Metallo-Supramolecular Hydrogels from the Copolymers of Acrylic Acid and 4-(2,2?:6?,2?-terpyridin-4?-yl)styrene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of 4-(2,2′:6′,2″-terpyridin-4′-yl)styrene (TPY)
2.2. Synthesis of 4-(2,2′:6′,2″-terpyridin-4′-yl)benzyl Bromide (2)
2.3. Synthesis of 4-(2,2′:6′,2″-terpyridin-4′-yl)benzyl triphenylphosphonium Bromide (3)
2.4. Synthesis of 4-(2,2′:6′,2″-terpyridin-4′-yl)styrene (TPY)
2.5. Synthesis of Poly (acrylic acid-co-4-(2,2′:6′,2″-terpyridin-4′-yl)styrene) (P(AA-co-TPY))
2.6. Preparation of the Metallo-Supramolecular Hydrogels
3. Results and Discussion
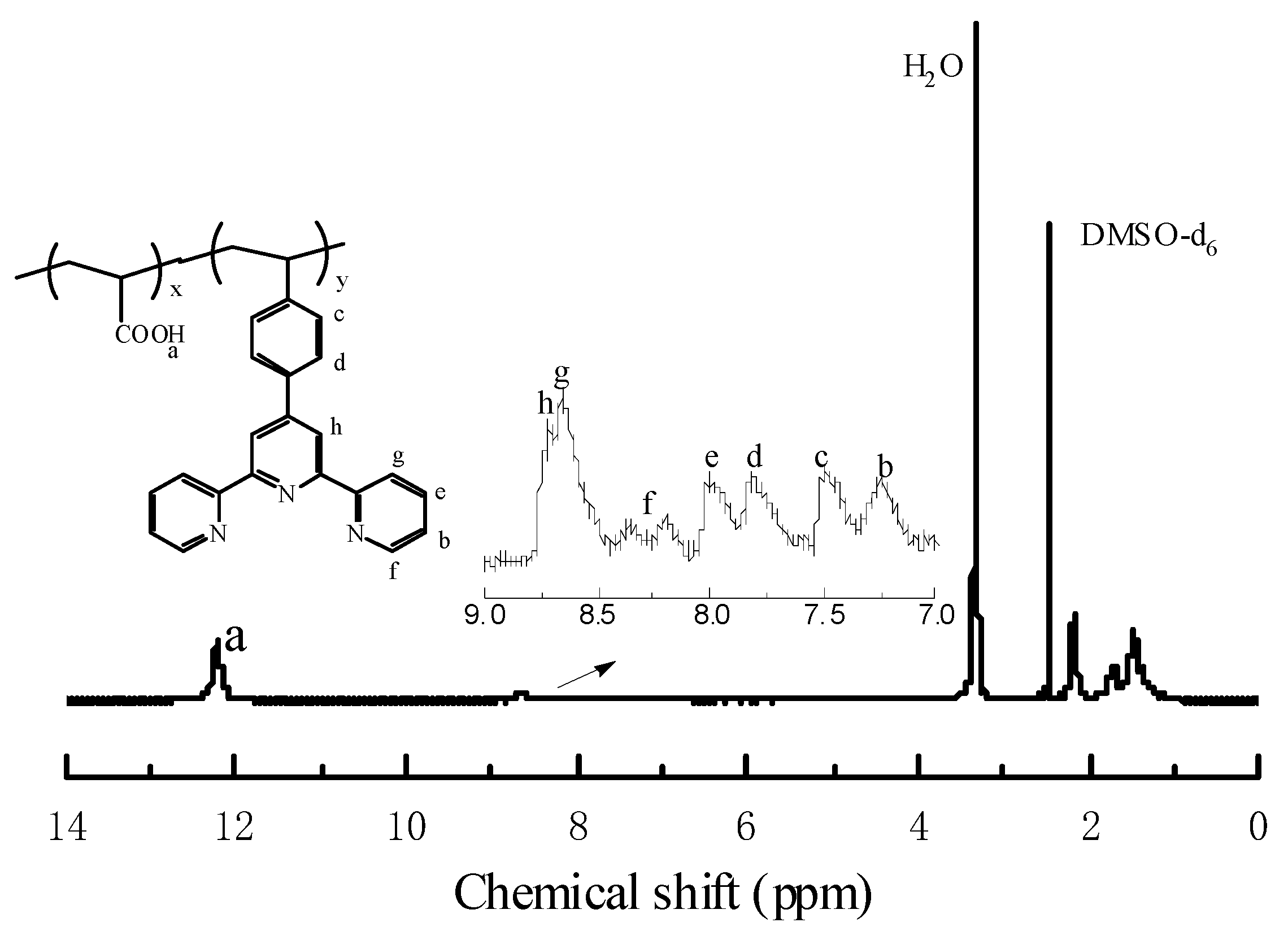
3.1. Synthesis and Characterization of the Copolymer Ligands
3.2. UV-Visible Properties of the Dilute Copolymer Solution and the Coordination Interaction with Transition Ions
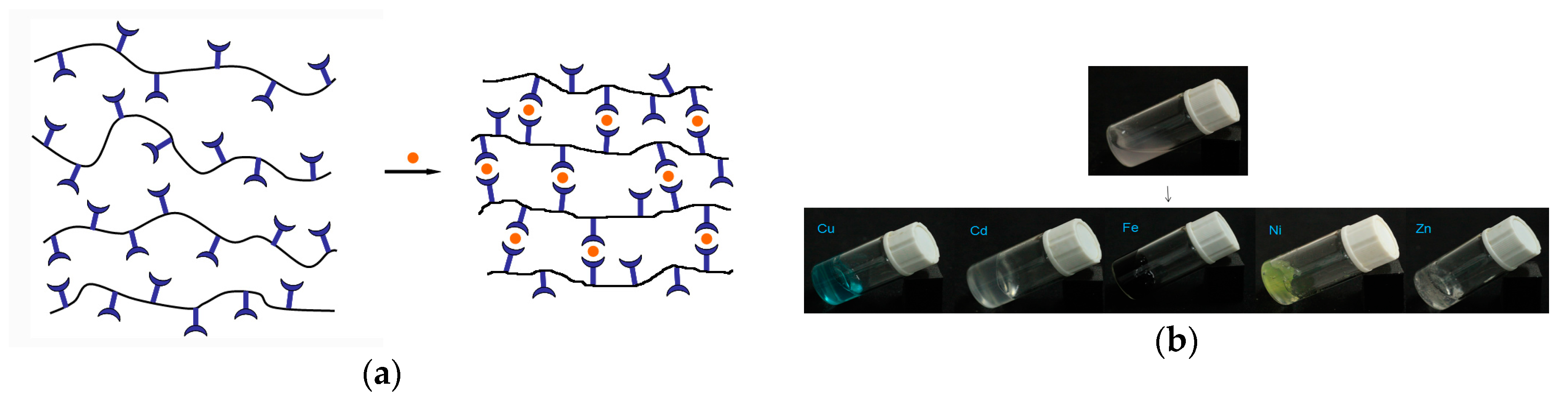
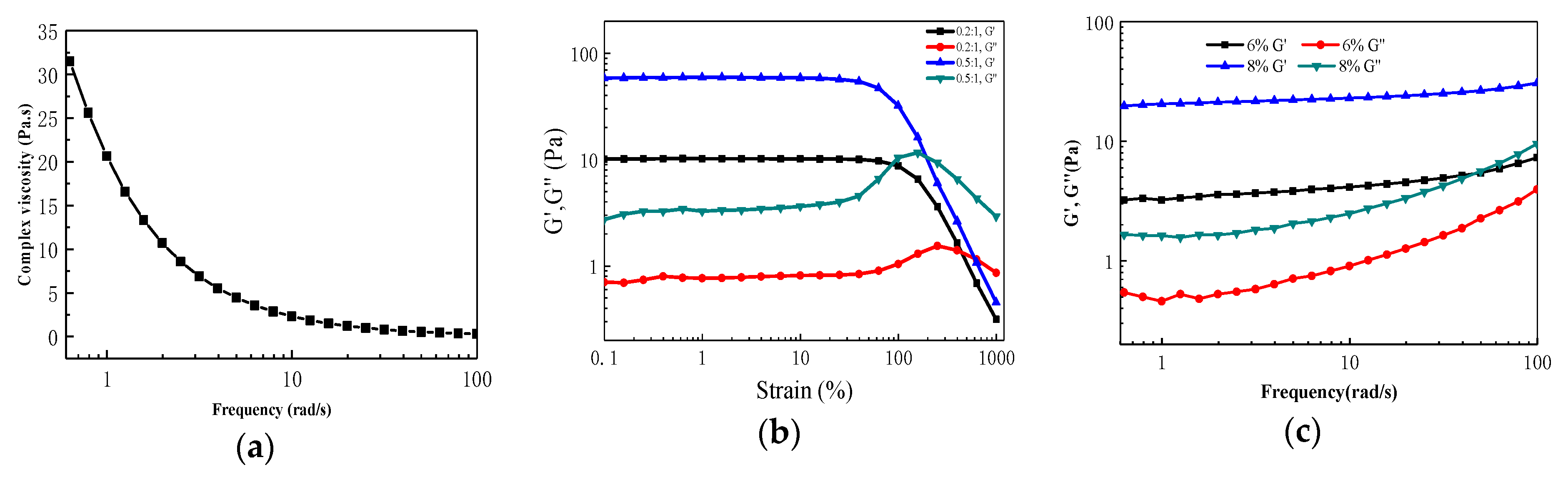
3.3. Preparation and Characterization of Metallo-Supramolecular Hydrogel
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lehn, J.-M. Supramolecular chemistry-scope and perspectives molecules, supermolecules, and molecular devices. Angew. Chem. Int. Ed. 1988, 27, 89–112. [Google Scholar] [CrossRef]
- Lehn, J.-M. Supramolecular Chemistry; VCH: Weinheim, Germany, 1995. [Google Scholar]
- De Greef, T.F.A.; Smulders, M.M.J.; Wolffs, M.; Schenning, A.P.H.J.; Sijbesma, R.P.; Meijer, E.W. Supramolecular Polymerization. Chem. Rev. 2009, 109, 5687–5754. [Google Scholar] [CrossRef] [PubMed]
- Burnworth, M.; Tang, L.; Kumpfer, J.R.; Duncan, A.J.; Beyer, F.L.; Fiore, G.L.; Rowan, S.J.; Weder, C. Optically healable supramolecular polymers. Nature 2011, 472, 334–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofmeier, H.; Schubert, U.S. Recent developments in the supramolecular chemistry of terpyridine-metal complexes. Chem. Soc. Rev. 2004, 33, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Beletskaya, I.; Tyurin, V.S.; Tsivadze, A.Y.; Guilard, R.; Stern, C. Supramolecular Chemistry of Metalloporphyrins. Chem. Rev. 2009, 109, 1659–1713. [Google Scholar] [CrossRef] [PubMed]
- Flory, P.J. Principles of Polymer Chemistry; Cornel University Press: New York, NY, USA, 1953. [Google Scholar]
- Peppas, N.A. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2010, 18, 1345–1360. [Google Scholar] [CrossRef]
- Bryant, S.J.; Anseth, K.S. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J. Biomed. Mater. Res. A 2010, 59, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Uragami, T.; Nakamae, K. Biomolecule-sensitive hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 79–98. [Google Scholar] [CrossRef]
- Wu, Y.L.; Chen, X.H.; Wang, W.Z. Engineering bioresponsive hydrogels toward healthcare applications. Macromol. Chem. Phys. 2016, 217, 175–188. [Google Scholar] [CrossRef]
- Eid, M. Preparation and magnetic investigation of magnetic nanoparticles entrapped hydrogels and its possible use as radiation shield. J. Inorg. Organomet. Polym. Mater. 2013, 23, 1255–1265. [Google Scholar] [CrossRef]
- Ma, M.; Huang, Y.; Gao, Y.; Zhang, Y.; Gao, P.; Xu, B. Aromatic-aromatic interactions induce the self-assembly of pentapeptidic derivatives in water to form nanofibers and supramolecular hydrogels. J. Am. Chem. Soc. 2010, 132, 2719–2728. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Liu, L.; Yuan, S.; Sun, J.; Li, Z. pH-Responsive peptide supramolecular hydrogels with antibacterial activity. Langmuir 2017, 33, 3234–3240. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Hu, J.; Wang, H.; Huang, J.; Yu, Y.; Zhang, Q.; Cheng, Y. Dynamic softening or stiffening a supramolecular hydrogel by ultraviolet or near-infrared light. ACS Appl. Mater. Interfaces 2017, 9, 24511–24517. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Dufresne, A. Supramolecular hydrogels from in situ host−guest inclusion between chemically modified cellulose nanocrystals and cyclodextrin. Biomacromolecules 2013, 14, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.; Wang, H.-S.; Zhong, C.; Chu, L.-Q. Facile fabrication of moldable antibacterial carboxymethyl chitosansupramolecular hydrogels crosslinked by metal ions complexation. Carbohydr. Polym. 2017, 165, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, Z.; Li, Y.; Geng, L.; Ren, J.; Feng, G. Fluorescent and electrochemical supramolecular coordination polymer hydrogels formed from ion-tuned self-assembly of small bis-terpyridine monomer. Inorg. Chem. 2017, 56, 7512–7518. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Le, X.; Zhang, J.; Huang, Y.; Chen, T. Supramolecular shape memory hydrogels: A new bridge between stimuli-responsive polymers and supramolecular chemistry. Chem. Soc. Rev. 2017, 46, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Wang, L.; Feng, X.; Bu, Y.; Wu, D.; Jin, Z. Supramolecular Hydrogel Formation Based on Tannic Acid. Macromolecules 2017, 50, 666–676. [Google Scholar] [CrossRef]
- Arnedo-Sánchez, L.; Bhowmik, S.; Hietala, S.; Puttreddy, R.; Lahtinen, M.; De Cola, L.; Rissanen, K. Rapid self-healing and anion selectivity in metallosupramolecular gels assisted by fluorine-fluorine interactions. Dalton Trans. 2017, 46, 7309–7331. [Google Scholar] [CrossRef]
- Gasnier, A.; Bucher, C.; Moutet, J.C. Redox-responsive metallo-supramolecular polymers and gels containing bis-terpyridine appended cyclam ligand. Macromol. Symp. 2011, 304, 87–92. [Google Scholar] [CrossRef]
- Yuan, J.C.; Fang, X.L.; Zhang, L.X. Multi-responsive self-healing metallo-supramolecular gels based on “click” ligand. J. Mater. Chem. 2012, 22, 11515–11522. [Google Scholar] [CrossRef]
- Theis, S.; Iturmendi, A.; Gorsche, C. Metallo-supramolecular photocleavable gels sensitive to visible and near infrared irradiation. Angew. Chem. Int. Ed. 2017, 56, 15857–15860. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.Y.; Chen, X.L.; Wang, L. Metallo-supramolecular hydrogels based on amphiphilic polymers bearing hydrophobic schiff base ligand with rapid self-healing and multi-stimuli responsive properties. Polym. Chem. 2017, 8, 4680–4687. [Google Scholar] [CrossRef]
- Whiteoak, C.J.; Salassa, G.; Kleij, A.W. Recent advances with π-conjugated salen systems. Chem. Soc. Rev. 2012, 41, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Yuan, J. Schiff’s base as a stimuli-responsive linker in polymer chemistry. Polym. Chem. 2012, 3, 3045–3055. [Google Scholar] [CrossRef]
- Andruh, M. Compartmental Schiff-base ligands-a rich library of tectons in designing magnetic and luminescent materials. Chem. Commun. 2011, 47, 3025–3042. [Google Scholar] [CrossRef] [PubMed]
- Jochum, F.D.; Brassinne, J.; Fustin, C.-A.; Gohy, J.F. Metallo-supramolecular hydrogels based on copolymers bearing terpyridine side-chain ligands. Soft Matter 2013, 9, 2314–2320. [Google Scholar] [CrossRef]
- Duerrbeck, A.; Gorelik, S. New solid-state Eu(III)-containing metallo-supramolecular polymers: Morphology control and optical wave-guiding properties. Mater. Chem. C 2015, 3, 8992–9002. [Google Scholar] [CrossRef]
- Hanabusa, K.; Nakamura, A.; Koyama, T. Synthesis, polymerization, copolymerization, and transition-metal coordination of 4-(2,2′:6′,2″-terpyridin-4′-yl)styrene and its polymers and copolymers. Macromol. Chem. Phys. 1992, 193, 1309–1319. [Google Scholar] [CrossRef]
- Holyer, R.H.; Hubbard, C.D.; Kettle, S.F.; Wilkins, A.R.G. The kinetics of replacement reactions of complexes of the transition metals with 2,2′,2″-terpyridine. Inorg. Chem. 1966, 5, 622–625. [Google Scholar] [CrossRef]









| Concentration of Fe2+ | Lower Wavelength | Longer Wavelength | ||
|---|---|---|---|---|
| λmax,abs (nm) | ε (L/mol.cm) | λmax,abs (nm) | ε (L/mol.cm) | |
| 0 | 305 | 29,790 | 570 | 0 |
| 0.2 | 308 | 34,820 | 570 | 6280 |
| 0.4 | 311 | 41,660 | 570 | 10,760 |
| 0.6 | 322 | 47,460 | 570 | 15,000 |
| 0.8 | 323 | 53,870 | 570 | 18,990 |
| 1.0 | 326 | 62,280 | 570 | 24,140 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Liu, P.; Chi, Z.; Chen, X. Metallo-Supramolecular Hydrogels from the Copolymers of Acrylic Acid and 4-(2,2?:6?,2?-terpyridin-4?-yl)styrene. Polymers 2019, 11, 1152. https://doi.org/10.3390/polym11071152
Zhang H, Liu P, Chi Z, Chen X. Metallo-Supramolecular Hydrogels from the Copolymers of Acrylic Acid and 4-(2,2?:6?,2?-terpyridin-4?-yl)styrene. Polymers. 2019; 11(7):1152. https://doi.org/10.3390/polym11071152
Chicago/Turabian StyleZhang, Huiqin, Pan Liu, Zheng Chi, and Xuegang Chen. 2019. "Metallo-Supramolecular Hydrogels from the Copolymers of Acrylic Acid and 4-(2,2?:6?,2?-terpyridin-4?-yl)styrene" Polymers 11, no. 7: 1152. https://doi.org/10.3390/polym11071152
APA StyleZhang, H., Liu, P., Chi, Z., & Chen, X. (2019). Metallo-Supramolecular Hydrogels from the Copolymers of Acrylic Acid and 4-(2,2?:6?,2?-terpyridin-4?-yl)styrene. Polymers, 11(7), 1152. https://doi.org/10.3390/polym11071152




