3D Printing of Amino Resin-based Photosensitive Materials on Multi-parameter Optimization Design for Vascular Engineering Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Preparation of Amino Resin-based Composites
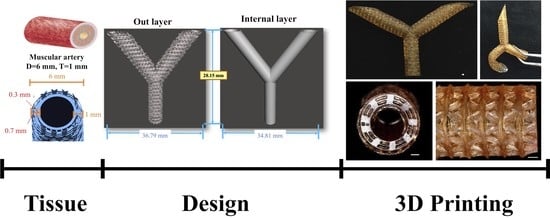
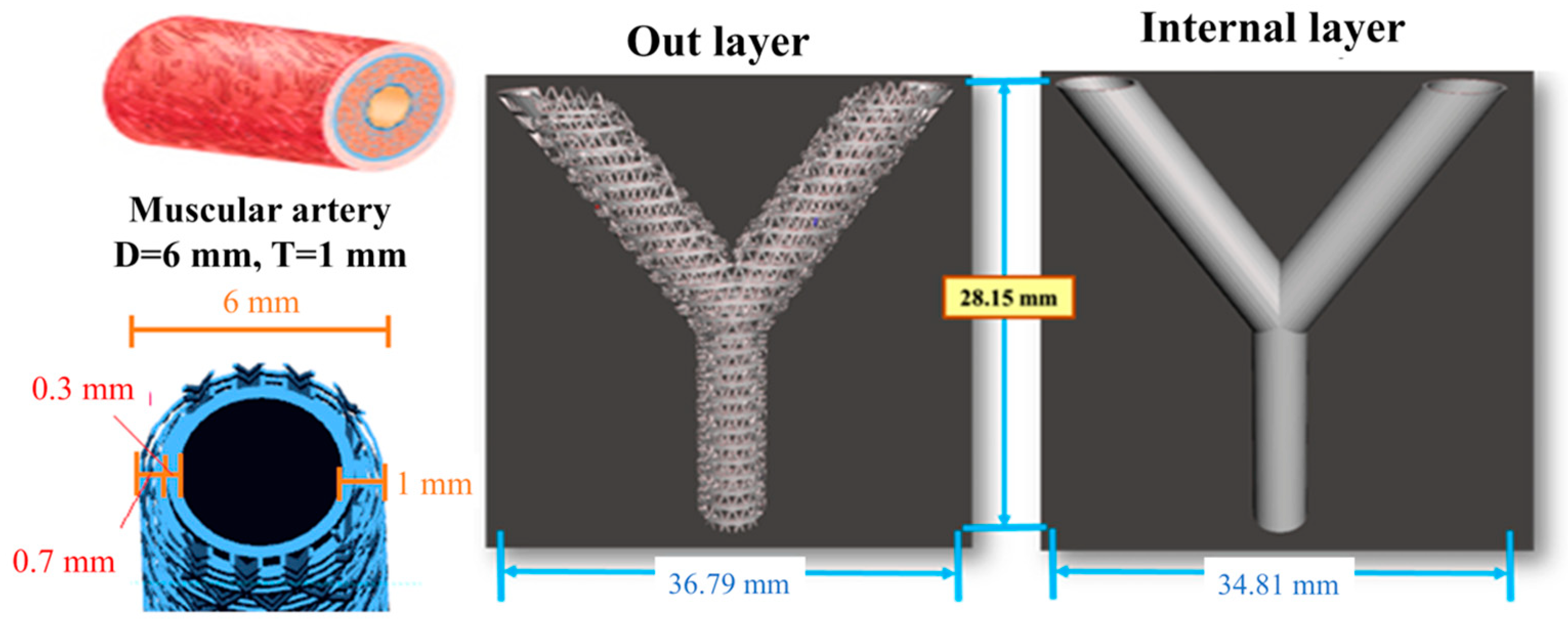
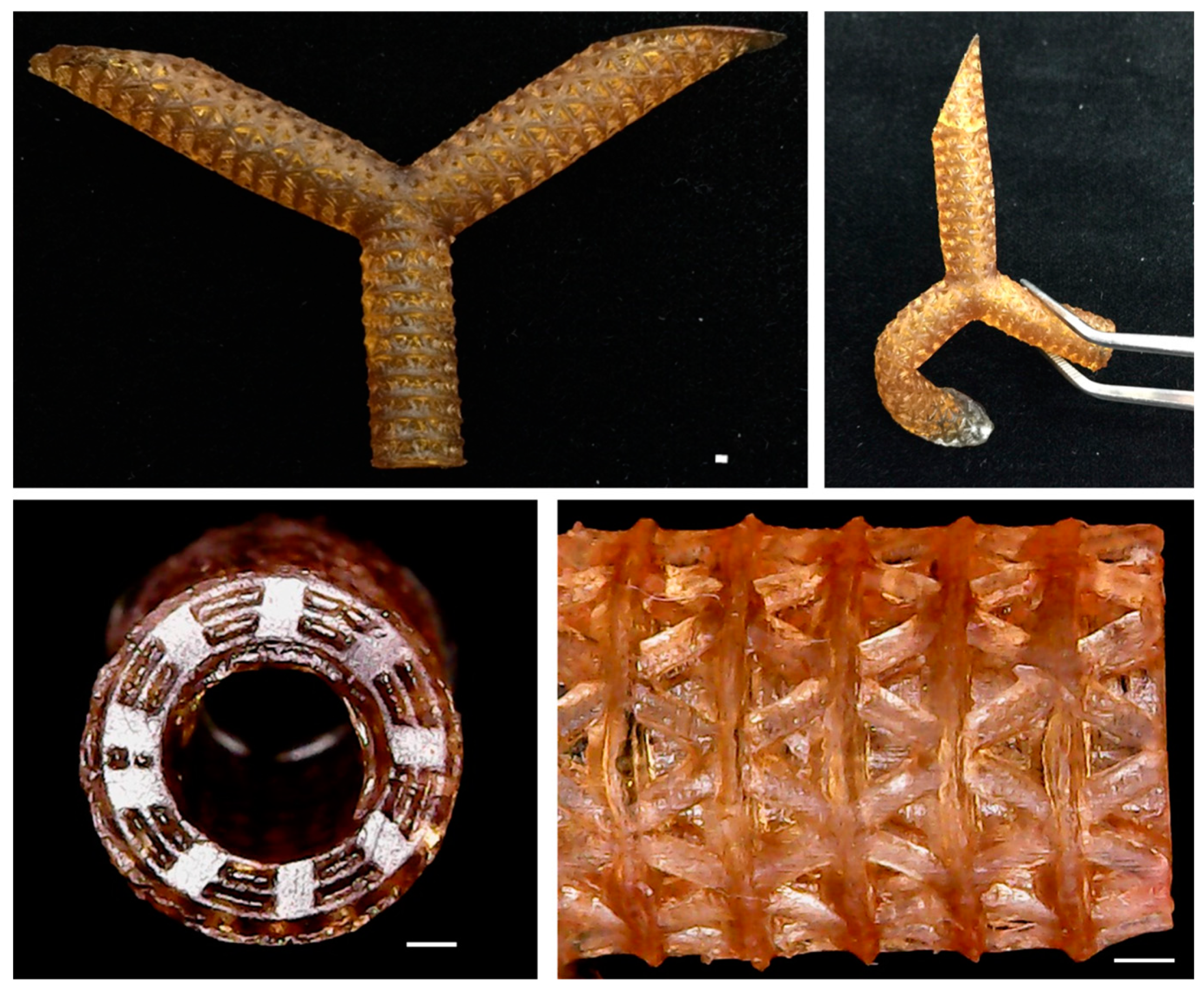
2.3. Artificial Blood Vessel Fabrication
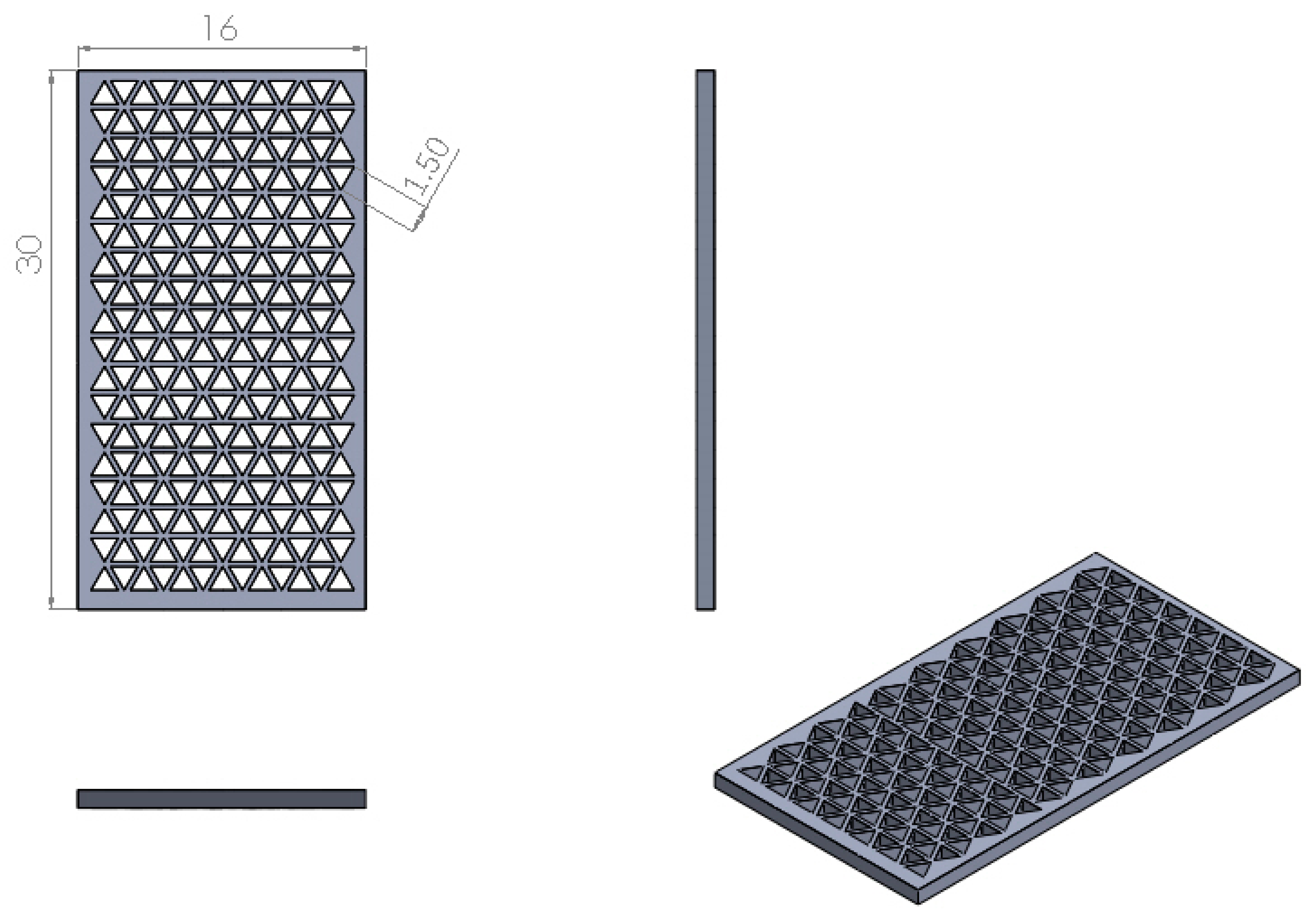
2.4. Printing Accuracy Analysis
2.5. Mechanical Testing
2.6. Cell Viability
2.7. Statistical Analysis
3. Results and Discussion
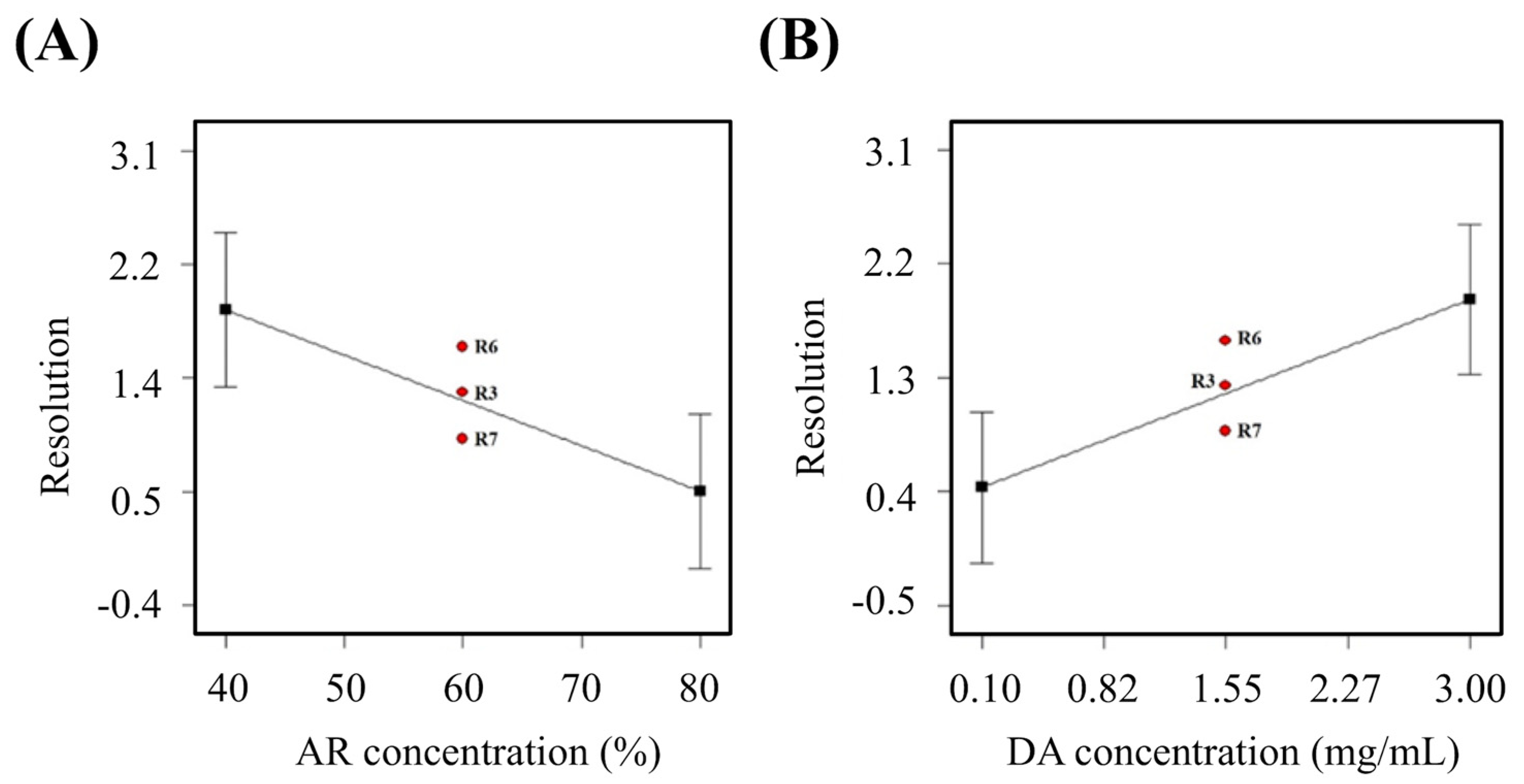
3.1. Resolution
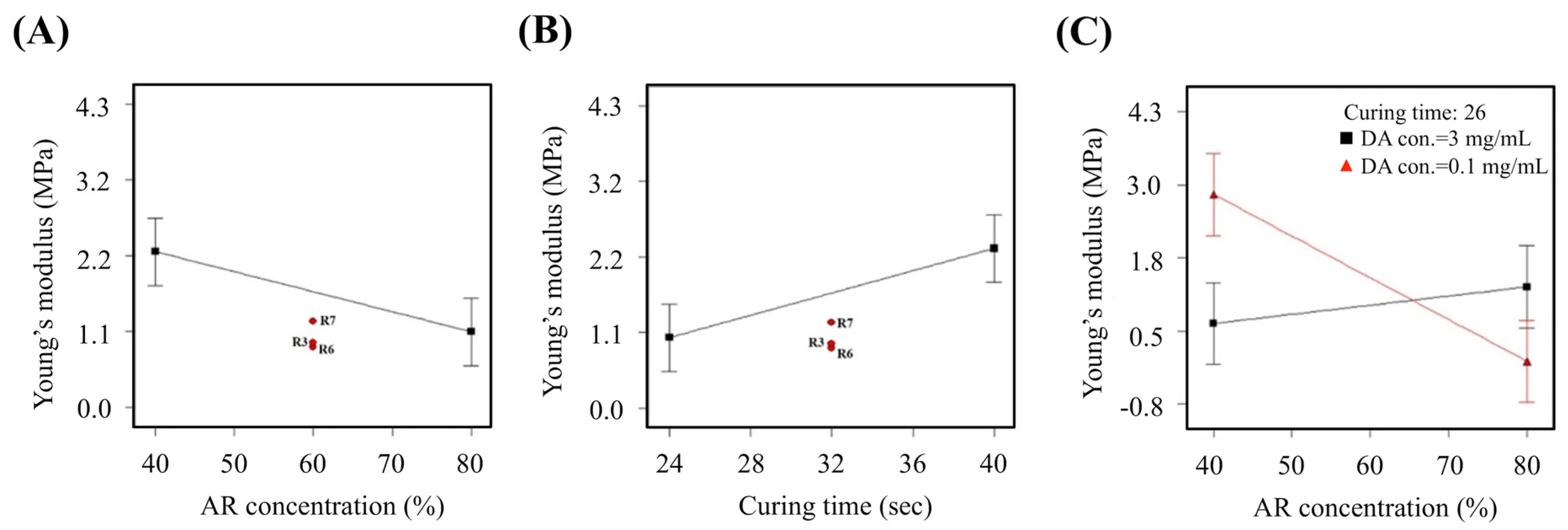
3.2. Young’s Modulus
3.3. Cell Viability
3.4. Comprehensive Comparison of Experimental Models
3.5. The Predicted Optimal Factor-level Combination for Verification
3.6. Optimization Parameter and Fabrication
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Yu, Y.; Akkouch, A.; Dababneh, A.; Dolati, F.; Ozbolat, I.T. In vitro study of directly bioprinted perfusable vasculature conduits. Biomater. Sci. 2015, 3, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Kucukgul, C.; Ozler, S.B.; Inci, I.; Karakas, E.; Irmak, S.; Gozuacik, D.; Taralp, A.; Koç, B. 3D bioprinting of biomimetic aortic vascular constructs with self-supporting cells. Biotechnol. Bioeng. 2015, 112, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.Y.; Lee, J.H.; Kim, B.J.; Kang, J.A.; Hong, J.M.; Kim, B.S.; Cha, H.J.; Rhie, J.W.; Cho, D.W. In vivo endothelization of tubular vascular grafts through in siturecruitment of endothelial and endothelial progenitor cells by RGD-fused mussel adhesive proteins. Biofabrication 2015, 7, 015007. [Google Scholar] [CrossRef] [PubMed]
- Spadaccio, C.; Rainer, A.; Barbato, R.; Trombetta, M.; Chello, M.; Meyns, B. The long-term follow-up of large-diameter Dacron® vascular grafts in surgical practice: A review. J. Cardiovasc. Surg. 2019, 60, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tillman, B.; Go, C.; Cho, S.K.; Clark, W.W.; Hur, T.B.; Ding, Y.; Chun, Y. A novel customizable stent graft that contains a stretchable ePTFE with a laser-welded nitinol stent. J. Biomed. Mater. Res. Part A 2019, 107, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Gungor-Ozkerim, P.S.; Zhang, Y.S.; Yue, K.; Zhu, K.; Liu, W.; Pi, Q.; Byambaa, B.; Dokmeci, M.R.; Shin, S.R.; et al. Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 2016, 106, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Tu, F.; Liu, Y.; Li, H.; Shi, P.; Hao, Y.; Wu, Y.; Yi, H.; Yin, Y.; Wang, J. Vascular cell co-culture on silk fibroin matrix. Polymers 2018, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Yu, C.; Xing, M.; Wu, Y.; Hu, X.; Wang, H.; Wang, L. Hydrogel small-diameter vascular graft reinforced with a braided fiber strut with improved mechanical properties. Polymers 2019, 11, 810. [Google Scholar] [CrossRef]
- Zhang, H.; Dalisson, B.; Tran, S.; Barralet, J. Preservation of blood vessels with an oxygen generating composite. Adv. Healthc. Mater. 2018, 7, 1701338. [Google Scholar] [CrossRef]
- Hong, S.; Kang, E.Y.; Byeon, J.; Jung, S.H.; Hwang, C. Embossed membranes with vascular patterns guide vascularization in a 3D tissue model. Polymers 2019, 11, 792. [Google Scholar] [CrossRef]
- Liu, F.; Chen, Q.; Liu, C.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Natural polymers for organ 3D bioprinting. Polymers 2018, 10, 1278. [Google Scholar] [CrossRef]
- Shie, M.Y.; Chiang, W.H.; Chen, I.W.P.; Liu, W.Y.; Chen, Y.W. Synergistic acceleration in the osteogenic and angiogenic differentiation of human mesenchymal stem cells by calcium silicate–graphene composites. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 73, 726–735. [Google Scholar] [CrossRef]
- Sarkar, S.; Schmitz-Rixen, T.; Hamilton, G.; Seifalian, A.M. Achieving the ideal properties for vascular bypass grafts using a tissue engineered approach: A review. Med. Biol. Eng. Comput. 2007, 45, 327–336. [Google Scholar] [CrossRef]
- Garreta, E.; Oria, R.; Tarantino, C.; Pla-Roca, M.; Prado, P.; Fernández-Avilés, F.; Campistol, J.M.; Samitier, J.; Montserrat, N. Tissue engineering by decellularization and 3D bioprinting. Mater. Today 2017, 20, 166–178. [Google Scholar] [CrossRef]
- Maiti, B.; Díaz Díaz, D. 3D printed polymeric hydrogels for nerve regeneration. Polymers 2018, 10, 1041. [Google Scholar] [CrossRef]
- Kankala, R.K.; Xu, X.M.; Liu, C.G.; Chen, A.Z.; Wang, S.B. 3D-printing of microfibrous porous scaffolds based on hybrid approaches for bone tissue engineering. Polymers 2018, 10, 807. [Google Scholar] [CrossRef]
- Shojaie, S.; Ermini, L.; Ackerley, C.; Wang, J.; Chin, S.; Yeganeh, B.; Bilodeau, M.; Sambi, M.; Rogers, I.; Rossant, J.; et al. Acellular lung scaffolds direct differentiation of endoderm to functional airway epithelial cells: Requirement of matrix-bound HS proteoglycans. Stem Cell Rep. 2015, 4, 419–430. [Google Scholar] [CrossRef]
- Dong, C.; Lv, Y. Application of collagen scaffold in tissue engineering: Recent advances and new perspectives. Polymers 2016, 8, 42. [Google Scholar] [CrossRef]
- Chen, J.Y.; Hwang, J.V.; Ao-Ieong, W.S.; Lin, Y.C.; Hsieh, Y.K.; Cheng, Y.L.; Wang, J. Study of physical and degradation properties of 3D-printed biodegradable, photocurable copolymers, PGSA-co-PEGDA and PGSA-co-PCLDA. Polymers 2018, 10, 1263. [Google Scholar] [CrossRef]
- Chiu, Y.C.; Shie, M.Y.; Lin, Y.H.; Lee, K.X.; Chen, Y.W. Effect of strontium substitution on the physicochemical properties and bone regeneration potential of 3D printed calcium silicate scaffolds. Int. J. Mol. Sci. 2019, 20, 2729. [Google Scholar] [CrossRef]
- Chen, C.C.; Yu, J.; Ng, H.Y.; Lee, K.X.; Chen, C.C.; Chen, Y.S.; Shie, M.Y. The physicochemical properties of decellularized extracellular matrix-coated 3D printed poly(ε-caprolactone) nerve conduits for promoting Schwann cells proliferation and differentiation. Materials 2018, 11, 1665. [Google Scholar] [CrossRef]
- Ho, C.C.; Fang, H.Y.; Wang, B.; Huang, T.H.; Shie, M.Y. The effects of Biodentine/polycaprolactone 3D-scaffold with odontogenesis properties on human dental pulp cells. Int. Endod. J. 2018, 51, e291–e300. [Google Scholar] [CrossRef]
- Tsai, C.H.; Hung, C.H.; Kuo, C.N.; Chen, C.Y.; Peng, Y.N.; Shie, M.Y. Improved bioactivity of 3D printed porous titanium alloy scaffold with chitosan/magnesium-calcium silicate composite for orthopaedic applications. Materials 2019, 12, 203. [Google Scholar] [CrossRef]
- Lin, Y.H.; Chuang, T.Y.; Chiang, W.H.; Chen, I.W.P.; Wang, K.; Shie, M.Y.; Chen, Y.W. The synergistic effects of graphene-contained 3D-printed calcium silicate/poly-ε-caprolactone scaffolds promote FGFR-induced osteogenic/angiogenic differentiation of mesenchymal stem cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 104, 109887. [Google Scholar] [CrossRef]
- Wu, Y.H.; Chiu, Y.C.; Lin, Y.H.; Ho, C.C.; Shie, M.Y.; Chen, Y.W. 3D-printed bioactive calcium silicate/poly-ε-caprolactone bioscaffolds modified with biomimetic extracellular matrices for bone regeneration. Int. J. Mol. Sci. 2019, 20, 942. [Google Scholar] [CrossRef]
- Chen, Y.W.; Shen, Y.F.; Ho, C.C.; Yu, J.; Wu, Y.H.; Wang, K.; Shih, C.T.; Shie, M.Y. Osteogenic and angiogenic potentials of the cell-laden hydrogel/mussel-inspired calcium silicate complex hierarchical porous scaffold fabricated by 3D bioprinting. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 679–687. [Google Scholar] [CrossRef]
- Derby, B. Printing and prototyping of tissues and scaffolds. Science 2012, 338, 921–926. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Chen, Y.W.; Wang, K.; Shie, M.Y. Enhanced adhesion and differentiation of human mesenchymal stem cell inside apatite-mineralized/poly(dopamine)-coated poly(ε-caprolactone) scaffolds by stereolithography. J. Mater. Chem. B 2016, 4, 6307–6315. [Google Scholar] [CrossRef]
- Ozbolat, I.T. Scaffold-based or scaffold-free bioprinting: Competing or complementing approaches? J. Nanotechnol. Eng. Med 2015, 6, 024701. [Google Scholar] [CrossRef]
- Datta, P.; Ayan, B.; Ozbolat, I.T. Bioprinting for vascular and vascularized tissue biofabrication. Acta Biomater. 2017, 51, 1–20. [Google Scholar] [CrossRef]
- Melchiorri, A.J.; Hibino, N.; Best, C.A.; Yi, T.; Lee, Y.U.; Kraynak, C.A.; Kimerer, L.K.; Krieger, A.; Kim, P.; Breuer, C.K.; et al. 3D-printed biodegradable polymeric vascular grafts. Adv. Healthc. Mater. 2016, 5, 319–325. [Google Scholar] [CrossRef]
- Kroll, E.; Buchris, E. Weight reduction of 3D-printed cylindrical and toroidal pressure vessels through shape modification. Procedia Manuf. 2018, 21, 133–140. [Google Scholar] [CrossRef]
- Anada, T.; Pan, C.C.; Stahl, A.M.; Mori, S.; Fukuda, J.; Suzuki, O.; Yang, Y. Vascularized bone-mimetic hydrogel constructs by 3D bioprinting to promote osteogenesis and angiogenesis. Int. J. Mol. Sci. 2019, 20, 1096. [Google Scholar] [CrossRef]
- Ware, H.O.T.; Farsheed, A.C.; Akar, B.; Duan, C.; Chen, X.; Ameer, G.; Sun, C. High-speed on-demand 3D printed bioresorbable vascular scaffolds. Mater. Today Chem. 2018, 7, 25–34. [Google Scholar] [CrossRef]
- Goh, T.N. A pragmatic approach to experimental design in industry. J. Appl. Stat. 2001, 28, 391–398. [Google Scholar] [CrossRef]
- Avasatthi, V.; Pawar, H.; Dora, C.P.; Bansod, P.; Gill, M.S.; Suresh, S. A novel nanogel formulation of methotrexate for topical treatment of psoriasis: Optimization, in vitro and in vivo evaluation. Pharm. Dev. Technol. 2016, 21, 554–562. [Google Scholar]
- Silva, J.M.; Custódio, C.A.; Reis, R.L.; Mano, J.F. Multilayered hollow tubes as blood vessel substitutes. ACS Biomater. Sci. Eng. 2016, 2, 2304–2314. [Google Scholar] [CrossRef]
- Al Kayal, T.; Maniglio, D.; Bonani, W.; Losi, P.; Migliaresi, C.; Soldani, G. A combined method for bilayered vascular graft fabrication. J. Mater. Sci. Mater. Med. 2015, 26, 96. [Google Scholar] [CrossRef]
- Kao, C.T.; Lin, C.C.; Chen, Y.W.; Yeh, C.H.; Fang, H.Y.; Shie, M.Y. Poly(dopamine) coating of 3D printed poly(lactic acid) scaffolds for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 56, 165–173. [Google Scholar] [CrossRef]
- Antonova, L.V.; Silnikov, V.N.; Sevostyanova, V.V.; Yuzhalin, A.E.; Koroleva, L.S.; Velikanova, E.A.; Mironov, A.V.; Godovikova, T.S.; Kutikhin, A.G.; Glushkova, T.V.; et al. Biocompatibility of small-diameter vascular grafts in different modes of RGD modification. Polymers 2019, 11, 174. [Google Scholar] [CrossRef]


| Experimental Factor | AR con. (%) | DA con. (mg/mL) | Curing time(sec) |
|---|---|---|---|
| Low level | 40 | 0.1 | 23 |
| High Level | 80 | 3 | 40 |
| Run# | AR con. (%) | DA con. (mg/mL) | Curing Time (sec) | Resolution (mm2) | Young’s Modulus (MPa) | Cell Viability (%) |
|---|---|---|---|---|---|---|
| 1 | 40.00 | 3.00 | 40.00 | 2.76 | 1.33 | 67.26 |
| 2 | 40.00 | 3.00 | 24.00 | 3.05 | 2.71 | 65.76 |
| 3 | 60.00 | 1.55 | 32.00 | 1.24 | 0.92 | 70.14 |
| 4 | 80.00 | 0.10 | 24.00 | 0.05 | 0.82 | 68.64 |
| 5 | 80.00 | 3.00 | 40.00 | 1.86 | 3.46 | 77.30 |
| 6 | 60.00 | 1.55 | 32.00 | 1.59 | 0.86 | 74.28 |
| 7 | 60.00 | 1.55 | 32.00 | 0.88 | 1.22 | 81.02 |
| 8 | 40.00 | 0.10 | 40.00 | 0.00 | 4.30 | 89.23 |
| 9 | 80.00 | 0.10 | 40.00 | 0.00 | 0.00 | 72.76 |
| 10 | 40.00 | 0.10 | 24.00 | 1.68 | 0.48 | 76.06 |
| 11 | 80.00 | 3.00 | 24.00 | 0.00 | 0.00 | 0.00 |
| Run# | X: Resolution (mm2) | Y: Young’s Modulus (MPa) | Z: Cell Viability (%) | Distance | Order |
|---|---|---|---|---|---|
| 1 | 2.76 | 1.333 | 67.26 | 32.79 | 9 |
| 2 | 3.05 | 2.707 | 65.76 | 34.34 | 10 |
| 3 | 1.24 | 0.917 | 70.14 | 29.86 | 7 |
| 4 | 0.05 | 0.822 | 68.64 | 31.37 | 8 |
| 5 | 1.86 | 3.456 | 77.30 | 22.85 | 3 |
| 6 | 1.59 | 0.856 | 74.28 | 25.73 | 5 |
| 7 | 0.88 | 1.218 | 81.02 | 18.98 | 2 |
| 8 | 0.00 | 4.298 | 89.23 | 11.31 | 1 |
| 9 | 0.00 | 0.00 | 72.76 | 27.26 | 6 |
| 10 | 1.68 | 0.484 | 76.06 | 23.95 | 4 |
| 11 | 0.00 | 0.00 | 0.00 | 100 | 11 |
| Run# | AR con. (%) | DA con. (mg/mL) | Curing time (sec) | Resolution (mm2) | Young’s Modulus (MPa) | Cell Viability (%) |
|---|---|---|---|---|---|---|
| 1 | 40.00 | 0.10 | 26.62 | 1.39 | 0.98 | 82.19 |
| 2 | 40.00 | 0.10 | 26.54 | 1.39 | 0.96 | 82.12 |
| 3 | 40.00 | 0.10 | 26.47 | 1.40 | 0.94 | 82.06 |
| 4 | 41.75 | 0.10 | 26.53 | 1.34 | 0.98 | 81.37 |
| 5 | 40.00 | 0.10 | 26.74 | 1.48 | 0.78 | 81.47 |
| Parameter | AR con. (%) | DA con. (mg/mL) | Curing Time (sec) | Resolution (mm2) | Young’s Modulus (MPa) | Cell Viability (%) |
|---|---|---|---|---|---|---|
| Expectation value | 40.00 | 0.10 | 26.62 | 1.39 | 0.983 | 82.19 |
| Experimental value | 40.00 | 0.10 | 26.60 | 1.35 | 0.988 | 88.37 |
| Deviation (%) | 0 | 0 | 0.75 | -2.68 | 0.55 | 6.99 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, Y.-C.; Shen, Y.-F.; Lee, A.K.-X.; Lin, S.-H.; Wu, Y.-C.; Chen, Y.-W. 3D Printing of Amino Resin-based Photosensitive Materials on Multi-parameter Optimization Design for Vascular Engineering Applications. Polymers 2019, 11, 1394. https://doi.org/10.3390/polym11091394
Chiu Y-C, Shen Y-F, Lee AK-X, Lin S-H, Wu Y-C, Chen Y-W. 3D Printing of Amino Resin-based Photosensitive Materials on Multi-parameter Optimization Design for Vascular Engineering Applications. Polymers. 2019; 11(9):1394. https://doi.org/10.3390/polym11091394
Chicago/Turabian StyleChiu, Yung-Cheng, Yu-Fang Shen, Alvin Kai-Xing Lee, Shu-Hsien Lin, Yu-Chen Wu, and Yi-Wen Chen. 2019. "3D Printing of Amino Resin-based Photosensitive Materials on Multi-parameter Optimization Design for Vascular Engineering Applications" Polymers 11, no. 9: 1394. https://doi.org/10.3390/polym11091394
APA StyleChiu, Y.-C., Shen, Y.-F., Lee, A. K.-X., Lin, S.-H., Wu, Y.-C., & Chen, Y.-W. (2019). 3D Printing of Amino Resin-based Photosensitive Materials on Multi-parameter Optimization Design for Vascular Engineering Applications. Polymers, 11(9), 1394. https://doi.org/10.3390/polym11091394