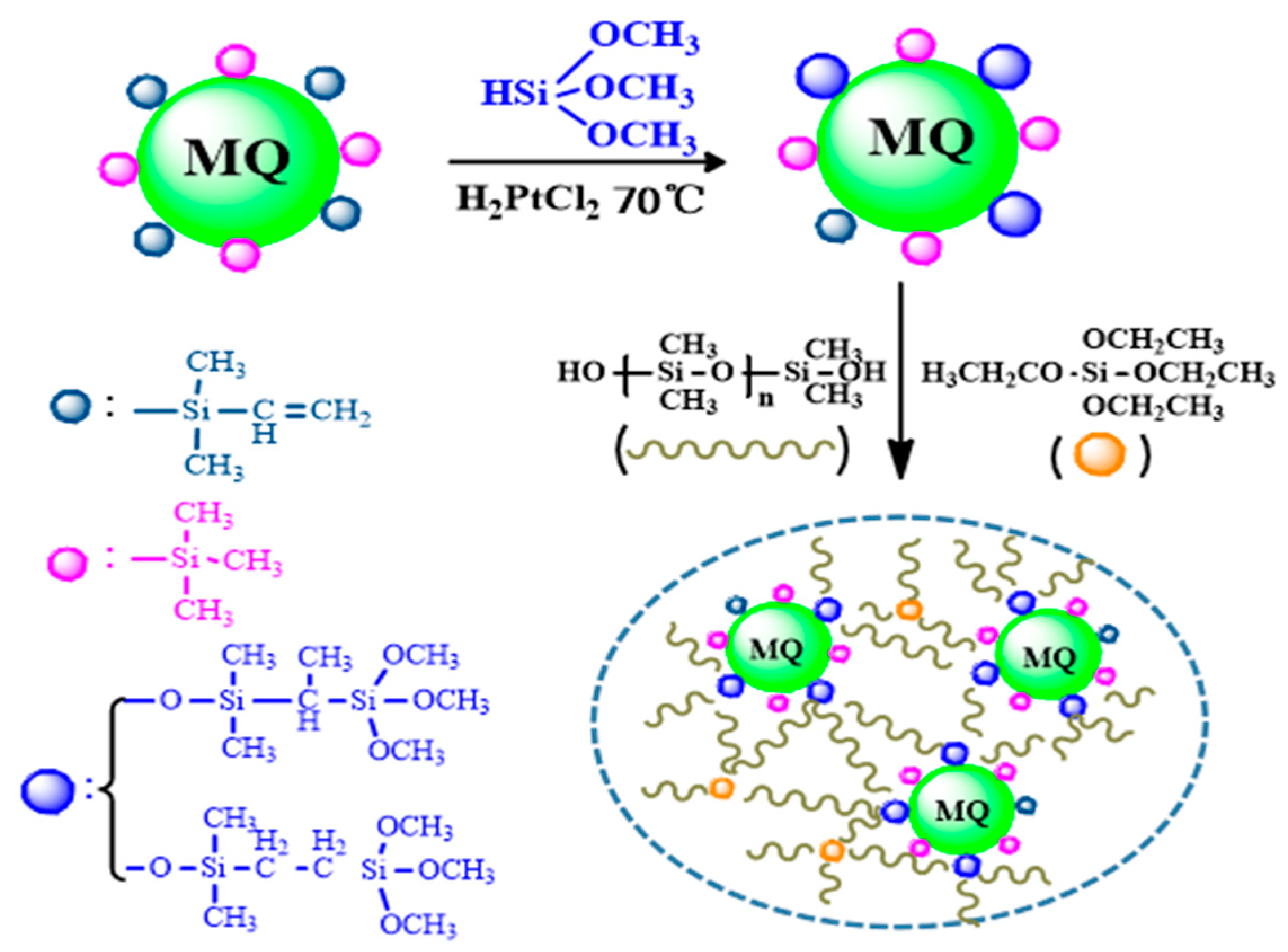
2.2. Synthesis of the MMQ
2.3. Synthesis of the Condensed RTV Silicone Rubber
2.4. Characterizations and Measurements
2.5. The Conversion of Vinyl in VMQ
2.6. Mass Fraction of Methoxy Groups in MMQ
2.7. Swelling Experiment
3. Results and Discussion
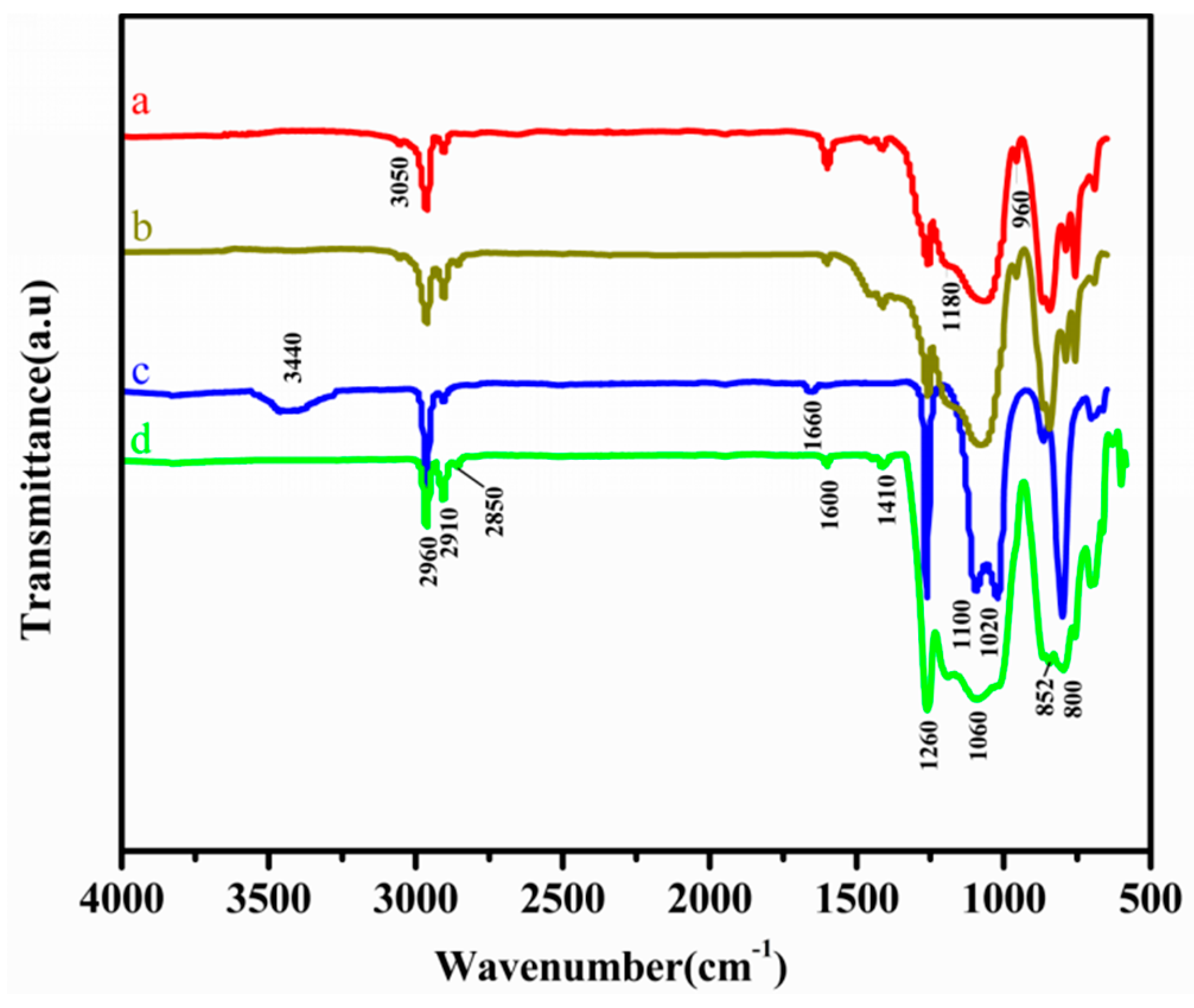
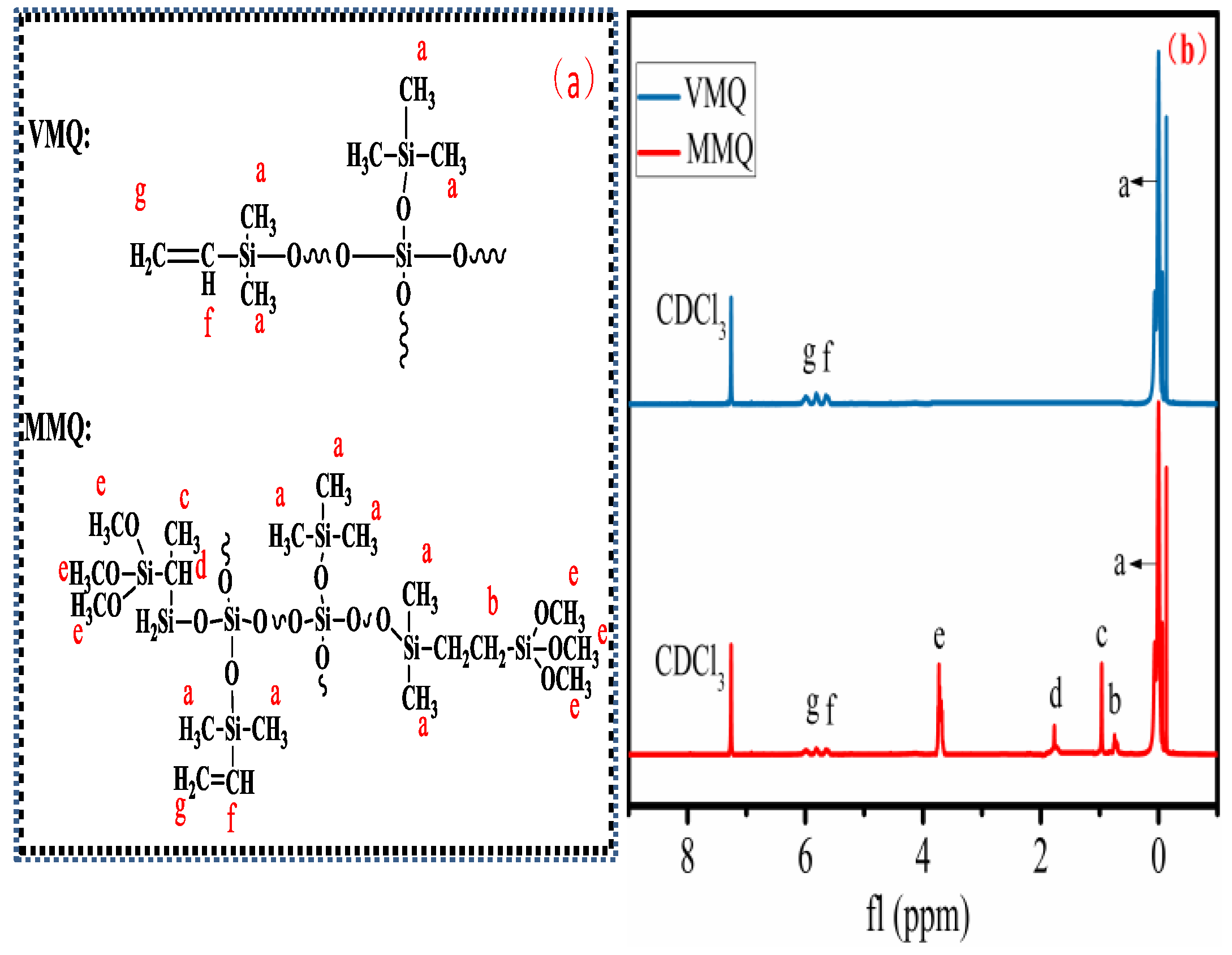
3.1. Structural Analysis
3.2. Morphology of the Silicone Rubber
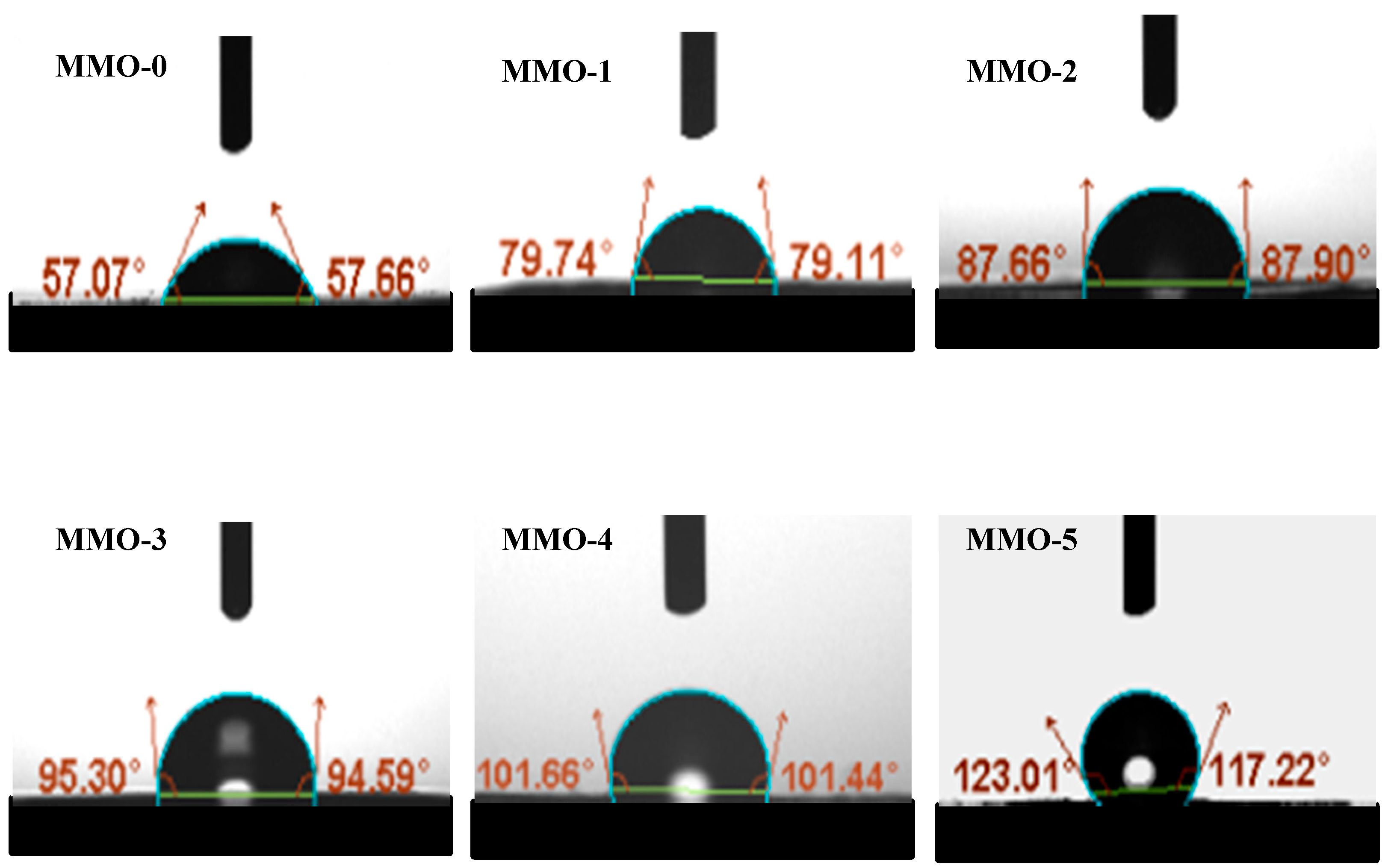
3.3. Hydrophobicity of the RTV Silicone Rubber
3.4. Cross-Linking Density of the RTV Rubber
3.5. Mechanical Properties of RTV Silicone Rubber
3.6. Dynamic Mechanical Properties Analysis
3.7. The Transmittance of RTV Silicone Rubber
3.8. Thermal Stability of RTV Silicone Rubber
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shit, S.C.; Shah, P. A review on silicone rubber. Natl. Acad. Sci. Lett. 2013, 36, 355–365. [Google Scholar] [CrossRef]
- Sun, F.; Hu, Y.; Du, H. Synthesis and characterization of MQ silicone resins. J. Appl. Polym. Sci. 2012, 125, 3532–3536. [Google Scholar] [CrossRef]
- Seyedmehdi, S.A.; Zhang, H.; Zhu, J. Superhydrophobic RTV silicone rubber insulator coatings. Appl. Surf. Sci. 2012, 258, 2972–2976. [Google Scholar] [CrossRef]
- Gu, J.; Meng, X.; Tang, Y.; Li, Y.; Zhuang, Q.; Kong, J. Hexagonal boron nitride/polymethyl-vinyl siloxane rubber dielectric thermally conductive composites with ideal thermal stabilities. Compos. Part A Appl. Sci. Manuf. 2017, 92, 27–32. [Google Scholar] [CrossRef]
- Shankar, B.S.; Ramakrishnan, I.; Satapathy, B.K. Rheological behavior and network dynamics of silica filled vinyl-terminated polydimethylsiloxane suspensions. Polym. Eng. Sci. 2017, 57, 973–981. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, G.; Shi, Y.; Zhang, Y.; Liu, F. Polyhedral oligomeric silsesquioxane/silica/polydimet hylsiloxane rubber composites with enhanced mechanical and thermal properties. J. Appl. Polym. Sci. 2015, 132, 42173–42180. [Google Scholar]
- Wang, J.; Ji, C.; Yan, Y.; Zhao, D.; Shi, L. Mechanical and ceramifiable properties of silicone rubber filled with different inorganic fillers. Polym. Degrad. Stabil. 2015, 121, 149–156. [Google Scholar] [CrossRef]
- Liang, W.; Ge, X.; Ge, J.; Li, T.; Zhao, T.; Chen, X.; Song, Y.; Cui, Y.; Khan, M.; Ji, J.; et al. Reduced Graphene Oxide Embedded with MQ Silicone Resin Nano-Aggregates for Silicone Rubber Composites with Enhanced Thermal Conductivity and Mechanical Performance. Polymers 2018, 10, 1254. [Google Scholar] [CrossRef]
- Yang, H.; Gao, Q.; Xie, Y.; Chen, Q.; Ouyang, C.; Xu, Y.; Ji, X. Effect of SiO2 and TiO2 nanoparticle on the properties of phenyl silicone rubber. J. Appl. Polym. Sci. 2015, 132, 42806–42814. [Google Scholar] [CrossRef]
- Ramli, M.R.; Othman, M.B.H.; Arifin, A.; Ahmad, Z. Cross-link network of polydim ethy-lsiloxane via addition and condensation (RTV) mechanisms. Part I Synth. Therm. Prop. Polym. Degrad. Stabil. 2011, 96, 2064–2070. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, J.; Yang, Q.; Shi, L.; Qi, S.; Jin, R. Novel polymethoxylsiloxane-based crosslinking reagent and its in-situ improvement for thermal and mechanical properties of siloxane elastomer. Appl. Surf. Sci. 2008, 107, 3788–3795. [Google Scholar] [CrossRef]
- Li, Q.; Huang, X.; Liu, H.; Shang, S.; Song, Z.; Song, J. Properties enhancement of room temperature vulcanized silicone rubber by rosin modified aminopropylt- riethoxysilane as a cross-linking agent. ACS Sustain. Chem. Eng. 2017, 5, 10002–10010. [Google Scholar] [CrossRef]
- Sirin, H.; Kodal, M.; Karaagac, B.; Ozkoc, G. Effects of octamaleamic acid-POSS used as the adhesion enhancer on the properties of silicone rubber/silica nanocomposites. Compos. Part B Eng. 2016, 98, 370–381. [Google Scholar] [CrossRef]
- Chen, D.; Chen, X.; Hu, Y.; Zhang, W.; Yin, Z.; Zhou, S. Thermal stability, mechanical and optical properties of novel addition cured PDMS composites with nano-silica sol and MQ silicone resin. Compos. Sci. Technol. 2015, 117, 307–314. [Google Scholar] [CrossRef]
- Di, H.; Wang, L.; Zhang, Y.; Luo, L.; Li, H.; Huang, R. Improved processibility of silicone composites by MQ silicone resins. Appl. Surf. Sci. 2018, 135, 46445. [Google Scholar]
- Flagg, D.H.; Mccarthy, T.J. Rediscovering silicones: MQ Copolymers. Macromo Lecules 2016, 49, 8581–8592. [Google Scholar] [CrossRef]
- He, M.; Zhang, Q.Y.; Guo, J.Y. Synthesis and Characterization of Silicone Based Pressure Sensitive Adhesive. Adv. Mater. Res. 2011, 306–307, 1773–1778. [Google Scholar] [CrossRef]
- Abe, T.; Konishi, M.; Hayakawa, C.; Kamei, M. Organic Group-Modified Organosilicon Resin, Making Method, and Cosmetics. U.S. Patent 15,923,478, 20 September 2018. [Google Scholar]
- Li, H.Y. A rapid accurate volumetric analysis for Si-OEt-Perchloric acid acetylated determination. Silicone Mater. 1996, 6, 18–21. (In Chinese) [Google Scholar]
- Chen, D.; Yi, S.; Fang, P.; Zhong, Y.; Huang, C.; Wu, X. Synthesis and characterization of novel room temperature vulcanized (RTV) silicone rubbers using octa[(trimethoxysilyl)ethyl]-POSS as cross-linker. React. Funct. Polym. 2011, 71, 502–511. [Google Scholar] [CrossRef]
- Chen, J.; Fu, Z.; Huang, H.; Zeng, X.; Chen, Z. Preparation and properties of vinylphenyl-silicone resins and their application in LED packaging. RSC Adv. 2016, 6, 71924–71933. [Google Scholar] [CrossRef]
- Liu, T.; Sun, C.; Ma, F. Study on the synthesis and thermal degradation of vinylphenylpolysilsesquioxane. J. Anal. Appl. Pyrol. 2018, 130, 249–255. [Google Scholar] [CrossRef]
- Indulekha, K.; Behera, P.K.; Rajeev, R.S.; Gouri, C.; Ninan, K.N. Polyfluoroalkyl siloxanes with varying trifluoropropyl content: Synthesis, characterization and solvent resistance studies. J. Fluor. Chem. 2017, 200, 24–32. [Google Scholar] [CrossRef]
- Yang, Z.; Feng, L.; Diao, S.; Feng, S.; Zhang, C. Study on the synthesis and thermal degradation of silicone resin containing silphenylene units. Thermochim. Acta 2011, 521, 170–175. [Google Scholar] [CrossRef]
- Shi, Y.; Gao, X.; Zhang, D.; Liu, Y.; Huang, G. Synthesis and thermal properties of modified room temperature vulcanized (RTV) silicone rubber using polyhedral oligomeric silsesqui oxane(POSS) as across linking agent. RSC Adv. 2014, 4, 41453–41460. [Google Scholar] [CrossRef]
- Li, Z.; Han, W.; Kozodaev, D.; Brokken-Zijp, J.C.M.; With, G.; Thüne, P.C. Surface properties of poly(dimethylsiloxane)-based inorganic/organic hybrid materials. Polymer 2006, 47, 1150–1158. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, S.; Sun, W.; Chen, W.; Dong, J.; Wen, J.; Zhang, J.; Li, Z.; Zheng, L.; Chen, C.; et al. Discovering partially charged single-atom Pt for enhanced anti-markovnikov alkene hydros-ilylation. J. Am. Chem. Soc. 2018, 140, 7407–7410. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Zheng, S.; Nie, K. Morphology and thermal properties of inorganic–organic hybrids involving epoxy resin and polyhedral oligomeric silsesquioxanes. Polymer 2004, 45, 5557–5568. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, F.; Williams, A.J.; Qu, X.; Feng, J.J.; Chen, C.H. Self-propelled droplet removal from hydrophobic fiber-based coalescers. Phys. Rev. Lett. 2015, 115, 074502. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, J.; Shi, L.; Qi, S.; Cheng, J.; Jin, R. Improvement of thermal resistance of polydimethylsiloxanes with polymethylmethoxysiloxane as cross-linker. Polym. Degrad. Stabil. 2008, 93, 242–251. [Google Scholar] [CrossRef]
- Seyedmehdi, S.A.; Zhang, H.; Zhu, J. Influence of production method, silicone type and thickness on silicon rubber superhydrophobic coatings. Prog. Org. Coat. 2016, 90, 291–295. [Google Scholar] [CrossRef]
- Lee, J.N.; Park, C.; Whitesides, G.M. Solvent compatibility of poly (dimethylsilo xane)-based microfluid- ic devices. Anal. Chem. 2003, 75, 6544–6554. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Huang, G.; Liu, Y.; Qu, Y.; Zhang, D.; Dang, Y. Synthesis and thermal properties of novel room temperature vulcanized (RTV) silicone rubber containing POSS units in polysioxane main chains. J. Polym. Res. 2013, 20, 245–255. [Google Scholar] [CrossRef]
- Xu, T.; Liu, H.; Song, J.; Song, Z.; Zou, K.; Yang, C. Synthesis and characterization of novel fluorosilicone rubber using imide modified vinyl-containing fluorosilicone resin as cross-linker. J. Polym. Sci. Pol. Chem. 2015, 53, 1769–1776. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Chen, F.; Zhang, H.; Yin, X.; Liu, X.; Zhou, Y. Preparation and characterization of novel addition cured polydimethylsiloxane nanocomposites using nano-silica sol as reinforcing filler. Polym. Int. 2015, 64, 1741–1746. [Google Scholar] [CrossRef]
- Wu, J.; Dong, J.; Wang, Y.; Gond, B.K. Thermal oxidation ageing effects on silicone rubber sealing performance. Polym. Degrad. Stabil. 2017, 135, 43–53. [Google Scholar] [CrossRef]
- Teo, J.K.H.; Teo, K.C.; Pan, B.; Xiao, Y.; Lu, X. Epoxy/polyhedral oligomeric silsesquioxane (POSS) hybrid networks cured with an anhydride: Cure kinetics and thermal properties. Polymer 2007, 48, 5671–5680. [Google Scholar]
- Wang, H.; Wang, H.; Zhou, G. Synthesis of rosin-based imidoamine-type curing agents and curing behavior with epoxy resin. Polym. Int. 2011, 60, 557–563. [Google Scholar] [CrossRef]
- Lee, J.K.; Koh, W.K.; Chae, W.S.; Kim, Y.R. Novel synthesis of organic nanowires and their optical properties. Chem. Commun. 2002, 21, 138–139. [Google Scholar] [CrossRef]
- Krč, J.; Zeman, M.; Kluth, O.; Smole, F.; Topič, M. Effect of surface roughness of ZnO: Al films on light scattering in hydrogenated amorphous silicon solar cells. Thin Solid Films 2003, 426, 296–304. [Google Scholar] [CrossRef]
- Simionescu, B.C.; Bordianu, I.E.; Tudorachi, N.; Cotofana, C.; Ursu, L.; Coroaba, A.; Drobota, M.; Olaru, M. Effects of chemical structure and chain end groups on the thermal stability of new silsesquioxanes with methacrylate and/or vinyl units. Mater. Chem. Phys. 2013, 139, 719–733. [Google Scholar] [CrossRef]





| Sample | HPDMS/g | MMQ/g | TEOS/g | DBTDL/μL | MMQ/wt % | Alkoxy of MMQ/mmol | Alkoxy of TEOS/mmol | Total Alkoxy /mmol |
|---|---|---|---|---|---|---|---|---|
| MMQ-0 | 50 | 0 | 3.19 | 200 | 0 | 0 | 61.37 | 61.37 |
| MMQ-1 | 50 | 5.87 | 2.85 | 200 | 10 | 6.52 | 54.84 | 61.37 |
| MMQ-2 | 50 | 13.11 | 2.43 | 200 | 20 | 14.56 | 46.81 | 61.37 |
| MMQ-3 | 50 | 22.25 | 1.91 | 200 | 30 | 24.72 | 36.65 | 61.37 |
| MMQ-4 | 50 | 34.15 | 1.22 | 200 | 40 | 37.94 | 23.43 | 61.37 |
| MMQ-5 | 50 | 50.29 | 0.29 | 200 | 50 | 55.87 | 5.50 | 61.37 |
| Sample | Temperature of 10% Weight Loss/°C | Temperature at Maximum Degradation Rate/°C | Residual Yield at 800 °C/% |
|---|---|---|---|
| MMQ-0 | 353.5 | 408.9 | 1.2 |
| MMQ-1 | 438.5 | 426.3 | 15.9 |
| MMQ-2 | 449.8 | 509.2 | 19.1 |
| MMQ-3 | 454.3 | 510.7 | 21.9 |
| MMQ-4 | 477.1 | 528.4 | 27.7 |
| MMQ-5 | 468.8 | 515.4 | 23.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, J.; Ge, X.; Pang, X.; Liu, R.; Wen, S.; Sun, J.; Liang, W.; Ge, J.; Chen, X. Synthesis and Characterization of Room Temperature Vulcanized Silicone Rubber Using Methoxyl-Capped MQ Silicone Resin as Self-Reinforced Cross-Linker. Polymers 2019, 11, 1142. https://doi.org/10.3390/polym11071142
Ji J, Ge X, Pang X, Liu R, Wen S, Sun J, Liang W, Ge J, Chen X. Synthesis and Characterization of Room Temperature Vulcanized Silicone Rubber Using Methoxyl-Capped MQ Silicone Resin as Self-Reinforced Cross-Linker. Polymers. 2019; 11(7):1142. https://doi.org/10.3390/polym11071142
Chicago/Turabian StyleJi, Jianye, Xin Ge, Xiaoyan Pang, Ruoling Liu, Shuyi Wen, Jiaqi Sun, Weijie Liang, Jianfang Ge, and Xunjun Chen. 2019. "Synthesis and Characterization of Room Temperature Vulcanized Silicone Rubber Using Methoxyl-Capped MQ Silicone Resin as Self-Reinforced Cross-Linker" Polymers 11, no. 7: 1142. https://doi.org/10.3390/polym11071142
APA StyleJi, J., Ge, X., Pang, X., Liu, R., Wen, S., Sun, J., Liang, W., Ge, J., & Chen, X. (2019). Synthesis and Characterization of Room Temperature Vulcanized Silicone Rubber Using Methoxyl-Capped MQ Silicone Resin as Self-Reinforced Cross-Linker. Polymers, 11(7), 1142. https://doi.org/10.3390/polym11071142





