Purification of Polybutylene Terephthalate by Oligomer Removal Using a Compressed CO2 Antisolvent
Abstract
:1. Introduction
2. Materials and Methods
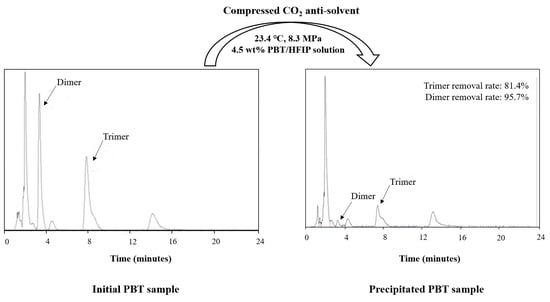
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Darda, P.J.; Hogendoorn, J.A.; Versteeg, G.F.; Souren, F. Reaction kinetics of polybutylene terephthalate polycondensation reaction. AIChE J. 2005, 51, 622–630. [Google Scholar] [CrossRef] [Green Version]
- Chopra, S.; Deshmukh, K.A.; Peshwe, D. Theoretical prediction of interfacial properties of PBT/CNT nanocomposites and its experimental evaluation. Mech. Mater. 2017, 109, 11–17. [Google Scholar] [CrossRef]
- Kalkar, A.K.; Deshpande, V.D.; Purkar, B.R. Evaluation of thermal transitions in poly(butylene terephthalate)/15A MMT nanocomposites: Nonisothermal experiments and modelling using isoconversional methods. Thermochim. Acta 2018, 660, 23–36. [Google Scholar] [CrossRef]
- Martínez De Ilarduya, A.; Muñoz-Guerra, S. Chemical structure and microstructure of poly(alkylene terephthalate)s, their copolyesters, and their blends as studied by NMR. Macromol. Chem. Phys. 2014, 215, 2138–2160. [Google Scholar] [CrossRef]
- Weijers, C.; De Rooij, R.D.; Agarwal, U.S.; De Wit, G.; Lemstra, P.J. Supercritical fluid extraction of cyclic oligomers from depolymerizing PBT. J. Appl. Polym. Sci. 2006, 101, 4487–4492. [Google Scholar] [CrossRef]
- Inagaki, J.; Hirae, K.; Sasaki, M.; Goto, M.; Ito, K. Removal of oligomers from poly(ethylene terephthalate) resins by hydrothermal extraction. Ind. Eng. Chem. Res. 2013, 52, 7640–7644. [Google Scholar] [CrossRef]
- Cotton, N.J.; Bartle, K.D.; Clifford, A.A.; Dowle, C.J. Rate and extent of supercritical fluid extraction of cyclic trimer from poly(ethylene terephthalate) at elevated temperatures. J. Chromatogr. Sci. 1993, 31, 157–161. [Google Scholar] [CrossRef]
- Goodman, I.; Nesbitt, B.F. The structures and reversible polymerization of cyclic oligomers from poly(ethylene terephthalate). Polymer (Guildf.) 1960, 1, 384–396. [Google Scholar] [CrossRef]
- Miao, H.; Chen, Z.; Xu, W.; Wang, W.; Song, Y.; Wang, Z. Preparation and characterization of naringenin microparticles via a supercritical anti-solvent process. J. Supercrit. Fluids 2018, 131, 19–25. [Google Scholar] [CrossRef]
- Chen, C.R.; Lin, D.M.; Chang, C.M.J.; Chou, H.N.; Wu, J.J. Supercritical carbon dioxide anti-solvent crystallization of fucoxanthin chromatographically purified from Hincksia mitchellae P.C. Silva. J. Supercrit. Fluids 2017, 119, 1–8. [Google Scholar] [CrossRef]
- Esfandiari, N. Production of micro and nanoparticles of pharmaceutical by supercritical carbon dioxide. J. Supercrit. Fluids 2015, 100, 129–141. [Google Scholar] [CrossRef]
- Lin, H.W.; Tan, C.S. Preparation of polyamic acid and polyimide nanoparticles by compressed fluid antisolvent and thermal imidization. J. Supercrit. Fluids 2015, 99, 103–111. [Google Scholar] [CrossRef]
- Chang, L.P.; Cheng, J.H.; Hsu, S.L.; Fu, Y.C.; Lin, K.L.; Shieh, C.J.; Zhou, X.Q.; Chang, C.M.J. Supercritical carbon dioxide anti-solvent purification of anti-oxidative compounds from Lycium barbarum fruits by using response surface methodology. Sep. Purif. Technol. 2012, 100, 66–73. [Google Scholar] [CrossRef]
- Lin, I.H.; Liang, P.F.; Tan, C.S. Preparation of polystyrene/poly(methyl methacrylate) blends by compressed fluid antisolvent technique. J. Supercrit. Fluids 2010, 51, 384–398. [Google Scholar] [CrossRef]
- Ahn, J.B.; Kim, D.H.; Lee, S.E.; Pyo, Y.C.; Park, J.S. Improvement of the dissolution rate and bioavailability of fenofibrate by the supercritical anti-solvent process. Int. J. Pharm. 2019, 564, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Ryan, K.M.; Padrela, L. From batch to continuous—New opportunities for supercritical CO2 technology in pharmaceutical manufacturing. Eur. J. Pharm. Sci. 2019, 137, 104971. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Randolph, A.D. Solvent expansion and solute solubility predictions in gas-expanded liquids. AIChE J. 1990, 36, 939–942. [Google Scholar] [CrossRef]
- Rajasingam, R.; Lioe, L.; Pham, Q.T.; Lucien, F.P. Solubility of carbon dioxide in dimethylsulfoxide and N-methyl-2-pyrrolidone at elevated pressure. J. Supercrit. Fluids 2004, 31, 227–234. [Google Scholar] [CrossRef]
- Villanueva Bermejo, D.; Ibáñez, E.; Reglero, G.; Turner, C.; Fornari, T.; Rodriguez-Meizoso, I. High catechins/low caffeine powder from green tea leaves by pressurized liquid extraction and supercritical antisolvent precipitation. Sep. Purif. Technol. 2015, 148, 49–56. [Google Scholar] [CrossRef]
- Paula, J.T.; Sousa, I.M.O.; Foglio, M.A.; Cabral, F.A. Selective fractionation of extracts of Arrabidaea chica Verlot using supercritical carbon dioxide as antisolvent. J. Supercrit. Fluids 2018, 133, 9–16. [Google Scholar] [CrossRef]
- Chang, C.M.J.; Wu, J.-J.; Shen, C.-T.; Jong, T.-T.; Young, C.-C.; Yang, H.-L.; Hsu, S.-L.; Shieh, C.-J. Supercritical carbon dioxide anti-solvent process for purification of micronized propolis particulates and associated anti-cancer activity. Sep. Purif. Technol. 2009, 70, 190–198. [Google Scholar]
- Langa, E.; Pardo, J.; Giménez-Rota, C.; González-Coloma, A.; MaríaJ.Hernáiz, M.J.; Mainar, A.M. Supercritical antisolvent fractionation of Artemisia absinthium L.conventional extracts: Tracking artemetin and casticin. J. Supercrit. Fluids 2019, 151, 15–23. [Google Scholar] [CrossRef]
- Yu, W.; Hsu, Y.P.; Tan, C.S. Synthesis of rhodium–platinum bimetallic catalysts supported on SBA-15 by chemical fluid deposition for the hydrogenation of terephthalic acid in water. Appl. Catal. B Environ. 2016, 196, 185–192. [Google Scholar] [CrossRef]
- Sarkari, M.; Darrat, I.; Knutson, B.L. Generation of microparticles using CO2 and CO2-philic antisolvents. AIChE J. 2000, 46, 1850–1859. [Google Scholar] [CrossRef]
- Knez, Z.; Pantić, M.; Cöa, D.; Novak, Z.; Hrnčič, M.K. Are supercritical fluids solvents for the future? Chem. Eng. Process. Process. Intensif. 2019, 141, 107532. [Google Scholar] [CrossRef]
- Ferrito, S. Polyesters. In Handbook of Chromatography; CRC Press: Boca Raton, FL, USA, 2005; pp. 1312–1314. [Google Scholar]
- Endo, H.; Marui, E. Fundamental studies on friction and wear of engineering plastics. Ind. Lubr. Tribol. 2004, 56, 283–287. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, S.H.; Jung, Y. TGF-β-3 encapsulated PLCL scaffold by a supercritical CO2-HFIP co-solvent system for cartilage tissue engineering. J. Control. Release 2015, 206, 101–107. [Google Scholar] [CrossRef]
- Campardelli, R.; Reverchon, E.; De Marco, I. Dependence of SAS particle morphologies on the ternary phase equilibria. J. Supercrit. Fluids 2017, 130, 273–281. [Google Scholar] [CrossRef]
- Prosapio, V.; Reverchon, E.; De Marco, I. Incorporation of liposoluble vitamins within PVP microparticles using supercritical antisolvent precipitation. J. CO2 Util. 2017, 19, 230–237. [Google Scholar] [CrossRef]
- Braeuer, A.; Dowy, S.; Torino, E.; Rossmann, M.; Luther, S.K.; Schluecker, E.; Leipertz, A.; Reverchon, E. Analysis of the supercritical antisolvent mechanisms governing particles precipitation and morphology by in situ laser scattering techniques. Chem. Eng. J. 2011, 173, 258–266. [Google Scholar] [CrossRef]
- Lin, I.H.; Tan, C.S. Diffusion of Benzonitrile in CO2-Expanded Ethanol. J. Chem. Eng. Data 2008, 53, 1886–1891. [Google Scholar] [CrossRef]
- Lin, I.H.; Tan, C.S. Measurement of Diffusion Coefficients of p-Chloronibtrobenzene in CO2-Expanded Methanol. J. Supercrit. Fluids 2008, 46, 112–117. [Google Scholar] [CrossRef]
- Lin, I.H.; Liang, P.F.; Tan, C.S. Precipitation of submicrometer-sized poly(methyl methacrylate) particles with a compressed fluid antisolvent. J. Appl. Polym. Sci. 2010, 117, 1197–1207. [Google Scholar] [CrossRef]







| Solvent | P (MPa) | T (°C) | Contact Time (h) | Removal Efficiency (wt %) |
|---|---|---|---|---|
| HFIP + CO2 | 6.9 | 40 | 2 | 15.3 |
| HFIP + CO2 | 6.9 | 40 | 4 | 24.8 |
| HFIP + CO2 | 6.9 | 40 | 8 | 28.2 |
| HFIP + CO2 | 10.3 | 40 | 2 | 18.9 |
| HFIP + CO2 | 10.3 | 40 | 4 | 28.8 |
| HFIP + CO2 | 10.3 | 40 | 8 | 33.2 |
| HFIP + CO2 | 13.1 | 40 | 8 | 38.1 |
| HFIP + CO2 | 10.3 | 50 | 8 | 31.7 |
| HFIP + CO2 | 13.1 | 50 | 8 | 36.1 |
| HFIP | 6.9 | 40 | 4 | 8.3 |
| HFIP + He | 6.9 | 40 | 4 | 8.2 |
| HFIP | 10.3 | 40 | 8 | 109 |
| HFIP + He | 10.3 | 40 | 8 | 11.2 |
| Entry | P (MPa) | T (°C) | (PBT in HFIP) (wt %) | Removal Efficiency (wt %) |
|---|---|---|---|---|
| 1 | 7.6 | 25.0 | 4.0 | 72.1 |
| 2 | 8.3 | 25.0 | 4.0 | 78.6 |
| 3 | 7.6 | 30.0 | 4.0 | 68.6 |
| 4 | 8.3 | 30.0 | 4.0 | 72.0 |
| 5 | 7.6 | 25.0 | 5.0 | 72.6 |
| 6 | 8.3 | 25.0 | 5.0 | 79.0 |
| 7 | 7.6 | 30.0 | 5.0 | 68.8 |
| 8 | 8.3 | 30.0 | 5.0 | 72.1 |
| 9 | 7.3 | 27.5 | 4.5 | 68.4 |
| 10 | 8.5 | 27.5 | 4.5 | 75.0 |
| 11 | 7.9 | 23.4 | 4.5 | 74.6 |
| 12 | 7.9 | 31.7 | 4.5 | 69.2 |
| 13 | 7.9 | 27.5 | 3.7 | 74.4 |
| 14 | 7.9 | 27.5 | 5.3 | 74.5 |
| 15 | 7.9 | 27.5 | 4.5 | 74.8 |
| 16 | 7.9 | 27.5 | 4.5 | 74.2 |
| 17 | 7.9 | 27.5 | 4.5 | 74.7 |
| 18 | 7.9 | 27.5 | 4.5 | 74.3 |
| 19 | 7.9 | 27.5 | 4.5 | 74.5 |
| 20 | 7.9 | 27.5 | 4.5 | 74.7 |
| Source | Degrees of Freedom | Sum of Square | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Regression | 9 | 160.79 | 17.87 | 36.90 | 0.000 |
| Linear | 3 | 135.32 | 45.11 | 93.17 | 0.000 |
| Pressure (X1) | 1 | 68.56 | 68.56 | 141.62 | 0.000 |
| Temperature (X2) | 1 | 66.56 | 66.56 | 137.48 | 0.000 |
| Concentration (X3) | 1 | 0.20 | 0.20 | 0.40 | 0.539 |
| Interaction | 3 | 4.67 | 1.56 | 3.22 | 0.071 |
| Pressure × temperature | 1 | 4.65 | 4.65 | 9.61 | 0.011 |
| Pressure × CO2 flow rate | 1 | 0.01 | 0.01 | 0.02 | 0.882 |
| Temperature × CO2 flow rate | 1 | 0.01 | 0.01 | 0.02 | 0.882 |
| Residual error | 10 | 4.84 | 0.48 | ||
| Lack-of-fit | 5 | 4.55 | 0.91 | 1.57 | 0.125 |
| Pure error | 5 | 0.29 | 0.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, W.; Huang, J.-H.; Tan, C.-S. Purification of Polybutylene Terephthalate by Oligomer Removal Using a Compressed CO2 Antisolvent. Polymers 2019, 11, 1230. https://doi.org/10.3390/polym11071230
Yu W, Huang J-H, Tan C-S. Purification of Polybutylene Terephthalate by Oligomer Removal Using a Compressed CO2 Antisolvent. Polymers. 2019; 11(7):1230. https://doi.org/10.3390/polym11071230
Chicago/Turabian StyleYu, Wen, Jian-Hao Huang, and Chung-Sung Tan. 2019. "Purification of Polybutylene Terephthalate by Oligomer Removal Using a Compressed CO2 Antisolvent" Polymers 11, no. 7: 1230. https://doi.org/10.3390/polym11071230
APA StyleYu, W., Huang, J. -H., & Tan, C. -S. (2019). Purification of Polybutylene Terephthalate by Oligomer Removal Using a Compressed CO2 Antisolvent. Polymers, 11(7), 1230. https://doi.org/10.3390/polym11071230