Biocompatibility of Polymer and Ceramic CAD/CAM Materials with Human Gingival Fibroblasts (HGFs)
Abstract
1. Introduction
2. Materials and Methods
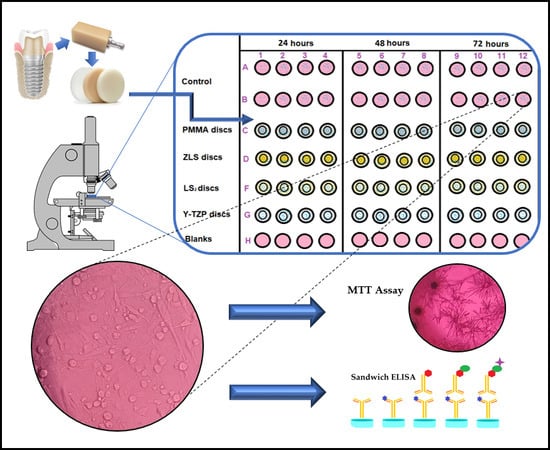
2.1. Sample Preparation
2.2. Cell Culture
2.3. Cytotoxicity Assay
2.4. Type I Collagen Secretion (ELISA)
2.5. Statistical Analysis
3. Results
3.1. Cytotoxicity of CAD/CAM Materials
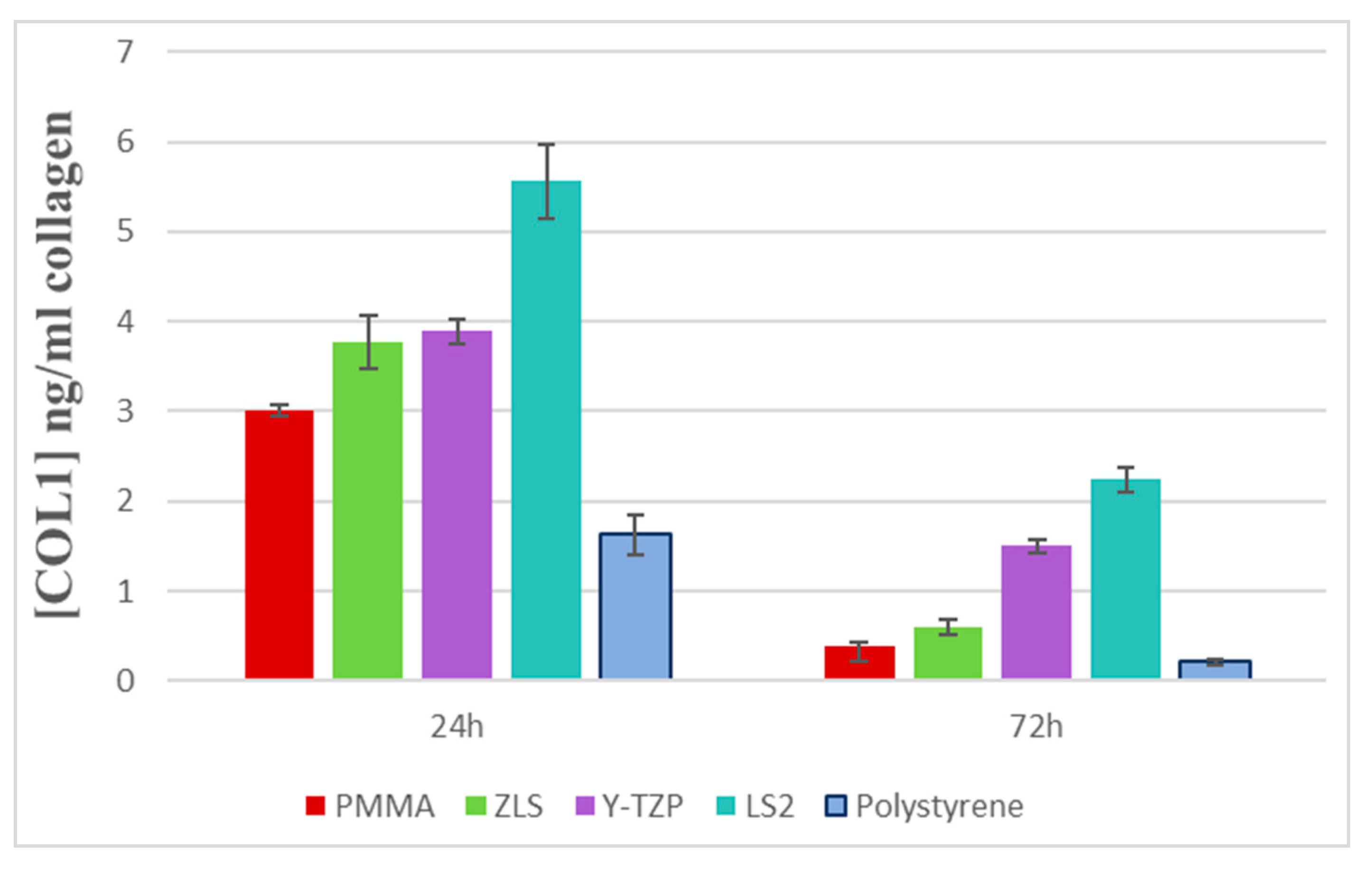
3.2. Evaluation of Collagen Type I Secretion (ELISA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grenade, C.; De Pauw-Gillet, M.-C.; Pirard, C.; Bertrand, V.; Charlier, C.; Vanheusden, A.; Mainjot, A. Biocompatibility of polymer-infiltrated-ceramic-network (PICN) materials with Human Gingival Keratinocytes (HGKs). Dent. Mater. 2017, 33, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Pabst, A.M.; Walter, C.; Grassmann, L.; Weyhrauch, M.; Brüllmann, D.D.; Ziebart, T.; Scheller, H.; Lehmann, K.M. Influence of CAD/CAM all-ceramic materials on cell viability, migration ability and adenylate kinase release of human gingival fibroblasts and oral keratinocytes. Clin. Oral Investig. 2014, 18, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Herráez-Galindo, C.; Rizo-Gorrita, M.; Luna-Oliva, I.; Serrera-Figallo, M.Á.; Castillo-Oyagüe, R.; Torres-Lagares, D. In vitro Comparative Study of Fibroblastic Behaviour o Polymethacrylate (PMMA) and Lithium Disilicate Polymer Surfaces. Polymers 2019, 11, 744. [Google Scholar] [CrossRef]
- Rizo-Gorrita, M.; Luna-Oliva, I.; Serrera-Figallo, M.Á.; Gutiérrez-Pérez, J.L.; Torres-Lagares, D. Comparison of Cytomorphometry and Early Cell Response of Human Gingival Fibroblast (HGFs) between Zirconium and New Zirconia-Reinforced Lithium Silicate Ceramics (ZLS). Int. J. Mol. Sci. 2018, 19, 2718. [Google Scholar] [CrossRef]
- Mehl, C.; Kern, M.; Schütte, A.M.; Kadem, L.F.; Selhuber-Unkel, C. Adhesion of living cells to abutment materials, dentin, and adhesive luting cement with different surface qualities. Dent. Mater. 2016, 32, 1524–1535. [Google Scholar] [CrossRef] [PubMed]
- Grenade, C.; de Pauw-Gillet, M.C.; Gailly, P.; Vanheusden, A.; Mainjot, A. Biocompatibility of polymerinfiltrated- ceramic-network (PICN) materials with Human Gingival Fibroblasts (HGFs). Dent. Mater. 2016, 32, 1152–1164. [Google Scholar] [CrossRef]
- Nothdurft, F.P.; Fontana, D.; Ruppenthal, S.; May, A.; Aktas, C.; Mehraein, Y.; Lipp, P.; Kaestner, L. Differential Behavior of Fibroblasts and Epithelial Cells on Structured Implant Abutment Materials: A Comparison of Materials and Surface Topographies. Clin. Implant Dent. Relat. Res. 2015, 17, 1237–1249. [Google Scholar] [CrossRef]
- D’Addona, A.; Ghassemian, M.; Raffaelli, L.; Manicone, P.F. Soft and hard tissue management in implant therapy-part I: Surgical concepts. Int. J. Biomater. 2012, 2012, 531202. [Google Scholar] [CrossRef][Green Version]
- Fischer, N.G.; Wong, J.; Baruth, A.; Cerutis, D.R. Effect of Clinically Relevant CAD/CAM Zirconia Polishing on Gingival Fibroblast Proliferation and Focal Adhesions. Materials 2017, 10, 1358. [Google Scholar] [CrossRef]
- Miyazaki, T.; Nakamura, T.; Matsumura, H.; Ban, S.; Kobayashi, T. Current status of zirconia restoration. J. Prosthodont. Res. 2013, 57, 236–261. [Google Scholar] [CrossRef]
- Elshahawy, W.; Shohieb, F.; Yehia, H.; Etman, W.; Watanabe, I.; Kramer, P. Cytotoxic effect of elements released clinically from gold and CAD-CAM fabricated ceramic crowns. Tanta Dent. J. 2014, 11, 189–193. [Google Scholar]
- Srinivasan, M.; Gjengedal, H.; Cattani-Lorente, M.; Moussa, M.; Durual, S.; Schimmel, M.; Müller, F. CAD/CAM milled complete removable dental prostheses: An in vitro evaluation of biocompatibility, mechanical properties, and surface roughness. Dent. Mater. J. 2018. [Google Scholar] [CrossRef]
- Tetè, S.; Zizzari, V.L.; Borelli, B.; De Colli, M.; Zara, S.; Sorrentino, R.; Scarano, A.; Gherlone, E.; Cataldi, A.; Zarone, F. Proliferation and adhesion capability of human gingival fibroblasts onto zirconia, lithium disilicate and feldspathic veneering ceramic in vitro. Dent. Mater. J. 2014, 33, 7–15. [Google Scholar] [PubMed]
- Awada, A.; Nathanson, D. Mechanical properties of resin-ceramic CAD/CAM restorative materials. J. Prosthet. Dent. 2015, 114, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Zarone, F.; Ferrari, M.; Mangano, F.G.; Leone, R.; Sorrentino, R. “Digitally Oriented Materials”: Focus on Lithium Disilicate Ceramics. Int. J. Dent. 2016, 2016, 9840594. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S.D. Key Parameters of Hybrid Materials for CAD/CAM-Based Restorative Dentistry. Compend. Contin. Educ. Dent. 2016, 37, 638–643. [Google Scholar] [PubMed]
- Atay, A.; Gürdal, I.; Çetıntas, V.B.; Üşümez, A.; Cal, E. Effects of New Generation All-Ceramic and Provisional Materials on Fibroblast Cells. J. Prosthodont. 2018, 28, e383–e394. [Google Scholar] [PubMed]
- Chevalier, J. Critical effect of cubic phase on aging in 3mol% yttria-stabilized zirconia ceramics for hip replacement prosthesis. Biomaterials 2004, 25, 5539–5545. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, J.; Gremillard, L.; Virkar, A.; Clarke, D.R. The Tetragonal-Monoclinic Transformation in Zirconia: Lessons Learned and Future Trends. J. Am. Ceram. Soc. 2009, 92, 1901–1920. [Google Scholar]
- Kelly, J.R.; Denry, I. Stabilized zirconia as a structural ceramic: An overview. Dent. Mater. 2008, 24, 289–298. [Google Scholar] [CrossRef]
- Gupta, T.K.; Bechtold, J.H.; Kuznicki, R.C.; Cadoff, L.H.; Rossing, B.R. Stabilization of tetragonal phase in polycrystalline zirconia. J. Mater. Sci. 1977, 12, 2421. [Google Scholar]
- El-Ghany, O.S.A.; Sherief, A.H. Zirconia based ceramics, some clinical and biological aspects: Review. Futur. Dent. J. 2016, 2, 55–64. [Google Scholar]
- Willard, A.; Chu, T.M.G. The science and application of IPS e. Max dental ceramic. Kaohsiung J. Med. Sci. 2018, 34, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Brackett, M.G.; Lockwood, P.E.; Messer, R.L.; Lewis, J.B.; Bouillaguet, S.; Wataha, J.C. In vitro cytotoxic response to lithium disilicate dental ceramics. Dent. Mater. 2008, 24, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Li, R.W.K.; Chow, T.W.; Matinlinna, J.P. Ceramic dental biomaterials and CAD/CAM technology: State of the art. J. Prosthodont. Res. 2014, 58, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Simba, B.G.; Ribeiro, M.V.; Suzuki, P.A.; Alves, M.F.R.P.; Strecker, K.; Santos, C.D. Mechanical properties of lithium metasilicate after short-term thermal treatments. J. Mech. Behav. Biomed. Mater. 2019, 98, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.L.; Borrero-López, O.; Guiberteau, F.; Zhang, Y. Microstructural development during heat treatment of a commercially available dental-grade lithium disilicate glass-ceramic. Dent. Mater. 2019, 35, 697–708. [Google Scholar] [CrossRef]
- Kilic, K.; Kesim, B.; Sumer, Z.; Polat, Z.; Kesim, S. In vitro cytotoxicity of all-ceramic substructural materials after aging. J. Dent. Sci. 2013, 8, 231–238. [Google Scholar]
- Barone, S.; Freulon, A.; Malard, B.; Dehmas, M. Solid-state phase transformation in a lithium disilicate-based glass-ceramic. J. Non-Crystalline Solids 2019, 513, 9–14. [Google Scholar]
- IPS e.max ® CAD Scientific Documentation; Ivoclar Vivadent AG: Liechtenstein, France, 2017.
- Silva, L.H.D.; Lima, E.; Miranda, R.B.P.; Favero, S.S.; Lohbauer, U.; Cesar, P.F. Dental ceramics: A review of new materials and processing methods. Braz. Oral Res. 2017, 31, 58. [Google Scholar] [CrossRef]
- Rinke, S.; Pabel, A.-K.; Rödiger, M.; Ziebolz, D. Chairside Fabrication of an All-Ceramic Partial Crown Using a Zirconia-Reinforced Lithium Silicate Ceramic. Case Rep. Dent. 2016, 2016, 1354186. [Google Scholar] [CrossRef]
- Sieper, K.; Wille, S.; Kern, M. Fracture strength of lithium disilicate crowns compared to polymer-infiltrated ceramic-network and zirconia reinforced lithium silicate crowns. J. Mech. Behav. Biomed. Mater. 2017, 74, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Lambert, H.; Durand, J.-C.; Jacquot, B.; Fages, M. Dental biomaterials for chairside CAD/CAM: State of the art. J. Adv. Prosthodont. 2017, 9, 486–495. [Google Scholar] [CrossRef]
- Elsaka, S.E.; Elnaghy, A.M. Mechanical properties of zirconia reinforced lithium silicate glass-ceramic. Dent. Mater. 2016, 32, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Sen, N.; Us, Y.O. Mechanical and optical properties of monolithic CAD-CAM restorative materials. J. Prosthet. Dent. 2018, 119, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Celtra® Duo Zirconia-Reinforced Lithium Silicate (ZLS) Block; Technical Monograph; Dentsply Sirona: Hanau-Wolfgang, Germany, 2016.
- Abdullah, A.O.; Pollington, S.; Liu, Y. Comparison between direct chairside and digitally fabricated temporary crowns. Dent. Mater. J. 2018, 37, 957–963. [Google Scholar] [CrossRef]
- Yao, J.; Li, J.; Wang, Y.; Huang, H. Comparison of the flexural strength and marginal accuracy of traditional and CAD/CAM interim materials before and after thermal cycling. J. Prosthet. Dent. 2014, 112, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Akin, H.; Tugut, F.; Polat, Z.A. In vitro comparison of the cytotoxicity and water sorption of two different denture base systems. J. Prosthodont. 2015, 24, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Salido, M.; Vilches, J.I.; Gutiérrez, J.L. Actin cytoskeletal organization in human osteoblasts grown on different dental titanium implant surfaces. Histol. Histopathol. 2007, 22, 1355–1364. [Google Scholar] [CrossRef]
- Alp, G.; Murat, S.; Yilmaz, B. Comparison of Flexural Strength of Different CAD/CAM PMMA-Based Polymers. J. Prosthodont. 2019, 28, e491–e495. [Google Scholar] [CrossRef]
- Huettig, F.; Prutscher, A.; Goldammer, C.; Kreutzer, C.A.; Weber, H. First clinical experiences with CAD/CAM-fabricated PMMA-based fixed dental prostheses as long-term temporaries. Clin. Oral Investig. 2016, 20, 161–168. [Google Scholar] [CrossRef]
- Lo Giudice, G.; Cicciù, M.; Cervino, G.; Lizio, A.; Visco, A.M. Flowable resin andmarginal gap on tooth third medial cavity involving enamel and radicularcementum: A SEM evaluation of two restoration techniques. Indian J. Dent. Res. 2012, 23, 763–769. [Google Scholar] [CrossRef]
- Shim, J.S.; Kim, H.C.; Park, S.I.; Yun, H.J.; Ryu, J.J. Comparison of Various Implant Provisional Resin Materials for Cytotoxicity and Attachment to Human Gingival Fibroblasts. Int. J. Oral Maxillofac. Implants 2019, 34, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Joda, T.; Ferrari, M.; Braegger, U. A digital approach for one-step formation of the supra-implant emergence profile with an individualized CAD/CAM healing abutment. J. Prosthodont. Res. 2016, 60, 220–223. [Google Scholar] [CrossRef]
- Özçelik, T.B.; Yilmaz, B.; ¸Seker, E.; Shah, K. Marginal Adaptation of Provisional CAD/CAM Restorations Fabricated Using Various Simulated Digital Cement Space Settings. Int. J. Oral Maxillofac. Implants 2018, 33, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Dal Piva, A.; Contreras, L.; Ribeiro, F.C.; Anami, L.C.; Camargo, S.; Jorge, A.; Bottino, M.A. Monolithic Ceramics: Effect of Finishing Techniques on Surface Properties, Bacterial Adhesion and Cell Viability. Oper. Dent. 2018, 43, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Rutkunas, V.; Bukelskiene, V.; Sabaliauskas, V.; Balciunas, E.; Malinauskas, M.; Baltriukiene, D. Assessment of human gingival fibroblast interaction with dental implant abutment materials. J. Mater. Sci. Mater. Med. 2015, 26, 169. [Google Scholar] [CrossRef] [PubMed]
- Pae, A.; Lee, H.; Kim, H.-S.; Kwon, Y.-D.; Woo, Y.-H. Attachment and growth behaviour of human gingival fibroblasts on titanium and zirconia ceramic surfaces. Biomed. Mater. 2009, 4, 25005. [Google Scholar] [CrossRef]
- Raffaelli, L.; Iommetti, P.R.; Piccioni, E.; Toesca, A.; Serini, S.; Resci, F.; Missori, M.; De Spirito, M.; Manicone, P.F.; Calviello, G. Growth, viability, adhesion potential, and fibronectin expression in fibroblasts cultured on zirconia or feldspatic ceramics in vitro. J. Biomed. Mater. Res. Part A 2008, 86, 959–968. [Google Scholar] [CrossRef] [PubMed]
- International Standards Organization (ISO). Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; 10993–5:2009; International Standards Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Elshahawy, W.M.; Watanabe, I.; Kramer, P. In vitro cytotoxicity evaluation of elemental ions released from different prosthodontic materials. Dent. Mater. 2009, 25, 1551–1555. [Google Scholar] [CrossRef] [PubMed]
- Roffel, S.; Wu, G.; Nedeljkovic, I.; Meyer, M.; Razafiarison, T.; Gibbs, S. Evaluation ofa novel oral mucosa in vitro implantation model for analysis of molecularinteractions with dental abutment surfaces. Clin. Implant Dent. Relat. Res. 2019, 21 (Suppl. 1), 25–33. [Google Scholar] [CrossRef] [PubMed]
- Pabst, A.M.; Walter, C.; Bell, A.; Weyhrauch, M.; Schmidtmann, I.; Scheller, H.; Lehmann, K.M. Influence of CAD/CAM zirconia for implant-abutment manufacturing on gingival fibroblasts and oral keratinocytes. Clin. Oral Investig. 2016, 20, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-D.; Choi, H.-J.; Lee, H.; Lee, J.-W.; Weber, H.-P.; Pae, A. Cellular viability and genetic expression of human gingival fibroblasts to zirconia with enamel matrix derivative (Emdogain®). J. Adv. Prosthodont. 2014, 6, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Knetsh, L.W.M. Chapter 13: Evolution of Current and Future Concepts of Biocompatibility Testing. In Polymeric Biomaterials: Structure and Function, 1st ed.; Dumitriu, S., Popa, V., Eds.; CRC Press: Boca Raton, FL, USA, 2013; Volume 1, pp. 385–396. [Google Scholar]
- Sjögren, G.; Sletten, G.; Dahl, J.E. Cytotoxicity of dental alloys, metals, and ceramics assessed by Millipore filter, agar overlay, and MTT tests. J. Prosthet. Dent. 2000, 84, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Wataha, J.; Craig, R.; Hanks, C. Precision of and new methods for testing in vitro alloy cytotoxicity. Dent. Mater. 1992, 8, 65–70. [Google Scholar] [CrossRef]
- Imirzalioglu, P.; Alaaddinoglu, E.; Yilmaz, Z.; Oduncuoglu, B.; Yilmaz, B.; Rosenstiel, S. Influence of recasting different types of dental alloys on gingival fibroblast cytotoxicity. J. Prosthet. Dent. 2012, 107, 24–33. [Google Scholar] [CrossRef]
- Nakonieczny, D.S.; Ziębowicz, A.; Paszenda, Z.K.; Krawczyk, C. Trends and perspectives in modification of zirconium oxide for a dental prosthetic applications—A review. Biocybern. Biomed. Eng. 2017, 37, 229–245. [Google Scholar] [CrossRef]
- Forster, A.; Ungvári, K.; Györgyey, Á.; Kukovecz, Á.; Turzó, K.; Nagy, K. Human epithelial tissue culture study on restorative materials. J. Dent. 2014, 42, 7–14. [Google Scholar] [CrossRef]
- Yamano, S.; Ma, A.K.-Y.; Shanti, R.M.; Kim, S.-W.; Wada, K.; Sukotjo, C. The influence of different implant materials on human gingival fibroblast morphology, proliferation, and gene expression. Int. J. Oral Maxillofac. Implants 2011, 26, 1247–1255. [Google Scholar]
- Cho, Y.D.; Shin, J.C.; Yoon, H.I.; Ku, Y.; Ryoo, H.M.; Kim, D.J.; Kim, D.G.; Han, J.S. Characterization of Human Gingival Fibroblasts on Zirconia Surfaces Containing Niobium Oxide. Materials 2015, 8, 6018–6028. [Google Scholar] [CrossRef]
- Elshahawy, W. Chapter 15: Biocompatibility. In Advances in Ceramics Electric and Magnetic Ceramics, Bioceramics, Ceramics and Environment, 1st ed.; InTech Open: London, UK, 2011; pp. 359–374. [Google Scholar]
- Milleding, P.; Karlsson, S.; Nyborg, L. On the surface elemental composition of non-corroded and corroded dental ceramic materials in vitro. J. Mater. Sci. Mater. Med. 2003, 14, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Milleding, P.; Haraldsson, C.; Karlsson, S. Ion leaching from dental ceramics during static in vitro corrosion testing. J. Biomed. Mater. Res. 2002, 61, 541–550. [Google Scholar] [CrossRef]
- Sulaiman, T.A.; Abdulmajeed, A.A.; Shahramian, K.; Hupa, L.; Donovan, T.E.; Vallittu, P.; Närhi, T.O. Impact of gastric acidic challenge on surface topography and optical properties of monolithic zirconia. Dent. Mater. 2015, 31, 1445–1452. [Google Scholar] [PubMed]
- Seabra, A.B.; Durán, N. Nanotoxicology of Metal Oxide Nanoparticles. Metals 2015, 5, 934–975. [Google Scholar] [CrossRef]
- Van Landuyt, K.; Nawrot, T.; Geebelen, B.; De Munck, J.; Snauwaert, J.; Yoshihara, K.; Scheers, H.; Godderis, L.; Hoet, P.; Van Meerbeek, B. How much do resin-based dental materials release? A meta-analytical approach. Dent. Mater. 2011, 27, 723–747. [Google Scholar] [CrossRef] [PubMed]
- Ivković, N.; Božović, D.; Ristić, S.; Mirjanić, V.; Janković, O. The residual monomer in dental acrylic resin and its adverse effects. Contemp. Mater. 2013, 1, 84–91. [Google Scholar] [CrossRef]
- Brunot-Gohin, C.; Duval, J.-L.; Verbeke, S.; Belanger, K.; Pezron, I.; Kugel, G.; Laurent-Maquin, D.; Gangloff, S.; Egles, C. Biocompatibility study of lithium disilicate and zirconium oxide ceramics for esthetic dental abutments. J. Periodontal Implant. Sci. 2016, 46, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Liu, R.; Liu, X.; Feng, X.; Zhang, Y.; Lai, R. The Biocompatibility of Dental Graded Nano-Glass-Zirconia Material after Aging. Nanoscale Res. Lett. 2018, 13, 61. [Google Scholar] [CrossRef]
- Kramer, P.R.; Janikkeith, A.; Cai, Z.; Ma, S.; Watanabe, I. Integrin mediated attachment of periodontal ligament to titanium surfaces. Dent. Mater. 2009, 25, 877–883. [Google Scholar] [CrossRef]
- Iyer, P.; Walker, K.J.; Madihally, S.V. Increased matrix synthesis by fibroblasts with decreased proliferation on synthetic chitosan-gelatin porous structures. Biotechnol. Bioeng. 2012, 109, 1314–1325. [Google Scholar] [CrossRef]
- Häkkinen, L.; Larjava, H.; Fournier, B.P. Distinct phenotype and therapeutic potential of gingival fibroblasts. Cytotherapy 2014, 16, 1171–1186. [Google Scholar] [CrossRef] [PubMed]
- Somerman, M.; Archer, S.; Imm, G.; Foster, R. A Comparative Study of Human Periodontal Ligament Cells and Gingival Fibroblasts in vitro. J. Dent. Res. 1988, 67, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Palaiologou, A.A.; Yukna, R.A.; Moses, R.; Lallier, T.E. Gingival, dermal, and periodontal ligament fibroblasts express different extracellular matrix receptors. J. Periodontol. 2001, 72, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Nor, N.H.M.; Berahim, Z.; Azlina, A.; Mokhtar, K.I.; Kannan, T.P. Identification and Characterization of Intraoral and Dermal Fibroblasts Revisited. Curr. Stem Cell Res. Ther. 2017, 12, 675–681. [Google Scholar] [CrossRef]
- Kournetas, N.; Spintzyk, S.; Schweizer, E.; Sawada, T.; Said, F.; Schmid, P.; Geis-Gerstorfer, J.; Eliades, G.; Rupp, F. Comparative evaluation of topographical data of dental implant surfaces applying optical interferometry and scanning electron microscopy. Dent. Mater. 2017, 33, e317–e327. [Google Scholar] [CrossRef] [PubMed]




| Specimen | Material Type | Composition | Manufacturer | Lot No. |
|---|---|---|---|---|
| Vita CAD-Temp® monoColor (PMMA) | Polymethacrylate | C5O2H8, SiO2 and pigments | VITA Zahnfabrik, Bad Säckingen, Germany | 1M27/51750 |
| Celtra® Duo (ZLS) | Zirconia-reinforced lithium silicate | SiO2, Li2O, P2O5, Al2O3, ZrO2, CeO2, Tb2O3 | Degudent GmbH, Hanau-Wolfgang, Germany | HT-A1-C14/16002830 |
| IPS e.max® CAD (LS2) | Vitreous ceramic lithium disilicate | SiO2, Li2O, K2O, MgO, ZnO, Al2O3, P2O5 | Ivoclar Vivadent, Schaan, Liechtenstein | HT A1/C1 4 /V28352 |
| VITA YZ® (Y-TZP) | Zirconia partially stabilised with yttrium oxide | Al2O3, ZrO2, Y2O3, Fe2O3, Er2O3, Hf2O3 | VITA Zahnfabrik, Bad Säckingen, Germany | YZ Twhite/74970 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizo-Gorrita, M.; Herráez-Galindo, C.; Torres-Lagares, D.; Serrera-Figallo, M.-Á.; Gutiérre-Pérez, J.-L. Biocompatibility of Polymer and Ceramic CAD/CAM Materials with Human Gingival Fibroblasts (HGFs). Polymers 2019, 11, 1446. https://doi.org/10.3390/polym11091446
Rizo-Gorrita M, Herráez-Galindo C, Torres-Lagares D, Serrera-Figallo M-Á, Gutiérre-Pérez J-L. Biocompatibility of Polymer and Ceramic CAD/CAM Materials with Human Gingival Fibroblasts (HGFs). Polymers. 2019; 11(9):1446. https://doi.org/10.3390/polym11091446
Chicago/Turabian StyleRizo-Gorrita, María, Cristina Herráez-Galindo, Daniel Torres-Lagares, María-Ángeles Serrera-Figallo, and José-Luis Gutiérre-Pérez. 2019. "Biocompatibility of Polymer and Ceramic CAD/CAM Materials with Human Gingival Fibroblasts (HGFs)" Polymers 11, no. 9: 1446. https://doi.org/10.3390/polym11091446
APA StyleRizo-Gorrita, M., Herráez-Galindo, C., Torres-Lagares, D., Serrera-Figallo, M.-Á., & Gutiérre-Pérez, J.-L. (2019). Biocompatibility of Polymer and Ceramic CAD/CAM Materials with Human Gingival Fibroblasts (HGFs). Polymers, 11(9), 1446. https://doi.org/10.3390/polym11091446