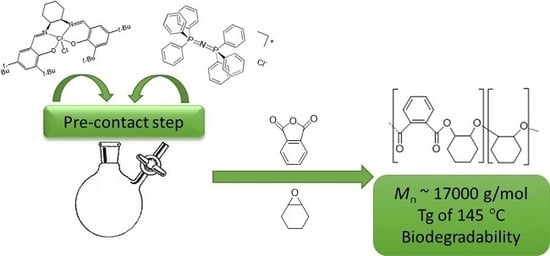
Influence of Co-Catalysts and Polymerization Conditions on Properties of Poly(anhydride-alt-epoxide)s from ROCOP Using Salen Complexes with Different Metals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.3. Methods
3. Results
ROCOP of CHO Using Complexes 1–3 in Presence of Different Co-Catalysts
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rabnawaz, M.; Wyman, I.; Auras, R.; Cheng, S. A roadmap towards green packaging: The current status and future outlook for polyesters in the packaging industry. Green Chem. 2017, 19, 4737–4753. [Google Scholar] [CrossRef]
- Hillmyer, M.A.; Tolman, W.B. Aliphatic Polyester Block Polymers: Renewable, Degradable, and Sustainable. Accounts Chem. Res. 2014, 47, 2390–2396. [Google Scholar] [CrossRef] [PubMed]
- Ajellal, N.; Carpentier, J.F.; Guillaume, C.; Guillaume, S.M.; Helou, M.; Poirier, V.; Sarazin, Y.; Trifonov, A. Metal-catalyzed immortal ring-opening polymerization of lactones, lactides and cyclic carbonates. Dalton Trans. 2010, 39, 8363–8376. [Google Scholar] [CrossRef] [PubMed]
- Mecerreyes, D.; Jerome, R.; Dubois, P. Novel Macromolecular Architectures Based on Aliphatic Polyesters: Relevance of the “Coordination-Insertion” Ring-Opening Polymerization. In Macromolecular Architectures. Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 1999; Volume 147, pp. 1–59. [Google Scholar]
- Dijkstra, P.J.; Du, H.; Feijen, J. Single site catalysts for stereoselective ring-opening polymerization of lactides. Polym. Chem. 2011, 2, 520–527. [Google Scholar] [CrossRef]
- Longo, J.M.; Sanford, M.J.; Coates, G.W. Ring-Opening Copolymerization of Epoxides and Cyclic Anhydrides with Discrete Metal Complexes: Structure–Property Relationships. Chem. Rev. 2016, 116, 15167–15197. [Google Scholar] [CrossRef] [PubMed]
- Sanford, M.J.; Carrodeguas, L.P.; Van Zee, N.J.; Kleij, A.W.; Coates, G.W. Alternating Copolymerization of Propylene Oxide and Cyclohexene Oxide with Tricyclic Anhydrides: Access to Partially Renewable Aliphatic Polyesters with High Glass Transition Temperatures. Macromolecules 2016, 49, 6394–6400. [Google Scholar] [CrossRef]
- DiCiccio, A.M.; Coates, G.W. Ring-Opening Copolymerization of Maleic Anhydride with Epoxides: A Chain-Growth Approach to Unsaturated Polyesters. J. Am. Chem. Soc. 2011, 133, 10724–10727. [Google Scholar] [CrossRef]
- Jeske, R.C.; DiCiccio, A.M.; Coates, G.W. Alternating Copolymerization of Epoxides and Cyclic Anhydrides: An Improved Route to Aliphatic Polyesters. J. Am. Chem. Soc. 2007, 129, 11330–11331. [Google Scholar] [CrossRef]
- Harrold, N.D.; Li, Y.; Chisholm, M.H. Studies of Ring-Opening Reactions of Styrene Oxide by Chromium Tetraphenylporphyrin Initiators. Mechanistic and Stereochemical Considerations. Macromolecules 2013, 46, 692–698. [Google Scholar] [CrossRef]
- Saini, P.K.; Romain, C.; Zhu, Y.; Williams, C.K. Di-magnesium and zinc catalysts for the copolymerization of phthalic anhydride and cyclohexene oxide. Polym. Chem. 2014, 5, 6068–6075. [Google Scholar] [CrossRef] [Green Version]
- Paoniasari, A.; Duchateau, R.; Nejad, E.H.; Koning, C.E. Semi-aromatic polyesters by alternating ring-opening copolymerisation of styrene oxide and anhydrides. Polym. Chem. 2012, 3, 1308–1313. [Google Scholar]
- Aida, T.; Inoue, S. Catalytic Reaction on Both Sides of a Metalloporphyrin Plane Alternating Copolymerization of Phthalic Anhydride and Epoxypropane with an Aluminum Porphyrin-Quaternary Salt System. J. Am. Chem. Soc. 1985, 107, 1358–1364. [Google Scholar] [CrossRef]
- Si, G.; Li, B.; Dong, J.; Zhang, L.; Han, B.; Duan, Z.; Liu, B. Novel chromium complexes with a [OSSO]-type bis(phenolato) dianionic ligand mediate the alternating ring-opening copolymerization of epoxides and phthalic anhydride. Polym. Chem. 2015, 6, 6372–6377. [Google Scholar] [CrossRef]
- Winkler, M.; Romain, C.; Meier, M.A.R.; Williams, C.K. Renewable polycarbonates and polyesters from 1,4-cyclohexadiene. Green Chem. 2015, 17, 300–306. [Google Scholar] [CrossRef]
- Biermann, U.; Sehlinger, A.; Meier, M.A.R.; Metzger, J.O. Catalytic Copolymerization of Methyl 9,10-Epoxystearate and Cyclic Anhydrides under Neat Conditions. Eur. J. Lipid Sci. Technol. 2016, 118, 104–110. [Google Scholar] [CrossRef]
- Carrodeguas, L.P.; Martín, C.; Kleij, A.W. Semiaromatic Polyesters Derived from Renewable Terpene Oxides with High Glass Transitions. Macromolecules 2017, 50, 5337–5345. [Google Scholar] [CrossRef]
- Han, B.; Zhang, L.; Liu, B.; Dong, X.; Kim, I.; Duan, Z.; Theato, P. Controllable Synthesis of Stereoregular Polyesters by Organocatalytic Alternating Copolymerizations of Cyclohexene Oxide and Norbornene Anhydrides. Macromolecules 2015, 48, 3431–3437. [Google Scholar] [CrossRef]
- Van Zee, N.J.; Sanford, M.J.; Coates, G.W. Electronic Effects of Aluminum Complexes in the Copolymerization of Propylene Oxide with Tricyclic Anhydrides: Access to Well-Defined, Functionalizable Aliphatic Polyesters. J. Am. Chem. Soc. 2016, 138, 2755–2761. [Google Scholar] [CrossRef]
- Ren, W.-M.; Lu, X.-B.; Li, J.; Liu, Y. Asymmetric Alternating Copolymerization of Meso-epoxides and Cyclic Anhydrides: Efficient Access to Enantiopure Polyesters. J. Am. Chem. Soc. 2016, 138, 11493–11496. [Google Scholar]
- Bernard, A.; Chatterjee, C.; Chisholm, M.H. The influence of the metal (Al, Cr and Co) and the substituents of the porphyrin in controlling the reactions involved in the copolymerization of propylene oxide and cyclic anhydrides by porphyrin metal(III) complexes. Polymer 2013, 54, 2639–2646. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, J.-Z.; Lu, H.W.; Wang, H.B.; Lu, X.B. Making Various Degradable Polymers from Epoxides Using a Versatile Dinuclear Chromium Catalyst. Macromolecules 2018, 51, 771–778. [Google Scholar] [CrossRef]
- Robert, C.; De Montigny, F.; Thomas, C.M. Tandem synthesis of alternating polyesters from renewable resources. Nat. Commun. 2011, 2, 586. [Google Scholar] [CrossRef] [PubMed]
- Poland, R.R.; Escobedo, C.; Darensbourg, D.J. Kinetic Studies of the Alternating Copolymerization of Cyclic Acid Anhydrides and Epoxides, and the Terpolymerization of Cyclic Acid Anhydrides, Epoxides, and CO2 Catalyzed by (salen)Cr III Cl. Macromolecules 2012, 45, 2242–2248. [Google Scholar]
- Bao, Y.Y.; Liu, Y.; Ren, W.M.; Lu, X.B.; Liu, J. Binuclear chromium–salan complex catalyzed alternating copolymerization of epoxides and cyclic anhydrides. Polym. Chem. 2013, 4, 1439–1444. [Google Scholar]
- Huijser, S.; Hosseininejad, E.; Sablong, R.; De Jong, C.; Koning, C.E.; Duchateau, R. Ring-Opening Co- and Terpolymerization of an Alicyclic Oxirane with Carboxylic Acid Anhydrides and CO2in the Presence of Chromium Porphyrinato and Salen Catalysts. Macromolecules 2011, 44, 1132–1139. [Google Scholar] [CrossRef]
- Liu, D.F.; Zhu, L.Q.; Wu, J.; Wu, L.Y.; Lü, X.Q. Ring-opening copolymerization of epoxides and anhydrides using manganese( III ) asymmetrical Schiff base complexes as catalysts. RSC Adv. 2015, 5, 3854–3859. [Google Scholar] [CrossRef]
- Robert, C.; Ohkawara, T.; Nozaki, K. Manganese-Corrole Complexes as Versatile Catalysts for the Ring-Opening Homo- and Co-Polymerization of Epoxide. Chem. Eur. J. 2014, 20, 4789–4795. [Google Scholar] [CrossRef] [PubMed]
- Mundil, R.; Hošt’álek, Z.; Šeděnková, I.; Merna, J. Alternating ring-opening copolymerization of cyclohexene oxide with phthalic anhydride catalyzed by iron(III) salen complexes. Macromol. Res. 2015, 23, 161–166. [Google Scholar] [CrossRef]
- Garden, J.A.; Saini, P.K.; Williams, C.K. Greater than the Sum of Its Parts: A Heterodinuclear Polymerization Catalyst. J. Am. Chem. Soc. 2015, 137, 15078–15081. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Romain, C.; Williams, C.K. Selective Polymerization Catalysis: Controlling the Metal Chain End Group to Prepare Block Copolyesters. J. Am. Chem. Soc. 2015, 137, 12179–12182. [Google Scholar] [CrossRef] [Green Version]
- Thevenon, A.; Garden, J.A.; White, A.J.P.; Williams, C.K. Dinuclear Zinc Salen Catalysts for the Ring Opening Copolymerization of Epoxides and Carbon Dioxide or Anhydrides. Inorg. Chem. 2015, 54, 11906–11915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, C.Y.; Chuang, H.J.; Ko, B.T. Bimetallic bis(benzotriazole iminophenolate) cobalt, nickel and zinc complexes as versatile catalysts for coupling of carbon dioxide with epoxides and copolymerization of phthalic anhydride with cyclohexene oxide. Catal. Sci. Technol. 2016, 6, 1779–1791. [Google Scholar] [CrossRef]
- Nejad, E.H.; Van Melis, C.G.W.; Vermeer, T.J.; Koning, C.E.; Duchateau, R. Alternating Ring-Opening Polymerization of Cyclohexene Oxide and Anhydrides: Effect of Catalyst, Cocatalyst, and Anhydride Structure. Macromolecules 2012, 45, 1770–1776. [Google Scholar] [CrossRef]
- Hošťálek, Z.; Trhlíková, O.; Walterová, Z.; Martinez, T.; Peruch, F.; Cramail, H.; Merna, J. Alternating copolymerization of epoxides with anhydrides initiated by organic bases. Eur. Polym. J. 2017, 88, 433–447. [Google Scholar] [CrossRef]
- Kummari, A.; Pappuru, S.; Chakraborty, D.; Kanji, A. Fully alternating and regioselective ring-opening copolymerization of phthalic anhydride with epoxides using highly active metal-free Lewis pairs as a catalyst. Polym. Chem. 2018, 9, 4052–4062. [Google Scholar] [CrossRef]
- Shi, Z.; Jiang, Q.; Song, Z.; Wang, Z.; Gao, C. Dinuclear iron(iii) complexes bearing phenylene-bridged bis(amino triphenolate) ligands as catalysts for the copolymerization of cyclohexene oxide with carbon dioxide or phthalic anhydride. Polym. Chem. 2018, 9, 4733–4743. [Google Scholar] [CrossRef]
- Darensbourg, D.J. Making Plastics from Carbon Dioxide: Salen Metal Complexes as Catalysts for the Production of Polycarbonates from Epoxides and CO2. Chem. Rev. 2007, 107, 2388–2410. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Ulusoy, M.; Karroonnirum, O.; Poland, R.R.; Reibenspies, J.H.; Çetinkaya, B. Highly Selective and Reactive (salan)CrCl Catalyst for the Copolymerization and Block Copolymerization of Epoxides with Carbon Dioxide. Macromolecules 2009, 42, 6992–6998. [Google Scholar] [CrossRef]
- Fieser, M.E.; Sanford, M.J.; Mitchell, L.A.; Dunbar, C.R.; Mandal, M.; Van Zee, N.J.; Urness, D.M.; Cramer, C.J.; Coates, G.W.; Tolman, W.B. Mechanistic Insights into the Alternating Copolymerization of Epoxides and Cyclic Anhydrides Using a (Salph)AICI and Iminium Salt Catalytic System. J. Am. Chem. Soc. 2017, 139, 15222–15231. [Google Scholar] [CrossRef] [PubMed]
- Van Zee, N.J.; Coates, G.W. Alternating Copolymerization of Propylene Oxide with Biorenewable Terpene-Based Cyclic Anhydrides: A Sustainable Route to Aliphatic Polyesters with High Glass Transition Temperatures. Angew. Chem. Int. Ed. 2015, 54, 2665–2668. [Google Scholar] [CrossRef] [PubMed]


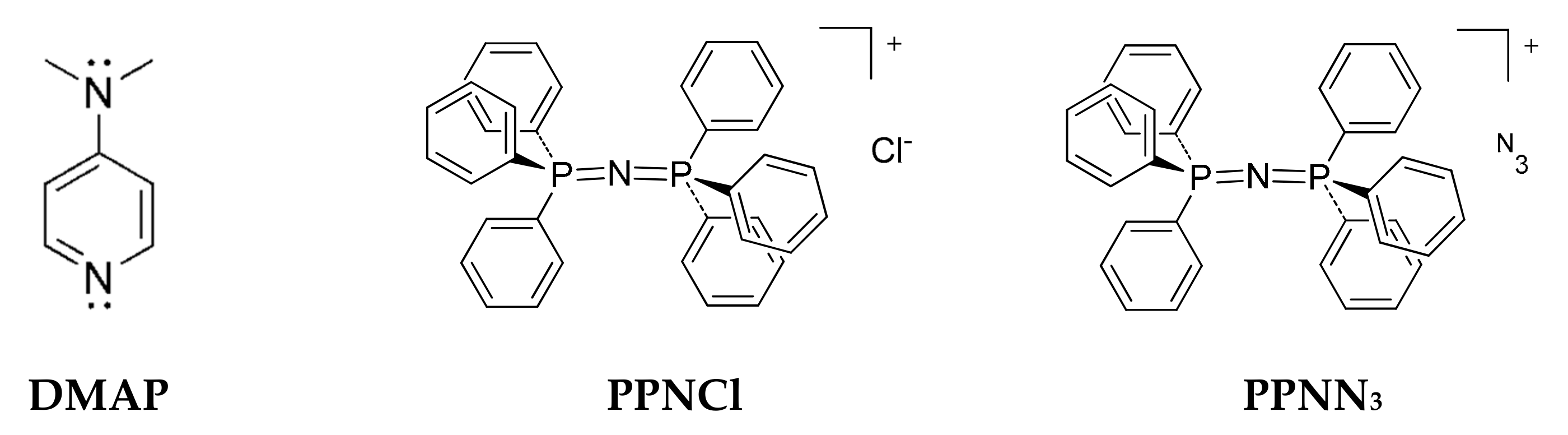

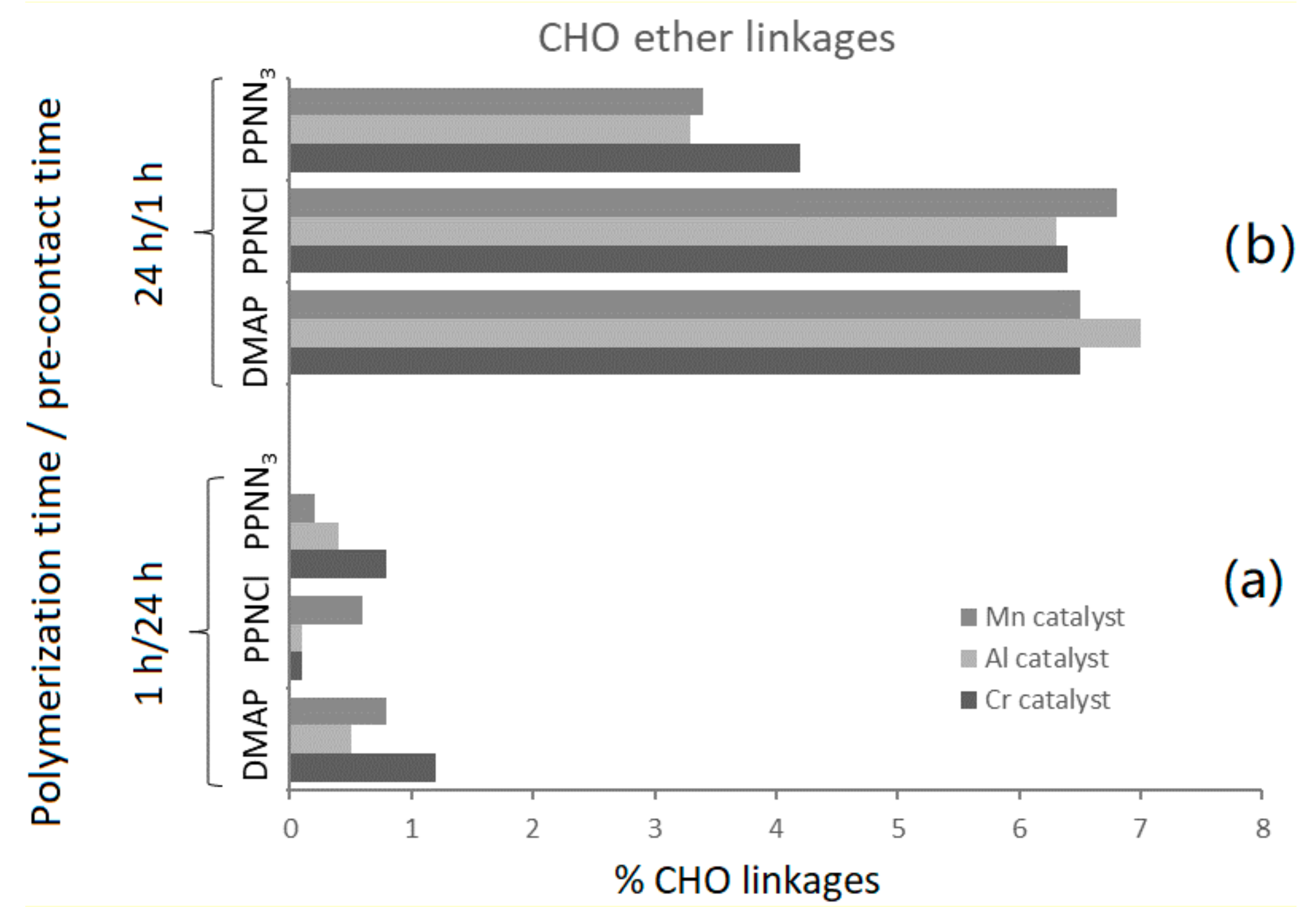
| Entry | Catalyst/Co-Catalyst | Time (h) | Yield (%) | Conversion (%) b | Ether Linkages (mol%) b | Mn (kg/mol) | Đ | Tg (°C) | |
|---|---|---|---|---|---|---|---|---|---|
| CHO | PA | ||||||||
| CHOPA 66 | 1/DMAP | 0.3 | 74 | 92 | 63 | 27 | 1.7 | 3.2 | 141 |
| CHOPA 69 | 1/PPNCl | 0.3 | 82 | 87 | 78 | 16 | 1.8 | 3.0 | 141 |
| CHOPA 68 | 2/DMAP | 1.1 | 71 | 78 | 66 | 18 | 2.3 | 2.5 | 136 |
| CHOPA 71 | 2/PPNCl | 1.1 | 57 | 64 | 53 | 20 | 1.9 | 2.9 | 137 |
| CHOPA 67 | 3/DMAP | 1.0 | 76 | 84 | 71 | 15 | 2.4 | 2.5 | 139 |
| CHOPA 70 | 3/PPNCl | 1.0 | 81 | 86 | 78 | 16 | 2.5 | 2.0 | 139 |
| Entry | Co-Catalyst | Time (h) | Yield (%) | Mn (kg/mol) | Đ | Tg (°C) |
|---|---|---|---|---|---|---|
| CHOPA 48 | DMAP | 1 | 76 | 15.5 | 1.2 | 147 |
| CHOPA 19 | DMAP | 3 | 91 | 17.5 | 1.2 | 146 |
| CHOPA 20 | DMAP | 24 | 82 | 15.4 | 1.3 | 146 |
| CHOPA 51 | PPNCl | 1 | 90 | 15.7 | 1.1 | 146 |
| CHOPA 17 | PPNCl | 3 | 90 | 16.1 | 1.2 | 145 |
| CHOPA 18 | PPNCl | 24 | 92 | 16.8 | 1.3 | 145 |
| CHOPA 54 | PPNN3 | 1 | 84 | 14.8 | 1.1 | 146 |
| CHOPA 36 | PPNN3 | 3 | 88 | 14.2 | 1.1 | 147 |
| CHOPA 37 | PPNN3 | 24 | 79 | 15.2 | 1.1 | 146 |
| Entry | Co-Catalyst | Time (h) | Yield (%) | Mn (kg/mol) a | Đ | Tg (°C) |
|---|---|---|---|---|---|---|
| CHOPA 50 | DMAP | 1 | 16 | 4.5 | 1.36 | 129 |
| CHOPA 23 | DMAP | 3 | 66 | 10.4 | 1.47 | 136 |
| CHOPA 24 | DMAP | 24 | 85 | 14.1 | 1.24 | 143 |
| CHOPA 53 | PPNCl | 1 | 40 | 9.4 | 1.10 | 133 |
| CHOPA 21 | PPNCl | 3 | 84 | 16.0 | 1.16 | 144 |
| CHOPA 22 | PPNCl | 24 | 95 | 15.8 | 1.25 | 145 |
| CHOPA 56 | PPNN3 | 1 | 41 | 8.8 | 1.10 | 140 |
| CHOPA 38 | PPNN3 | 3 | 76 | 12.8 | 1.17 | 144 |
| CHOPA 39 | PPNN3 | 24 | 78 | 9.3 | 1.24 | 144 |
| Entry | Co-Catalyst | Time (h) | Yield (%) | Mn (kg/mol) | Đ | Tg (°C) |
|---|---|---|---|---|---|---|
| CHOPA 49 | DMAP | 1 | 14 | 4.5 | 1.31 | 144 |
| CHOPA 27 | DMAP | 3 | 74 | 12.0 | 1.17 | / |
| CHOPA 28 | DMAP | 24 | 96 | 14.0 | 1.27 | / |
| CHOPA 52 | PPNCl | 1 | 35 | 7.8 | 1.21 | 137 |
| CHOPA 25 | PPNCl | 3 | 86 | 14.9 | 1.15 | 144 |
| CHOPA 26 | PPNCl | 24 | 92 | 15.1 | 1.26 | 144 |
| CHOPA 55 | PPNN3 | 1 | 39 | 7.5 | 1.19 | 136 |
| CHOPA 40 | PPNN3 | 3 | 68 | 12.6 | 1.20 | 138 |
| CHOPA 41 | PPNN3 | 24 | 81 | 15.7 | 1.22 | 144 |
| Entry | Catalyst/Co-Catalyst | Polym. Time (h) | Pre-Contact Time (h) | Yield (%) | Mn (kg/mol) | Đ | Tg (°C) |
|---|---|---|---|---|---|---|---|
| CHOPA 48 | 1/DMAP | 1 | 1 | 76 | 15.5 | 1.20 | 147 |
| CHOPA 57 | 1/DMAP | 1 | 24 | 64 | 16.3 | 1.20 | 147 |
| CHOPA 20 | 1/DMAP | 24 | 1 | 82 | 15.4 | 1.30 | 146 |
| CHOPA 51 | 1/PPNCl | 1 | 1 | 90 | 15.7 | 1.10 | 146 |
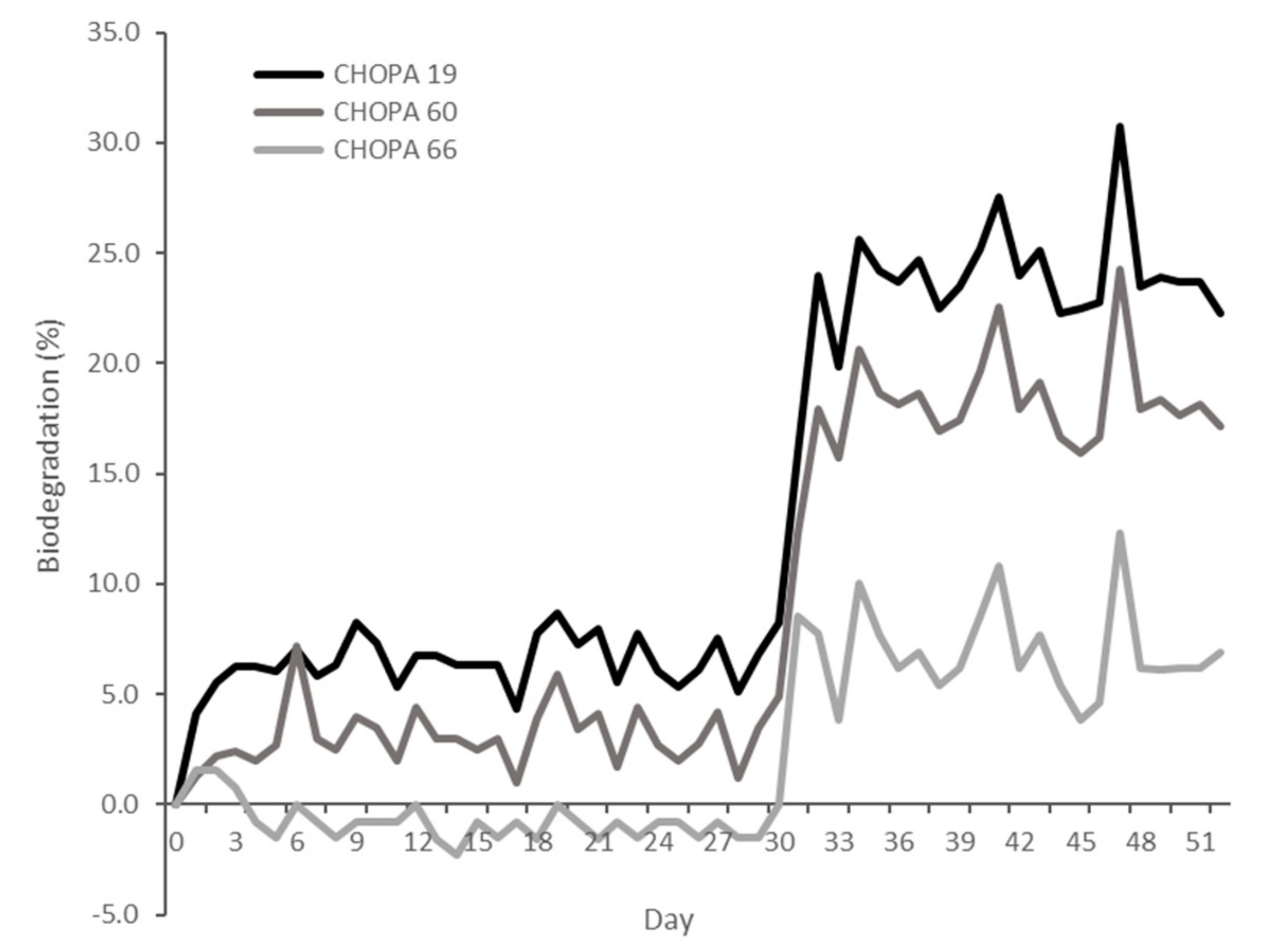
| CHOPA 60 | 1/PPNCl | 1 | 24 | 90 | 15.7 | 1.13 | 146 |
| CHOPA 18 | 1/PPNCl | 24 | 1 | 92 | 16.8 | 1.30 | 145 |
| CHOPA 54 | 1/PPNN3 | 1 | 1 | 84 | 14.8 | 1.10 | 146 |
| CHOPA 63 | 1/PPNN3 | 1 | 24 | 89 | 15.5 | 1.10 | 143 |
| CHOPA 37 | 1/PPNN3 | 24 | 1 | 79 | 15.2 | 1.10 | 146 |
| CHOPA 50 | 2/DMAP | 1 | 1 | 16 | 4.5 | 1.36 | 129 |
| CHOPA 59 | 2/DMAP | 1 | 24 | 80 | 14.2 | 1.13 | 145 |
| CHOPA 24 | 2/DMAP | 24 | 1 | 85 | 14.1 | 1.24 | 143 |
| CHOPA 53 | 2/PPNCl | 1 | 1 | 40 | 9.4 | 1.10 | 133 |
| CHOPA 62 | 2/PPNCl | 1 | 24 | 92 | 16.3 | 1.11 | 146 |
| CHOPA 22 | 2/PPNCl | 24 | 1 | 95 | 15.8 | 1.25 | 145 |
| CHOPA 56 | 2/PPNN3 | 1 | 1 | 41 | 8.8 | 1.10 | 140 |
| CHOPA 65 | 2/PPNN3 | 1 | 24 | 93 | 16.2 | 1.20 | 148 |
| CHOPA 39 | 2/PPNN3 | 24 | 1 | 78 | 9.3 | 1.24 | 144 |
| CHOPA 49 | 3/DMAP | 1 | 1 | 14 | 4.5 | 1.31 | 144 |
| CHOPA 58 | 3/DMAP | 1 | 24 | 84 | 15.4 | 1.13 | 147 |
| CHOPA 28 | 3/DMAP | 24 | 1 | 96 | 14.0 | 1.27 | 144 |
| CHOPA 52 | 3/PPNCl | 1 | 1 | 35 | 7.8 | 1.21 | 137 |
| CHOPA 61 | 3/PPNCl | 1 | 24 | 98 | 14.8 | 1.09 | 146 |
| CHOPA 26 | 3/PPNCl | 24 | 1 | 92 | 15.1 | 1.26 | 144 |
| CHOPA 55 | 3/PPNN3 | 1 | 1 | 39 | 7.5 | 1.19 | 136 |
| CHOPA 64 | 3/PPNN3 | 1 | 24 | 97 | 14.7 | 1.20 | 145 |
| CHOPA 41 | 3/PPNN3 | 24 | 1 | 81 | 15.7 | 1.22 | 144 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Proverbio, M.; Galotto Galotto, N.; Losio, S.; Tritto, I.; Boggioni, L. Influence of Co-Catalysts and Polymerization Conditions on Properties of Poly(anhydride-alt-epoxide)s from ROCOP Using Salen Complexes with Different Metals. Polymers 2019, 11, 1222. https://doi.org/10.3390/polym11071222
Proverbio M, Galotto Galotto N, Losio S, Tritto I, Boggioni L. Influence of Co-Catalysts and Polymerization Conditions on Properties of Poly(anhydride-alt-epoxide)s from ROCOP Using Salen Complexes with Different Metals. Polymers. 2019; 11(7):1222. https://doi.org/10.3390/polym11071222
Chicago/Turabian StyleProverbio, Matteo, Nella Galotto Galotto, Simona Losio, Incoronata Tritto, and Laura Boggioni. 2019. "Influence of Co-Catalysts and Polymerization Conditions on Properties of Poly(anhydride-alt-epoxide)s from ROCOP Using Salen Complexes with Different Metals" Polymers 11, no. 7: 1222. https://doi.org/10.3390/polym11071222
APA StyleProverbio, M., Galotto Galotto, N., Losio, S., Tritto, I., & Boggioni, L. (2019). Influence of Co-Catalysts and Polymerization Conditions on Properties of Poly(anhydride-alt-epoxide)s from ROCOP Using Salen Complexes with Different Metals. Polymers, 11(7), 1222. https://doi.org/10.3390/polym11071222