Effect of Sulfonic Groups Concentration on IEC Properties in New Fluorinated Copolyamides
Abstract
:1. Introduction
2. Materials
3. Methodology
3.1. Copolyamide Synthesis
3.1.1. ISO Purification Method
3.1.2. Synthesis of Sulfonated Copolyamides
3.2. Film Preparation
3.3. Characterization
3.3.1. Mechanical Properties
3.3.2. Water Absorption Capacity (WAC)
3.3.3. Ion Exchange Capacity (IEC)
3.3.4. Proton Conductivity Determination
4. Results
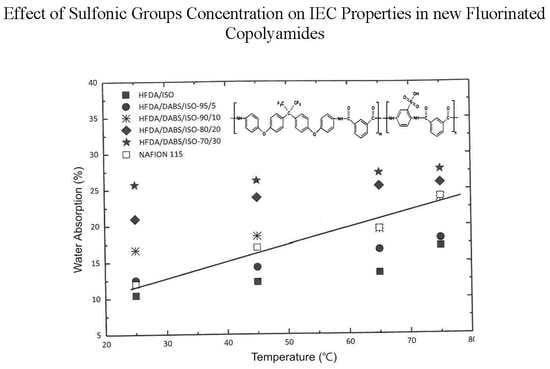
4.1. Water Absorption Capacity (WAC)
4.2. Ion Exchange Capacity (IEC)
4.3. Proton Conductivity
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Baroutaji, A.; Carton, J.G.; Sajjia, M.; Olabi, A.G. Materials in PEM Fuel Cells. Ref. Module Mater. Sci. Mater. Eng. 2015. [Google Scholar] [CrossRef]
- Hernández-Flores, G.; Poggi-Varaldo, H.M.; Solorza-Feria, O.; Romero-Castañón, T.; Ríos-Leal, E.; Galíndez-Mayer, J.; Esparza-García, F. Batch operation of a microbial fuel cell equipped with alternative proton exchange membrane. Inter. J. Hydrogen Energy 2015, 40, 17323–17331. [Google Scholar] [CrossRef]
- Carrete, L.; Friedrich, K.A.; Stimming, U. Fuel Cells: Principles, Types, Fuels, and Applications. ChemPhysChem 2000, 200, 162–193. [Google Scholar] [CrossRef]
- Borup, R.; Meyers, J.; Pivovar, B.; Kim, Y.S.; Mukundan, R.; Garland, N.; Myers, D.; Wilson, M.; Garzon, F.; Wood, D. Scientific Aspects of Polymers Fuel Cell Durability and Degradation. Chem. Rev. 2007, 107, 3904–3951. [Google Scholar] [CrossRef] [PubMed]
- Gubler, L.; Scherer, G.G. A Proton-Conducting Polymer Membrane as Solid Electrolyte—Function and Required Properties. In Fuel Cells I. Advances in Polymer Science; Scherer, G.G., Ed.; Springer: Berlin, Heidelberg, Germany, 2008; Volume 215, pp. 1–14. [Google Scholar]
- Wilkinson, D.P.; Zhang, J.; Hui, R.; Fergus, J.; Li, X. Proton Exchange Membrane Fuel Cell. In Materials Properties and Performance; CRC Press: Boca Raton, FL, USA, 2009; Chapter 1; pp. 1–60. [Google Scholar]
- Eisnla, R.B. High Temperature Polymers for Proton Exchange for Fuel Cells. Ph.D. Thesis, Faculty of Virginia Politechnic Institute and State University, Blacksburg, VA, USA, 2005; pp. 1–307. [Google Scholar]
- Zhao, T.S.; Xu, C.; Chen, R.; Yang, W.W. Mass Transport Phenomenain Direct Methnol Fuel Cells. Prog Energyand Combus. Sci. 2009, 35, 275–292. [Google Scholar] [CrossRef]
- Helen, M.; Viswanathan, B.; Himakumar, L.; Srinivasamurthy, S. Strategies for the design of membranes for fuel cells. In Photo/Electrochemistry and Photobiology in the Environment, Energy and Fuel; Funasaka, K., Satoshi Kaneco, Y.A., Eds.; Research Signpost: Kerala, India, 2006; Volume 5, pp. 1–42. [Google Scholar]
- Cai, H.; Shao, K.; Zhong, S.; Zhao, C.; Zhang, G.; Li, X.; Na, H. Properties of Composite Membranes Based on Sulfonated Poly(ether ether ketone)s(SPEEK)/Phenoxy Resin (PHR) for Direct Methanol Fuel Cells Usages. J. Membr. Sci. 2007, 297, 162–173. [Google Scholar] [CrossRef]
- Basile, A.; Paturzo, L.; Iulianelli, A.; Gatto, I.; Passalacqua, E. Sulfonated PEEK-WC Membranes for Proton-Exchange Membrane Fuel Cell: Effect of the Increasing Level of Sulfonation on Electrochemical Performances. J. Membr. Sci. 2006, 281, 377–385. [Google Scholar] [CrossRef]
- Li, X.; Hao, X.; Xu, D.; Zhang, G.; Zhong, S.; Na, H.; Wang, D. Fabrication of Sulfonated Poly(ether ether ketone ketone) Membranes with High Proton Conductivity. J. Membr. Sci. 2006, 281, 1–6. [Google Scholar] [CrossRef]
- Zhong, S.; Liu, C.; Dou, Z.; Li, X.; Zhao, C.; Fu, T.; Na, H. Synthesis and Properties of Sulfonated Poly(ether ether ketone ketone) Containing Tert-Butyl Groups as Proton Exchange Membrane Materials. J. Membr. Sci. 2006, 285, 404–411. [Google Scholar] [CrossRef]
- Goh, Y.T.; Patel, R.; Im, S.J.; Kim, J.H.; Min, B.R. Synthesis and Characterization of Poly(ether sulfone) Grafted Poly(styrene sulfonic acid) for Proton Conducting Membranes. Korean J. Chem. Eng. 2009, 26, 518–522. [Google Scholar] [CrossRef]
- Yu, J.; Dong, C.; Liu, J.; Li, C.; Fang, J.; Guan, R. Crosslinked Sulfonated Poly(bis- A)-Sulfones as Proton Exchange Membrane for PEM Fuel Cell Application. J. Mater. Sci. 2010, 45, 1017–1024. [Google Scholar] [CrossRef]
- Shang, X.; Fang, S.; Meng, Y. Synthesis and Characterization of Poly(arylene ether ketone) with Sulfonated Fluorene Pendants For Proton Exchange Membrane. J. Membr. Sci. 2007, 297, 90–97. [Google Scholar] [CrossRef]
- Wiles, K.B.; De Diego, C.M.; De Abajo, J.; McGrath, J.E. Directly Copolymerized Partially Fluorinated Disulfonated Poly(Arylene Ether Sulfone) Random Copolymers for PEM Fuel Cell Systems: Synthesis, Fabrication and Characterization of Membranes and Membrane–Electrode Assemblies for Fuel Cell Applications. J. Membr. Sci. 2007, 294, 22–29. [Google Scholar] [CrossRef]
- Sosa-González, W.E.; Palí Casanova, R.J.; Aguilar Vega M., J. Sulfonated Aromatic Copoly(ether-amide) Membranes: Preparation and Characterization for Possible Application in Polymer Electrolyte Membrane Fuel Cells. High Perform. Polym. 2014, 26, 997–1006. [Google Scholar] [CrossRef]
- Maier, G.; Meier-Haack, J. Sulfonated Aromatic Polymers for Fuel Cell Membranes. In Fuel Cells II. Advances in Polymer Science; Scherer, G.G., Ed.; Springer: Berlin, Heidelberg, Germany, 2008; Volume 216, pp. 1–62. [Google Scholar]
- Grubb, J.W.T. Fuel Cell. U.S. Patent No. 2,913,511, 1959. [Google Scholar]
- Shang, X.Y.; Shu, D.; Wang, S.J.; Xiao, M.; Meng, Y.Z. Fluorene-Containing Sulfonated Poly(arylene ether 1,3,4-oxadiazole) as Proton-Exchange Membrane for PEM Fuel Cell Application. J. Membr. Sci. 2007, 291, 140–147. [Google Scholar] [CrossRef]
- Pérez-Francisco, J.M.; Herrera-Kao, W.; González-Díaz, M.O.; Aguilar-Vega, M.; Santiago-García, J.L. Assessment of Random Aromatic Co-polyamides Containing Two Different Bulky Pendant Groups. J. Appl. Polym. Sci. 2017, 135, 45884. [Google Scholar] [CrossRef]
- Palί, R.; Aguilar-Vega, M.; Angulo, J.L.; Vázquez, H. Synthesis and Characterization of Aromatic Polyamides Obtained from 4-4′-(9-Fluorenylidene)Diamine, 4-4′-(Hexafluoro-Isopropylidene)Dianiline and 4-4′-Diamine-Benzophenone. High Perform. Polym. 2002, 14, 77–91. [Google Scholar] [CrossRef]
- Satterfield, M.B.; Benziger, J.B. Viscoelastic Properties of Nafion at Elevated Temperature and Humidity. J. Polym. Sci. B. 2008, 47, 11–24. [Google Scholar] [CrossRef]
- Majsztrik, P.W.; Bocarsly, A.B.; Benziger, J.B. Viscoelastic Response of Nafion. Effects of Temperature and Hydration on Tensile Creep. Macromolecules 2008, 41, 9849–9862. [Google Scholar] [CrossRef] [Green Version]
- Allahyarov, E.; Taylor, P.L.; Lowen, H. Simulation study of Sulfonate Cluster Swelling in Ionomers. Phys. Rev. E 2009, 80, 061802. [Google Scholar] [CrossRef]
- Berezina, N.P.; Kononenko, N.A.; Dyomina, O.A.; Gnusin, N.P. Characterization of Ion-Exchange Membrane Materials: Properties vs Structure. Adv. Colloid Interface Sci. 2008, 139, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Kornyshev, A.A.; Spohr, E. Proton Transport in Polymer Electrolyte Membranes Using Theory and Classical Molecular Dynamics. In Device and Materials Modeling in PEM Fuel Cells; Springer: New York, NY, USA, 2009; pp. 349–363. [Google Scholar]
- Choi, P.; Jalani, N.H.; Datta, R. Thermodynamics and Proton Transport in Nfion II: Proton Diffusion Mechanism And Conductivity. J. Electrochem. Soc. 2005, 152, E123–E130. [Google Scholar] [CrossRef]
- Watari, T.; Fang, J.; Guo, X.; Tanaka, K.; Kita, H. Proton Conductivity and Vapor Permeation Properties of Polyimides Containing Sulfonic Acid Groups. In ACS SYMPOSIUM SERIES; American Chemical Society: Washington, DC, USA, 2004; pp. 253–268. [Google Scholar]
- Fimrite, J.; Struchtrup, H.; Djilali, N. Transport Phenomena in Polymer Electrolyte Membranes I. Modeling Framework. J. Electrochem. Soc. 2005, 152, A1815–A1823. [Google Scholar] [CrossRef]





| Polymer | Product Obtained | Amount (g) | Film | ηinh (dL/g) |
|---|---|---|---|---|
| HFDA/ISO | FIBERS | 1.12 | ✓ | 0.5482 |
| HFDA/DABS/ISO-95/5 | FIBERS | 0.96 | ✓ | 0.4487 |
| HFDA/DABS/ISO-90/10 | FIBERS | 0.80 | ✓ | 0.483 |
| HFDA/DABS/ISO-80/20 | FIBERS | 0.72 | ✓ | 0.3659 |
| HFDA/DABS/ISO-70/30 | NON-DEFINED FIBERS | 0.66 | ✓ | 0.3163 |
| HFDA/DABS/ISO-60/40 | SEMI-POWDER | 0.60 | X | 0.3066 |
| HFDA/DABS/ISO-50/50 | POWDER | 0.54 | X | 0.2581 |
| Polymer | Thickness (mm) | σmax (MPa) | ɛmax (%) | Е (GPa) |
|---|---|---|---|---|
| HFDA/ISO | 0.047 | 60.36 | 11.11 | 1.069 |
| HFDA/DABS/ISO 95/5 | 0.037 | 53.15 | 7.45 | 1.002 |
| HFDA/DABS/ISO 90/10 | 0.041 | 42.24 | 5.51 | 0.954 |
| HFDA/DABS/ISO 80/20 | 0.043 | 50.97 | 5.85 | 1.061 |
| HFDA/DABS/ISO 70/30 | 0.052 | 34.4 | 2.34 | 0.788 |
| HFDA/DABS/ISO 60/40 | - | - | - | - |
| HFDA/DABS/ISO 50/50 | - | - | - | - |
| Polymer | 25 °C | 45 °C | 65 °C | 75 °C |
|---|---|---|---|---|
| HFDA/ISO | 10.44 | 12.24 | 13.46 | 17.11 |
| HFDA/DABS/ISO 95/5 | 12.5 | 14.28 | 16.66 | 18.18 |
| HFDA/DABS/ISO 90/10 | 16.66 | 18.51 | 19.42 | 23.80 |
| HFDA/DABS/ISO 80/20 | 20.96 | 23.88 | 25.42 | 25.92 |
| HFDA/DABS/ISO 70/30 | 25.71 | 26.22 | 27.27 | 27.77 |
| HFDA/DABS/ISO 60/40 | - | - | - | - |
| HFDA/DABS/ISO 50/50 | - | - | - | - |
| Nafion 115 | 10 | 14.95 | 15.25 | 21.55 |
| Polymer | 0.01 M NaOH Added (mL) | Membrane dry Weight(g) | IEC (mmol/g) |
|---|---|---|---|
| HFDA/ISO | 0.275 | 0.0141 | 0.1954 |
| HFDA/DABS/ISO 95/5 | 0.360 | 0.0158 | 0.2274 |
| HFDA/DABS/ISO 90/10 | 0.775 | 0.0160 | 0.4847 |
| HFDA/DABS/ISO 80/20 | 1.175 | 0.0180 | 0.6527 |
| HFDA/DABS/ISO 70/30 | 0.890 | 0.0119 | 0.7478 |
| HFDA/DABS/ISO 60/40 | - | - | - |
| HFDA/DABS/ISO 50/50 | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pali-Casanova, R.d.J.; Yam-Cervantes, M.A.; Zavala-Loría., J.d.C.; Loría-Bastarrachea, M.I.; Aguilar-Vega, M.d.J.; Dzul-López, L.A.; Sámano-Celorio, M.L.; Crespo-Álvarez, J.; García-Villena, E.; Agudo-Toyos, P.; et al. Effect of Sulfonic Groups Concentration on IEC Properties in New Fluorinated Copolyamides. Polymers 2019, 11, 1169. https://doi.org/10.3390/polym11071169
Pali-Casanova RdJ, Yam-Cervantes MA, Zavala-Loría. JdC, Loría-Bastarrachea MI, Aguilar-Vega MdJ, Dzul-López LA, Sámano-Celorio ML, Crespo-Álvarez J, García-Villena E, Agudo-Toyos P, et al. Effect of Sulfonic Groups Concentration on IEC Properties in New Fluorinated Copolyamides. Polymers. 2019; 11(7):1169. https://doi.org/10.3390/polym11071169
Chicago/Turabian StylePali-Casanova, Ramón de Jesús, Marcial Alfredo Yam-Cervantes, José del Carmen Zavala-Loría., María Isabel Loría-Bastarrachea, Manuel de Jesús Aguilar-Vega, Luis Alonso Dzul-López, María Luisa Sámano-Celorio, Jorge Crespo-Álvarez, Eduardo García-Villena, Pablo Agudo-Toyos, and et al. 2019. "Effect of Sulfonic Groups Concentration on IEC Properties in New Fluorinated Copolyamides" Polymers 11, no. 7: 1169. https://doi.org/10.3390/polym11071169
APA StylePali-Casanova, R. d. J., Yam-Cervantes, M. A., Zavala-Loría., J. d. C., Loría-Bastarrachea, M. I., Aguilar-Vega, M. d. J., Dzul-López, L. A., Sámano-Celorio, M. L., Crespo-Álvarez, J., García-Villena, E., Agudo-Toyos, P., & Méndez-Martínez, F. (2019). Effect of Sulfonic Groups Concentration on IEC Properties in New Fluorinated Copolyamides. Polymers, 11(7), 1169. https://doi.org/10.3390/polym11071169