Influence of Nano Titanium Dioxide and Clove Oil on Chitosan–Starch Film Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Preparation
2.3. Determination of Film Structure
2.4. Physico-Chemical Properties
2.5. Biological Properties
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Film
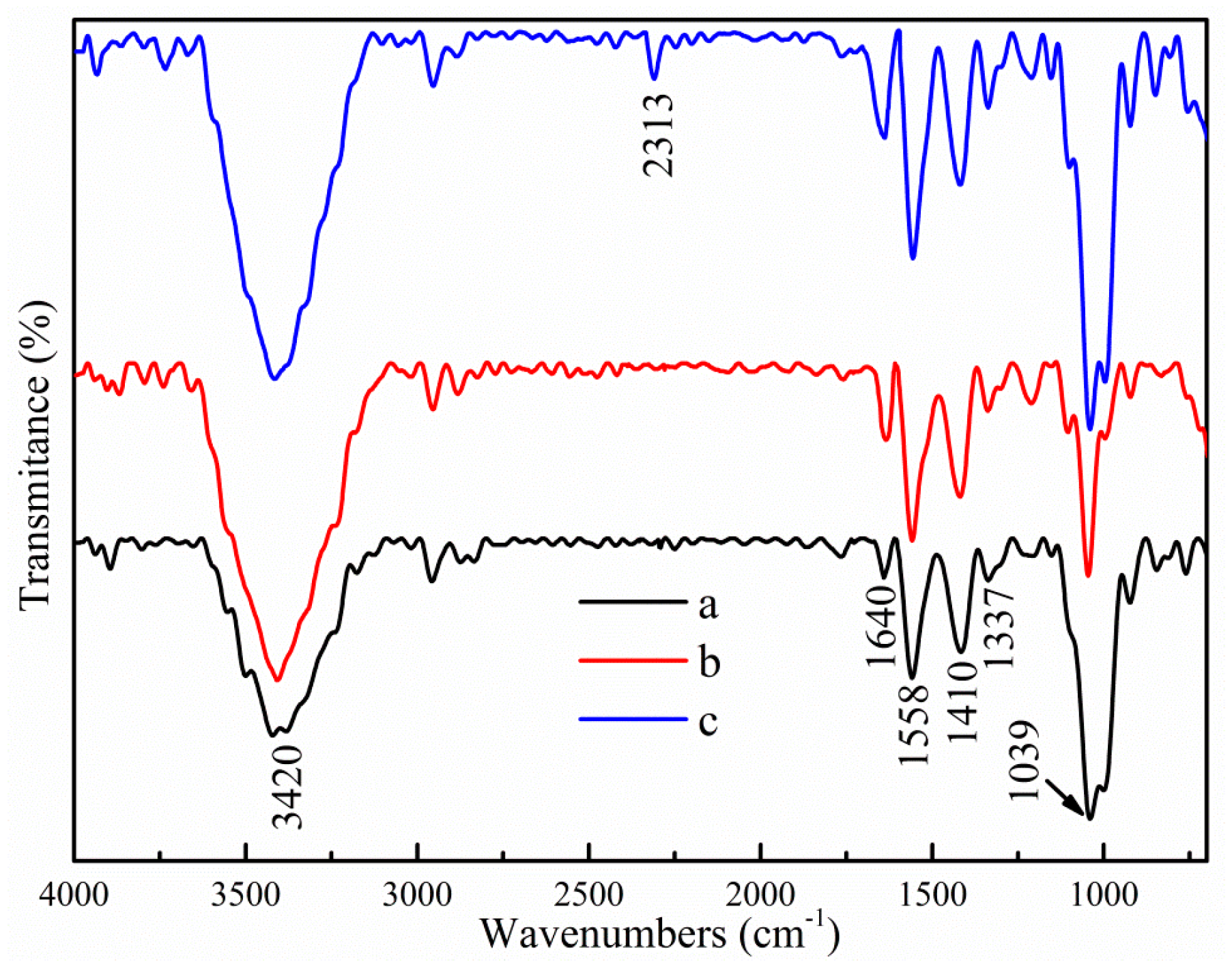
3.1.1. FTIR Spectroscopy
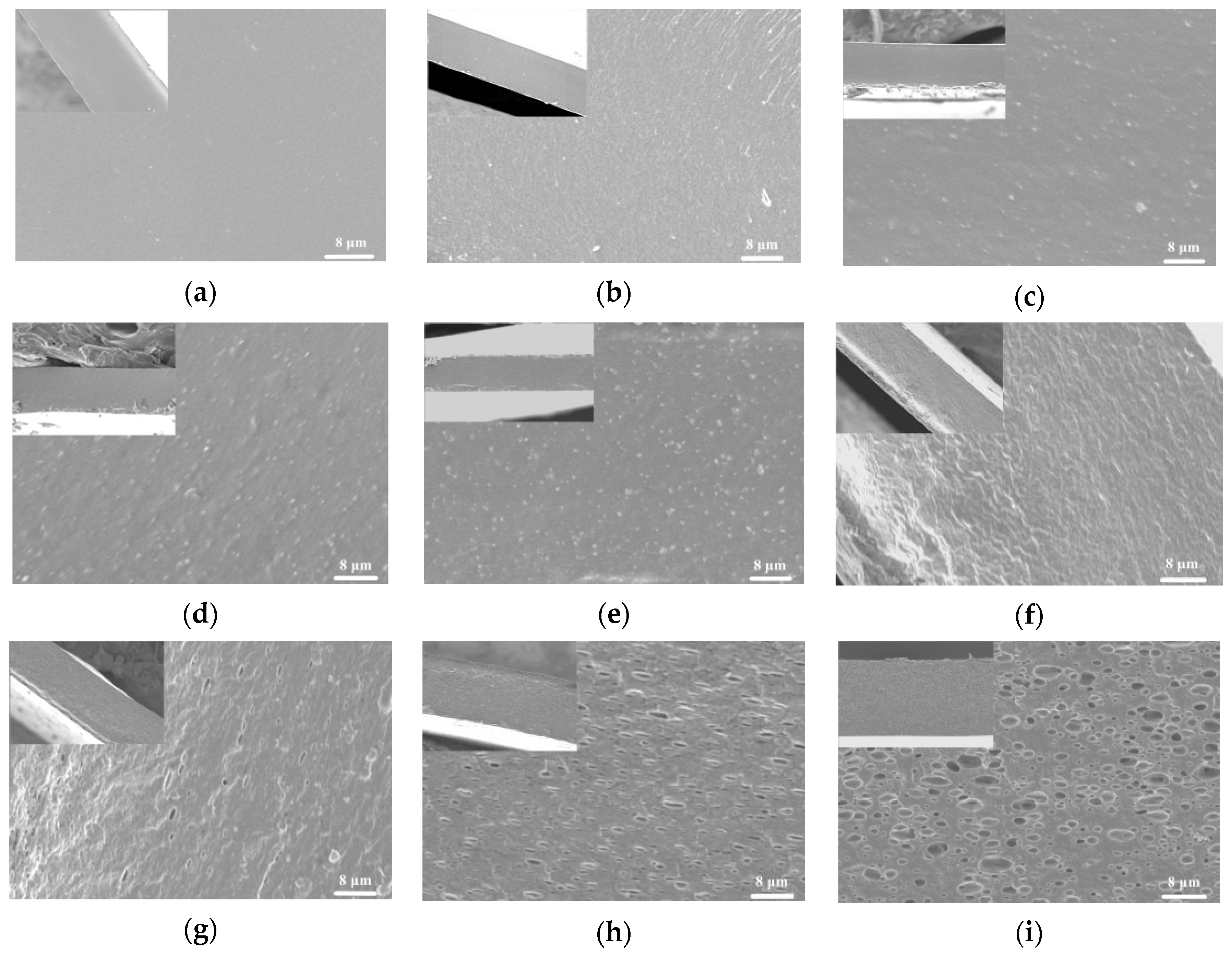
3.1.2. Microstructure
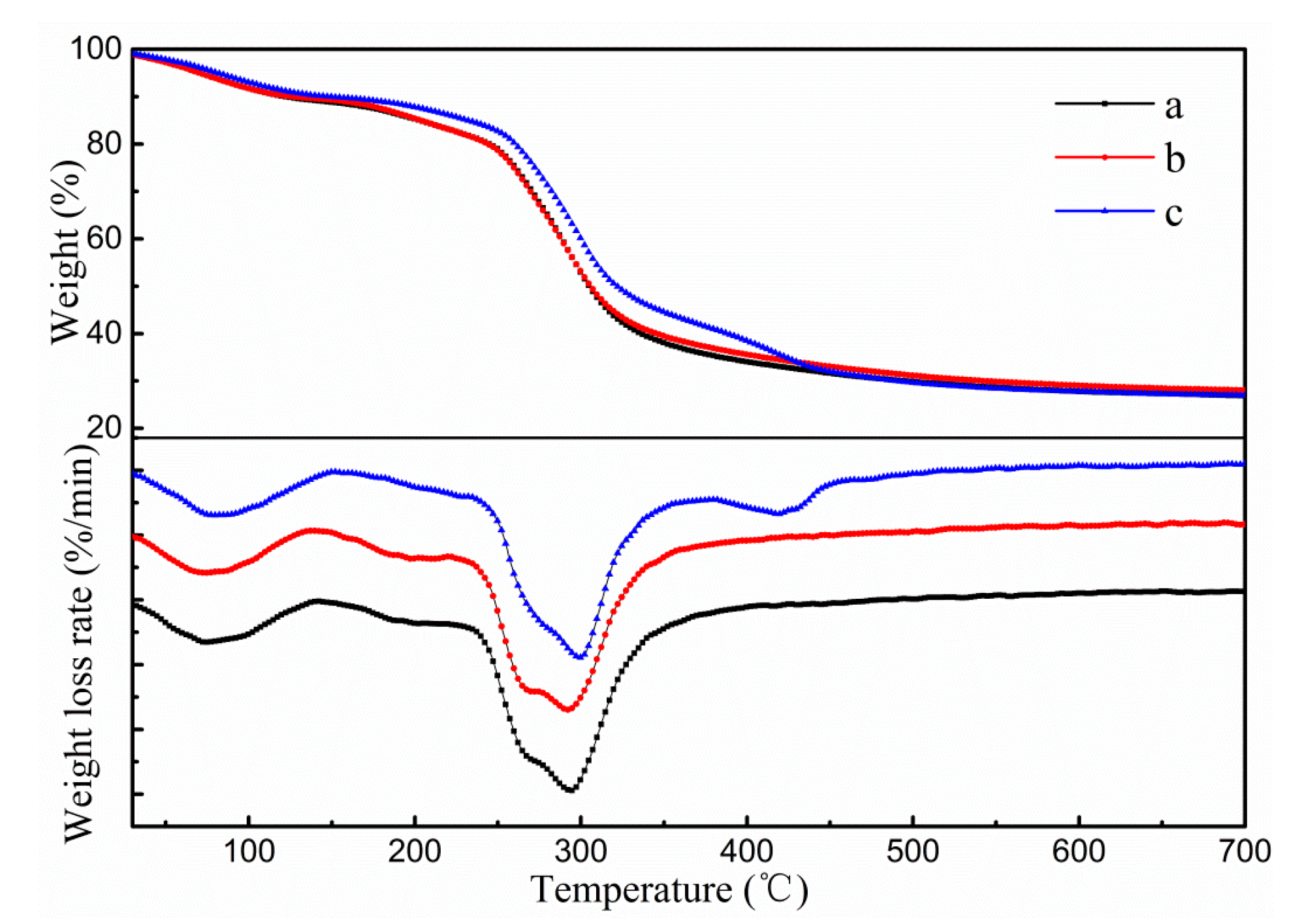
3.1.3. Thermal Stability
3.1.4. Morphology Analysis
3.2. Physico-Chemical Properties
3.2.1. Mechanical Properties
3.2.2. Moisture Content and Water Contact Angle
3.2.3. Water Vapour Permeability
3.2.4. Colour Difference and Opacity
3.3. Biological Properties
3.3.1. Antimicrobial Activity
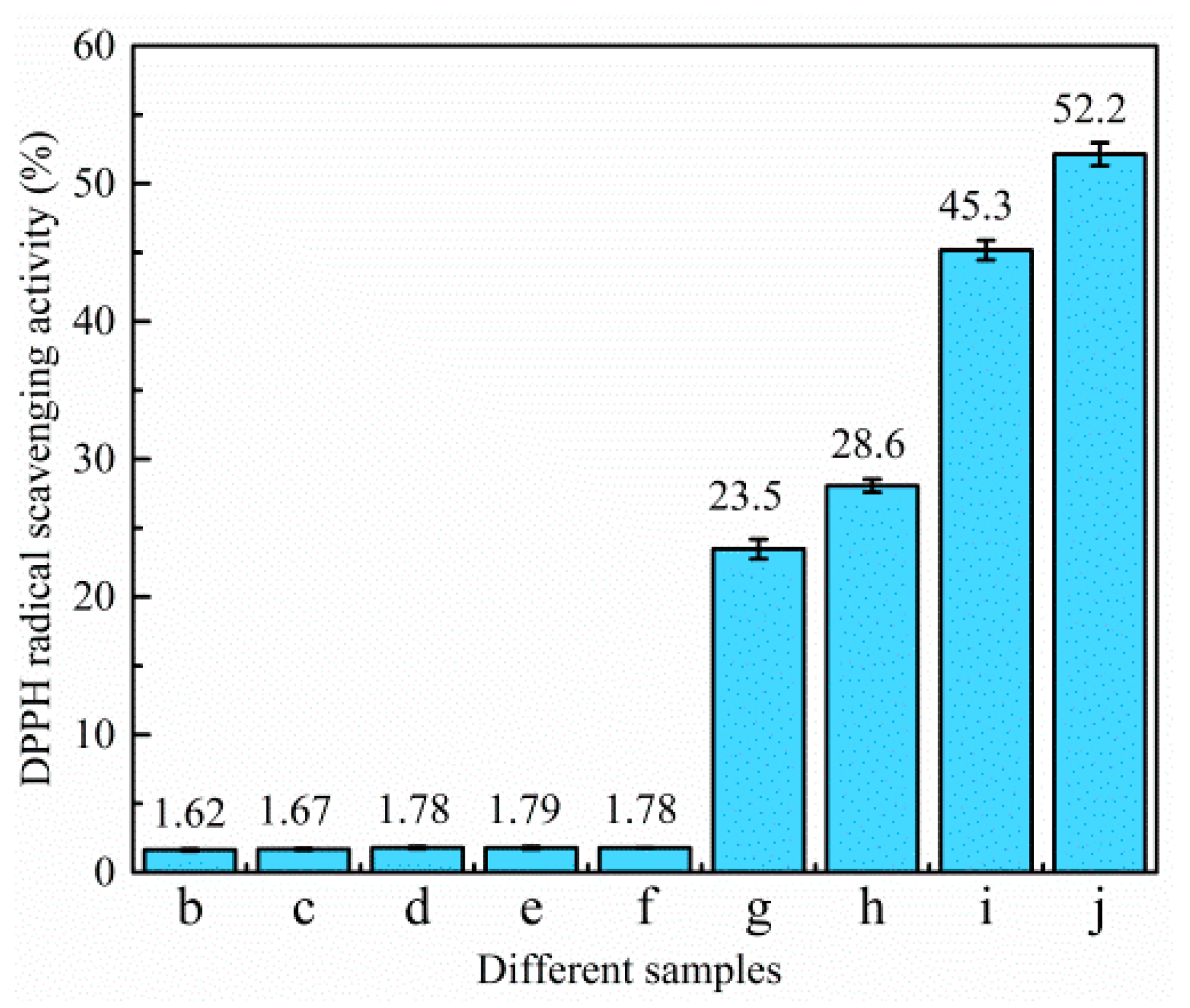
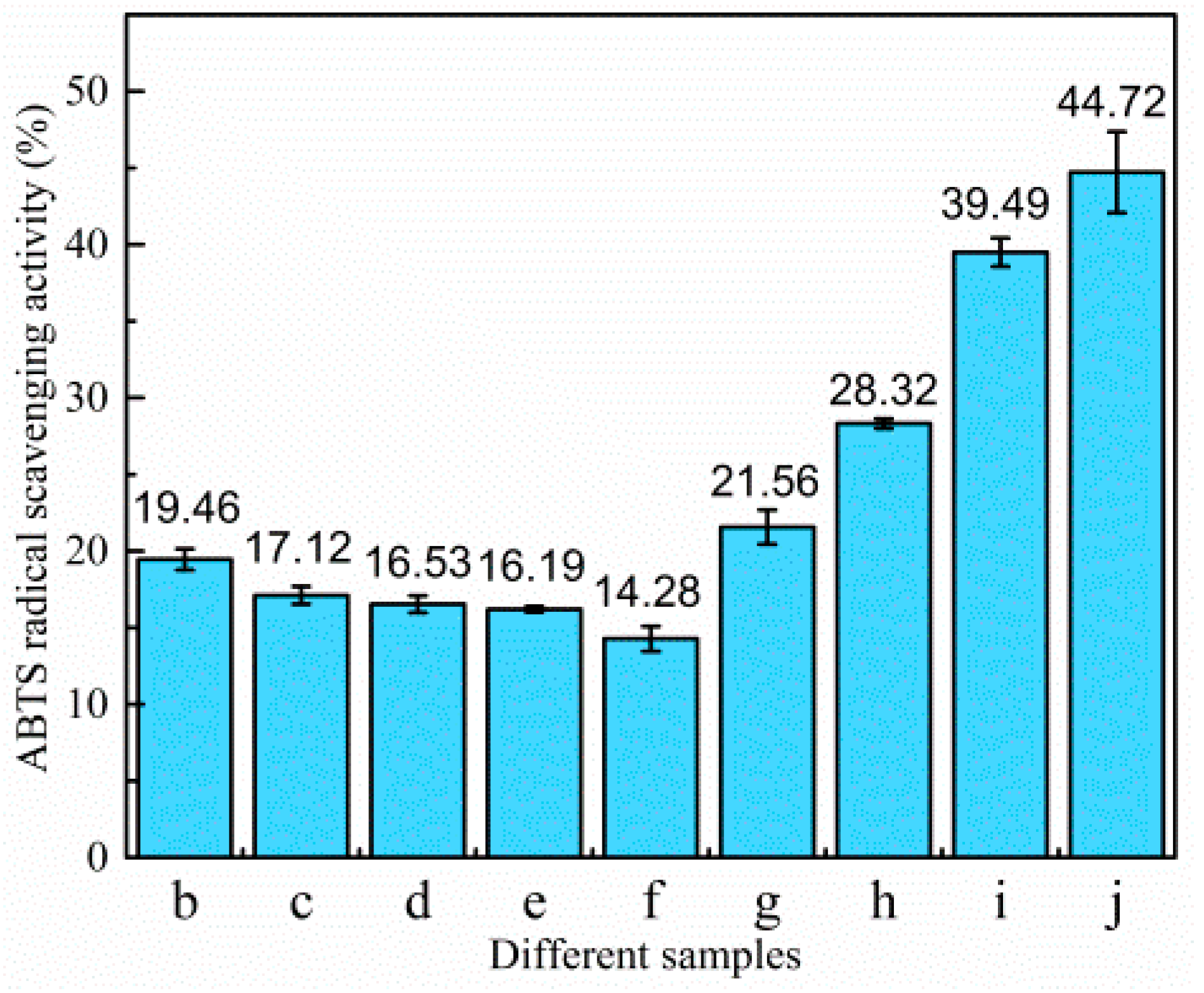
3.3.2. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CH | chitosan |
| ST | starch |
| TiO2-N | nano titanium dioxide |
| CO | clove oil |
References
- Elsabee, M.Z.; Abdou, E.S. Chitosan based edible films and coatings: A review. Mater. Sci. Eng. C. 2013, 33, 1819–1841. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, S.; Hosseini, S.V.; Regenstein, J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Chillo, S.; Flores, S.; Mastromatteo, M.; Conte, A.; Gerschenson, L.; Del Nobile, M.A. Influence of glycerol and chitosan on tapioca starch-based edible film properties. Int. J. Food Eng. 2008, 88, 159–168. [Google Scholar] [CrossRef]
- Kerch, G. Chitosan films and coatings prevent losses of fresh fruit nutritional quality: A review. Trends Food Sci. Technol. 2015, 46, 159–166. [Google Scholar] [CrossRef]
- Özdemir, K.S.; Gökmen, V. Extending the shelf-life of pomegranate arils with chitosan-ascorbic acid coating. LWT Food Sci. Technol. 2017, 76, 172–180. [Google Scholar] [CrossRef]
- Zhao, Y.; Teixeira, J.S.; Gänzle, M.M.; Saldaña, M.D.A. Development of Antimicrobial Films Based on Cassava Starch, Chitosan and Gallic Acid Using Subcritical Water Technology. J. Supercrit. Fluids 2018, 137, 101–110. [Google Scholar] [CrossRef]
- Pinzon, M.; Garcia, O.; Villa, C. The influence of Aloe vera gel incorporation on the physicochemical and mechanical properties of banana starch-chitosan edible films. J. Sci. Food Agric. 2018, 98, 4042–4049. [Google Scholar] [CrossRef]
- Aider, M. Chitosan application for active bio-based films production and potential in the food industry: Review. LWT Food Sci. Technol. 2010, 43, 837–842. [Google Scholar] [CrossRef]
- Schreiber, S.B.; Bozell, J.; Hayes, D.G.; Zivanovic, S. Introduction of primary antioxidant activity to chitosan for application as a multifunctional food packaging material. Food Hydrocoll. 2013, 33, 207–214. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Ren, L.; Yan, X.; Zhou, J.; Tong, J.; Su, X. Influence of chitosan concentration on mechanical and barrier properties of corn starch/chitosan films. Int. J. Biol. Macromol. 2017, 105, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Bonila, J.; Atarés, L.; Varges, M.; Chiralt, A. Properties of wheat starch film-forming dispersions and films as affected by chitosan addition. J. Food Eng. 2013, 114, 303–312. [Google Scholar] [CrossRef]
- Mohammadi, R.; Mohammadifar, M.A.; Rouhi, M.; Mohaddeseh, K.; Amir, M.M.; Ehsan, S.; Sara, H. Physico-mechanical and structural properties of eggshell membrane gelatin-chitosan blend edible films. Int. J. Biol. Macromol. 2017, 107, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Talón, E.; Trifkovic, K.T.; Nedovic, V.A.; Bugarski, B.M.; Vargas, M.; Chiralt, A.; González-Martínez, C. Antioxidant edible films based on chitosan and starch containing polyphenols from thyme extracts. Carbohydr. Polym. 2017, 157, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Córdoba, L.J.; Norton, L.T.; Batchelor, H.K.; Gkatzionis, K.; Spyropoulos, F.; Sobral, P.J.A. Physico-chemical, antimicrobial and antioxidant properties of gelatin-chitosan based films loaded with nanoemulsions encapsulating active compounds. Food Hydrocoll. 2018, 79, 544–559. [Google Scholar] [CrossRef]
- Yang, J.; Xiong, L.; Li, M.; Sun, Q.J. Chitosan–Sodium Phytate Films with a Strong Water Barrier and Antimicrobial Properties Produced via One-Step-Consecutive-Stripping and Layer-by-Layer-Casting Technologies. J. Agric. Food Chem. 2018, 66, 6104–6115. [Google Scholar] [CrossRef]
- Sadegh-Hassani, F.; Nafchi, A.M. Preparation and characterization of bionanocomposite films based on potato starch/halloysite nanoclay. Int. J. Biol. Macromol. 2014, 67, 458–462. [Google Scholar] [CrossRef]
- Akbariazam, M.; Ahmadi, M.; Javadian, N.; Nafchi, A.M. Fabrication and characterization of soluble soybean polysaccharide and nanorod-rich ZnO bionanocomposite. Int. J. Biol. Macromol. 2016, 89, 369–375. [Google Scholar] [CrossRef]
- Yin, C.; Huang, C.X.; Wang, J.; Liu, Y.; Lu, P.; Huang, L.J. Effect of Chitosan- and Alginate-Based Coatings Enriched with Cinnamon Essential Oil Microcapsules to Improve the Postharvest Quality of Mangoes. Materials 2019, 12, 2039. [Google Scholar] [CrossRef]
- Marvizadeh, M.M.; Oladzadabbasabadi, N.; Nafchi, A.M.; Jokar, M. Preparation and characterization of bionanocomposite film based on tapioca starch/bovine gelatin/nanorod zinc oxide. Int. J. Biol. Macromol. 2017, 99, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Rezaei, M.; Farzi, G. A novel active bionanocomposite film incorporating rosemary essential oil and nanoclay into chitosan. J. Food Eng. 2012, 111, 343–350. [Google Scholar] [CrossRef]
- Mei, J.; Yuan, Y.; Guo, Q.; Wu, Y.; Li, Y.; Yu, H. Characterization and antimicrobial properties of water chestnut starch-chitosan edible films. Int. J. Biol. Macromol. 2013, 61, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Altln, I.; Sökmen, M. Preparation of TiO2-polystyrene photocatalyst from waste material and its usability for removal of various pollutants. Appl. Catal. B Environ. 2014, 144, 694–701. [Google Scholar]
- Zhang, X.; Xiao, G.; Wang, Y.; Zhao, Y.; Su, H.; Tan, T. Preparation of chitosan-TiO2 composite film with efficient antimicrobial activities under visible light for food packaging applications. Carbohydr. Polym. 2017, 169, 101–107. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Huang, Y.; Lin, B.; Wang, S. A nanocomposite film fabricated with simultaneously extracted protein-polysaccharide from a marine alga and TiO2nanoparticles. J. Appl. Phycol. 2017, 29, 1541–1552. [Google Scholar] [CrossRef]
- Zheng, K.; Xiao, S.; Li, W.; Wang, W.; Chen, H.; Yang, F.; Qin, C. Chitosan-acorn starch-eugenol edible film: Physico-chemical, barrier, antimicrobial, antioxidant and structural properties. Int. J. Biol. Macromol. 2019, 135, 344–352. [Google Scholar] [CrossRef]
- Alparslan, Y. Antimicrobial and antioxidant capacity of biodegradable gelatin film forming solutions incorporated with different essential oils. J. Food Meas. Charact. 2018, 12, 317–322. [Google Scholar] [CrossRef]
- EI-Mesallamy, A.M.D.; EI-Gerby, M.; Azim, M.H.A.E.; Awad, A. Antioxidant, Antimicrobial Activities and Volatile Constituents of Clove Flower Buds Oil. J. Essent. Oil Bear. Plants 2012, 15, 900–907. [Google Scholar] [CrossRef]
- Aguilar-Sánchez, R.; Munguía-Pérez, R.; Reyes-Jurado, F.; Navarro-Cruz, A.R.; Cid-Pérez, T.S.; Hernándz-Carranza, P.; Beristain-Bauza, S.C.; Ochoa-Velasco, C.E.; Avila-Sosa, R. Structural, Physical, and Antifungal Characterization of Starch Edible Films Added with Nanocomposites and Mexican Oregano (Lippia berlandieri Schauer) Essential Oil. Molecules 2019, 24, 2340. [Google Scholar] [CrossRef]
- Wu, J.; Sun, Q.; Huang, H.; Duan, Y.; Xiao, G.; Le, T. Enhanced physico-mechanical, barrier and antifungal properties of soy protein isolate film by incorporating both plant-sourced cinnamaldehyde and facile synthesized zinc oxide nanosheets. Colloids Surf. B Biointerfaces 2019, 180, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhou, W.; Pang, C.; Deng, W.; Xu, C.; Wang, X. Multifunctional chitosan-based coating with liposomes containing laurel essential oils and nanosilver for pork preservation. Food Chem. 2019, 295, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Nafchi, A.M.; Moradpour, M.; Saeidi, M.; Alias, A.K. Thermoplastic starches: Properties, challenges, and prospects. Starch Stärke 2013, 65, 61–72. [Google Scholar] [CrossRef]
- Nafchi, A.M.; Alias, A.K.; Muhmud, S.; Robal, M. Antimicrobial, rheological, and physicochemical properties of sago starch films filled with nanorod-rich zinc oxide. J. Food Eng. 2012, 113, 511–519. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, M.; Xiao, C.; Zhao, Q.; Su, G. Practical problems when using ABTS assay to assess the radical-scavenging activity of peptides: Importance of controlling reaction pH and time. Food Chem. 2016, 192, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Ojagh, S.M.; Razavi, S.H.; Hosseini, S.M.H. Development and evaluation of a novel biodegradable film made from chitosan and cinnamon essential oil with low affinity toward water. Food Chem. 2010, 122, 161–166. [Google Scholar] [CrossRef]
- Silva-Pereira, M.C.; Teixeira, J.A.; Pereira-Júnior, V.A.; Stefani, R. Chitosan/corn starch blend films with extract from Brassica oleraceae (red cabbage) as a visual indicator of fish deterioration. LWT Food Sci. Technol. 2015, 61, 258–262. [Google Scholar] [CrossRef]
- Namazi, H.; Dadkhah, A. Convenient method for preparation of hydrophobically modified starch nanocrystals with using fatty acids. Carbohydr. Polym. 2010, 79, 731–737. [Google Scholar] [CrossRef]
- Nafchi, A.M.; Moradpour, M.; Saeidi, M.; Alias, A.K. Effects of nanorod-rich ZnO on rheological, sorption isotherm, and physicochemical properties of bovine gelatin films. LWT Food Sci. Technol. 2014, 58, 142–149. [Google Scholar] [CrossRef]
- Nafchi, A.M.; Nassiri, R.; Sheibani, S.; Ariffin, F.; Karim, A.A. Preparation and characterization of bionanocomposite films filled with nanorod-rich zinc oxide. Carbohydr. Polym. 2013, 96, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, Y. Combined effects of two kinds of essential oils on physical, mechanical and structural properties of chitosan films. Food Hydrocoll. 2014, 36, 287–293. [Google Scholar] [CrossRef]
- Silva, M.F.; Lopes, P.S.; Silva, C.F.; Yoshida, C.M.P. Active packaging material based on buriti oil—Mauritia flexuosa L.f. (Arecaceae) incorporated into chitosan films. J. App. Polym. Sci. 2016, 133, 43210–43218. [Google Scholar]
- Shen, Z.; Kamdem, D.P. Development and characterization of biodegradable chitosan films containing two essential oils. Int. J. Biol. Macromol. 2015, 74, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Teymourpour, S.; Nafchi, A.M.; Nahidi, F. Functional, thermal, and antimicrobial properties of soluble soybean polysaccharide biocomposites reinforced by nano TiO2. Carbohydr. Polym. 2015, 134, 726–731. [Google Scholar]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Moreover, the presence of CO droplets increased the distance travelled by water molecules diffusing through the film matrix. Food Chem. 2012, 134, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Valodkar, M.; Thakore, S. Isocyanate crosslinked reactive starch nanoparticles for thermo-responsive conducting applications. Carbohydr. Res. 2010, 345, 2354–2360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, Y.; Ding, Y.; Daskalakis, N. Mechanistic investigation into antibacterial behaviour of suspensions of ZnO nanoparticles against E. coli. J. Nanopart. Res. 2010, 12, 1625–1636. [Google Scholar] [CrossRef]





| Films | Thickness (mm) | TS (MPa) | EAB (%) | MC (%) | Contact Angle (°) |
|---|---|---|---|---|---|
| Control | 0.065 ± 0.009 a | 33.2 ± 1.65 d | 21.3 ± 5.2 d | 12.5 ± 0.12 d | 90.5 ± 1.37 a |
| 1% TiO2 | 0.068 ± 0.007 ab | 37.8 ± 1.59 e | 20.2 ± 3.5 cd | 11.4 ± 0.28 cd | 103.2 ± 1.25 b |
| 3% TiO2 | 0.067 ± 0.007 ab | 39.2 ± 1.21 f | 18.9 ± 4.3 c | 10.9 ± 0.20 c | 106.7 ± 0.58 c |
| 5% TiO2 | 0.066 ± 0.008 a | 39.4 ± 0.88 f | 16.2 ± 3.8 b | 10.0 ± 0.31 bc | 108.8 ± 1.08 d |
| 7% TiO2 | 0.067 ± 0.008 ab | 37.7 ± 2.11 e | 13.1 ± 4.5 a | 9.20 ± 0.09 b | 109.8 ± 1.25 de |
| 3% TiO2–3% CO | 0.068 ± 0.009 ab | 34.8 ± 2.21 de | 24.5 ± 3.8 e | 10.2 ± 0.11 bc | 108.2 ± 1.15 d |
| 3% TiO2–6% CO | 0.070 ± 0.006 b | 31.3 ± 2.32 c | 28.9 ± 1.9 f | 9.62 ± 0.14 b | 111.4 ± 0.51 e |
| 3% TiO2–9% CO | 0.071 ± 0.004 b | 29.1 ± 1.82 b | 30.8 ± 3.2 g | 8.91 ± 0.18 ab | 113.2 ± 0.92 f |
| 3% TiO2–12% CO | 0.072 ± 0.004 b | 26.3 ± 1.14 a | 26.2 ± 2.8 ef | 8.41 ± 0.16 a | 114.0 ± 0.86 f |
| Films | WVP (10−10 g/Pa·m·s) | Inhibitory Zone (mm2) | |
|---|---|---|---|
| S. aureus | E. coli | ||
| Control | 1.406 ± 0.008 a | - | - |
| 1% TiO2 | 1.300 ± 0.019 b | 30 ± 1.04 a | 23 ± 1.02 a |
| 3% TiO2 | 1.285 ± 0.027 bc | 43 ± 2.77 b | 29 ± 2.11 b |
| 5% TiO2 | 1.204 ± 0.014 c | 49 ± 3.14 c | 34 ± 2.15 c |
| 7% TiO2 | 1.144 ± 0.028 cd | 54 ± 0.89 d | 39 ± 1.73 d |
| 3% TiO2–3% CO | 1.202 ± 0.121 c | 45 ± 1.89 bc | 32 ± 3.88 bc |
| 3% TiO2–6% CO | 1.154 ± 0.098 cd | 48 ± 2.07 c | 34 ± 1.17 c |
| 3% TiO2–9% CO | 1.123 ± 0.201 cd | 50 ± 3.55 cd | 37 ± 2.04 cd |
| 3% TiO2–12% CO | 1.058 ± 0.109 d | 53 ± 3.73 d | 40 ± 3.21 d |
| Films | L* | a* | b* | WI | Opacity |
|---|---|---|---|---|---|
| Control | 66.43 ± 0.09 a | 4.69 ± 0.23 a | 8.67 ± 0.51 a | 65.01 ± 0.15 a | 0.74 ± 0.12 a |
| 1% TiO2 | 78.29 ± 0.16 a | 5.30 ± 0.09 a | 13.86 ± 0.79 b | 73.70 ± 0.12 a | 9.02 ± 0.18 b |
| 3% TiO2 | 81.33 ± 0.24 a | 5.96 ± 0.24 a | 13.56 ± 0.84 bc | 76.17 ± 0.29 ab | 19.4 ± 0.11 c |
| 5% TiO2 | 82.36 ± 0.09 a | 6.14 ± 0.10 a | 12.65 ± 0.42 bc | 77.44 ± 0.31 b | 29.3 ± 0.24 d |
| 7% TiO2 | 83.11 ± 0.09 a | 6.21 ± 0.53 a | 12.44 ± 0.72 bc | 78.12 ± 0.15 b | 34.6 ± 0.37 e |
| 3% TiO2–3% CO | 78.42 ±0.07 a | 6.98 ± 0.12 a | 16.47 ± 0.11 c | 72.51 ± 0.14 b | 22.4 ± 0.31 c |
| 3% TiO2–6% CO | 77.77 ± 0.47 a | 7.95 ± 0.18 a | 17.72 ± 0.84 bc | 70.48 ± 0.39 ab | 24.4 ± 0.14 cd |
| 3% TiO2–9% CO | 77.44 ± 0.17 a | 8.68 ± 0.11 a | 19.50 ± 0.14 bc | 68.94 ± 0.42 ab | 25.8 ± 0.22 cd |
| 3% TiO2–12% CO | 77.24 ± 0.09 a | 8.87 ± 0.36 a | 19.52 ± 0.33 b | 68.73 ± 0.45 a | 26.9 ± 0.34 cd |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Zheng, K.; Chen, H.; Feng, S.; Wang, W.; Qin, C. Influence of Nano Titanium Dioxide and Clove Oil on Chitosan–Starch Film Characteristics. Polymers 2019, 11, 1418. https://doi.org/10.3390/polym11091418
Li W, Zheng K, Chen H, Feng S, Wang W, Qin C. Influence of Nano Titanium Dioxide and Clove Oil on Chitosan–Starch Film Characteristics. Polymers. 2019; 11(9):1418. https://doi.org/10.3390/polym11091418
Chicago/Turabian StyleLi, Wei, Kewang Zheng, Hujian Chen, Shirong Feng, Wei Wang, and Caiqin Qin. 2019. "Influence of Nano Titanium Dioxide and Clove Oil on Chitosan–Starch Film Characteristics" Polymers 11, no. 9: 1418. https://doi.org/10.3390/polym11091418
APA StyleLi, W., Zheng, K., Chen, H., Feng, S., Wang, W., & Qin, C. (2019). Influence of Nano Titanium Dioxide and Clove Oil on Chitosan–Starch Film Characteristics. Polymers, 11(9), 1418. https://doi.org/10.3390/polym11091418






