Study on the Preparation and Conjugation Mechanism of the Phosvitin-Gallic Acid Complex with an Antioxidant and Emulsifying Capability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
2.3. Extraction of Pv
2.4. Preparation of Pv–GA Complexes
2.5. Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis
2.6. MALDI-TOF-MS
2.7. Fourier Transform Infrared (FTIR) Spectroscopy
2.8. Fluorescence Spectroscopy
2.9. Antioxidant Activity Assay
2.9.1. DPPH Radical Scavenging Ability
2.9.2. Reducing Power Assay
2.9.3. ABTS Free Radical Scavenging Ability
2.10. Measurement of Emulsifying Properties
2.11. Mechanism of Pv–GA Conjugation
2.12. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Pv–GA Complex
3.1.1. SDS-PAGE
3.1.2. MALDI-TOF-MS
3.1.3. FTIR
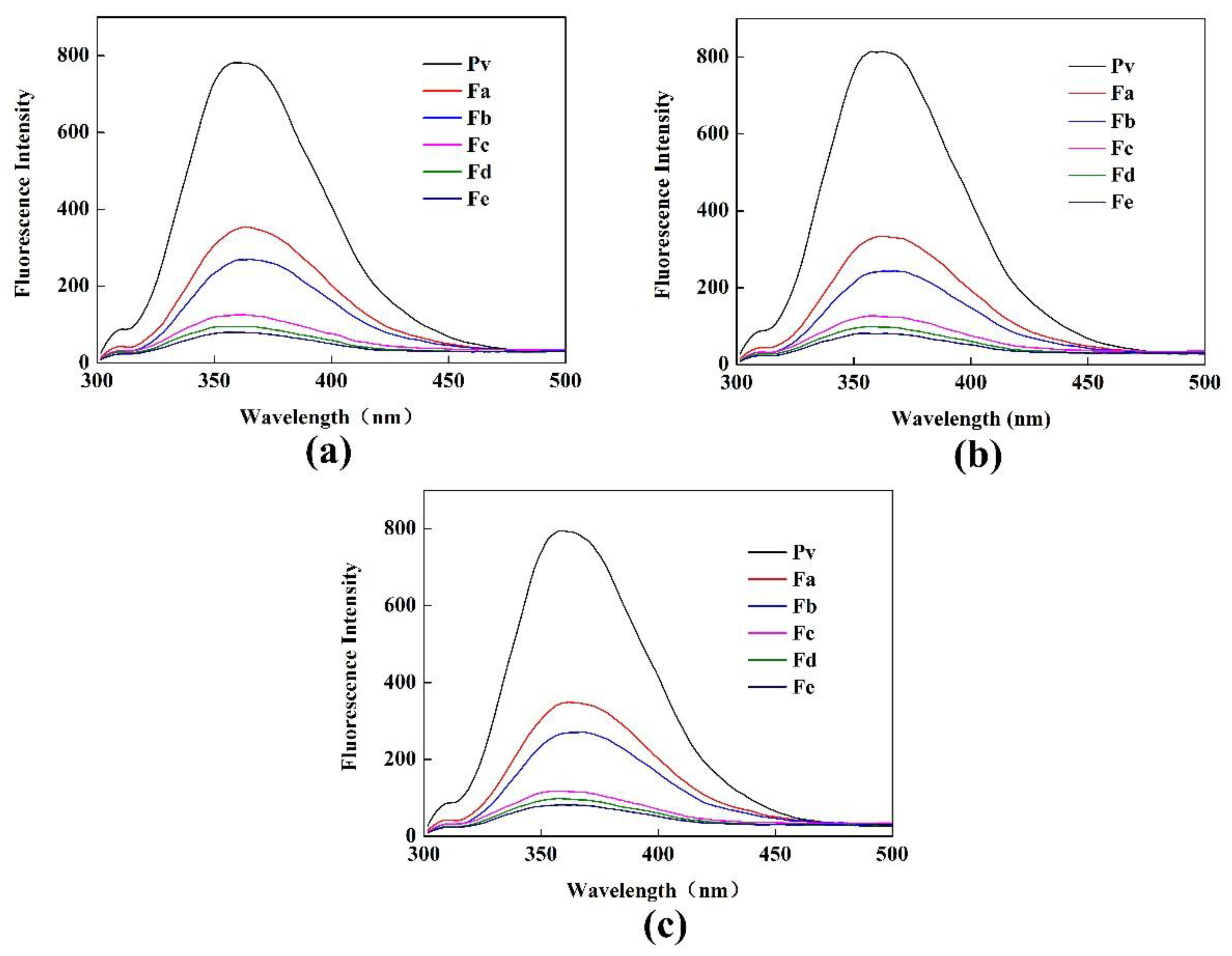
3.1.4. Fluorescence Spectroscopy Analysis
3.1.5. Analysis of Antioxidant Activities
3.1.6. Analysis of Emulsifying Properties
3.2. Mechanism of Pv–GA Conjugation
3.2.1. Fluorescence Spectra of Pv Interacting with GA
3.2.2. Analysis of the Fluorescence Quenching of Pv Induced by GA
3.2.3. Determination of the Binding Constants and Number of Binding Sites
3.2.4. Thermodynamic Parameters and the Nature of the Binding Forces
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Li, X.; Li, J.; Chang, C.; Wang, C.; Zhang, M.; Su, Y.; Yang, Y. Foaming characterization of fresh egg white proteins as a function of different proportions of egg yolk fractions. Food Hydrocolloid 2019, 90, 118–125. [Google Scholar] [CrossRef]
- Chen, H.; Jin, Y.; Ding, X.; Wu, F.; Bashari, M.; Chen, F.; Cui, Z.; Xu, X. Improved the emulsion stability of phosvitin from hen egg yolk against different ph by the covalent attachment with dextran. Food Hydrocolloid 2014, 39, 104–112. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, X.; Ma, M. Phosphorylated serine clusters of phosvitin plays a crucial role in the regulatory mineralization. Int. J. Biol. Macromol. 2018, 115, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, L.; Hu, S.; Liu, X.; Duan, X. Consequences of ball-milling treatment on the physicochemical, rheological and emulsifying properties of egg phosvitin. Food Hydrocolloid 2019, 95, 418–425. [Google Scholar] [CrossRef]
- Samaraweera, H.; Zhang, W.G.; Lee, E.J.; Ahn, D.U. Egg yolk phosvitin and functional phosphopeptides-review. J. Food Sci. 2011, 76, R143–R150. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Li, M.; Ma, H.; Xu, X.; Jin, Z.; Liu, X. Physicochemical properties and antioxidant potential of phosvitin-resveratrol complexes in emulsion system. Food Chem. 2016, 206, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Ogawa, M.; Nakai, S.; Kato, A.; Kitts, D.D. Antioxidant activity of a maillard-type phosvitin-galactomannan conjugate with emulsifying properties and heat stability. J. Agr. Food Chem. 1998, 46, 3958–3963. [Google Scholar] [CrossRef]
- Liu, F.; Sun, C.; Yang, W.; Yuan, F.; Gao, Y. Structural characterization and functional evaluation of lactoferrin-polyphenol conjugates formed by free-radical graft copolymerization. RSC Adv. 2015, 5, 15641–15651. [Google Scholar] [CrossRef]
- Yuksel, Z.; Avci, E.; Erdem, Y.K. Characterization of binding interactions between green tea flavanoids and milk proteins. Food Chem. 2010, 121, 450–456. [Google Scholar] [CrossRef]
- Dubeau, S.; Samson, G.; Tajmir-Riahi, H.-A. Dual effect of milk on the antioxidant capacity of green, darjeeling, and english breakfast teas. Food Chem. 2010, 122, 539–545. [Google Scholar] [CrossRef]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Cao, Y.; True, A.D.; Chen, J.; Xiong, Y.L. Dual role (anti- and pro-oxidant) of gallic acid in mediating myofibrillar protein gelation and gel in vitro digestion. J. Agric. Food Chem. 2016, 64, 3054–3061. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wu, F.; Duan, X.; Yang, N.; Xu, Y.; Xu, B.; Jin, Z.; Xu, X. Characterization of emulsions prepared by egg yolk phosvitin with pectin, glycerol and trehalose. Food Hydrocolloids 2013, 30, 123–129. [Google Scholar] [CrossRef]
- Liu, J.; Lu, J.F.; Kan, J.; Jin, C.H. Synthesis of chitosan-gallic acid conjugate: Structure characterization and in vitro anti-diabetic potential. Int. J. Biol. Macromol. 2013, 62, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, J.; Qian, C.; Kan, J.; Jin, C. Effect of grafting method on the physical property and antioxidant potential of chitosan film functionalized with gallic acid. Food Hydrocolloids 2019, 89, 1–10. [Google Scholar] [CrossRef]
- Gu, L.; Peng, N.; Chang, C.; McClements, D.J.; Su, Y.; Yang, Y. Fabrication of surface-active antioxidant food biopolymers: Conjugation of catechin polymers to egg white proteins. Food Biophys. 2017, 12, 198–210. [Google Scholar] [CrossRef]
- Curcio, M.; Puoci, F.; Iemma, F.; Parisi, O.I.; Cirillo, G.; Spizzirri, U.G.; Picci, N. Covalent insertion of antioxidant molecules on chitosan by a free radical grafting procedure. J. Agric. Food Chem. 2009, 57, 5933–5938. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, Q.H.; Wang, Z.; ZHAO, J. Study of the interaction of carbamazepine with bovine serum albumin by fluorescence quenching method. Anal. Sci. 2006, 22. [Google Scholar]
- Kanakis, C.D.; Hasni, I.; Bourassa, P.; Tarantilis, P.A.; Polissiou, M.G.; Tajmir-Riahi, H.A. Milk beta-lactoglobulin complexes with tea polyphenols. Food Chem. 2011, 127, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; He, Y.; Kong, Y.; Mei, X.; Huo, Y.; He, Y.; Liu, J. Elucidating the interaction mechanism of eriocitrin with β-casein by multi-spectroscopic and molecular simulation methods. Food Hydrocolloids 2019, 94, 63–70. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, L.; Wang, X.; Wu, S.; Li, D.; Liu, C.; Feng, Z. Ultrasonic thermal-assisted extraction of phosvitin from egg yolk and evaluation of its properties. Polymers 2019, 11, 1353. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Feng, Z.B.; Liu, C.H.; Xu, Y.C.; Li, D.M.; Ji, G. Extraction and purification of wheat-esterase using aqueous two-phase systems of ionic liquid and salt. J. Food Sci. Technol. 2015, 52, 2878–2885. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Yuan, Y.; Zhang, X.; Feng, Z.; Liu, C. Separation and enrichment of lectin from zihua snap-bean (phaseolus vulgaris) seeds by peg 600-ammonium sulfate aqueous two-phase system. Molecules 2017, 22, 1596. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Wang, L.; Na, J.; Zhang, X.; Yuan, Y.; Liu, C.; Feng, Z. Environmentally-friendly strategy for separation of α-lactalbumin from whey by aqueous two phase flotation. Arabian J. Chem. 2018. [CrossRef]
- Zhang, X.; Qiu, N.; Geng, F.; Ma, M. Simply and effectively preparing high-purity phosvitin using polyethylene glycol and anion-exchange chromatography. J. Sep. Sci. 2011, 34, 3295–3301. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Liu, Y.Y.; Jiang, J.Y.; Ji, Q.Y.; Fu, Y.; Zhao, L.X.; Li, C.Y.; Ye, F. Physicochemical properties and fungicidal activity of inclusion complexes of fungicide chlorothalonil with β-cyclodextrin and hydroxypropyl-β-cyclodextrin. J. Mol. Liq. 2019, 293, 111513. [Google Scholar] [CrossRef]
- Jiang, B.; Na, J.; Wang, L.; Li, D.; Liu, C.; Feng, Z. Eco-innovation in reusing food by-products: Separation of ovalbumin from salted egg white using aqueous two-phase system of PEG 1000/(NH4)2SO4. Polymers 2019, 11, 238. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Zhou, Y.; Li, M.; Wu, F.; Yang, N.; Xu, J.; Chen, H.; Jin, Z.; Xu, X. Postfertilization changes in conformation of egg yolk phosvitin and biological activities of phosphopeptides. Food Res. Int. 2014, 62, 1008–1014. [Google Scholar] [CrossRef]
- Jiang, B.; Na, J.; Wang, L.; Li, D.; Liu, C.; Feng, Z. Separation and enrichment of antioxidant peptides from whey protein isolate hydrolysate by aqueous two-phase extraction and aqueous two-phase flotation. Foods 2019, 8, 34. [Google Scholar] [CrossRef]
- Yang, H.; Deng, J.; Yuan, Y.; Fan, D.; Zhang, Y.; Zhang, R.; Han, B. Two novel exopolysaccharides from bacillus amyloliquefaciens c-1: Antioxidation and effect on oxidative stress. Curr. Microbiol. 2015, 70, 298–306. [Google Scholar] [CrossRef]
- Zampini, I.C.; Ordonez, R.M.; Isla, M.I. Autographic assay for the rapid detection of antioxidant capacity of liquid and semi-solid pharmaceutical formulations using ABTS+ immobilized by gel entrapment. AAPS PharmSciTech 2010, 11, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Xu, C.; Liu, F.; Yuan, F.; Gao, Y. Native and thermally modified protein-polyphenol coassemblies: Lactoferrin-based nanoparticles and submicrometer particles as protective vehicles for (-)-epigallocatechin-3-gallate. J. Agric. Food Chem. 2014, 62, 10816–10827. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, H.; Liu, M.; Liu, Z.; Xu, H.; Lai, F. Analysis of binding interaction between (−)-epigallocatechin (EGC) and β-lactoglobulin by multi-spectroscopic method. Spectrochim. Acta A 2011, 82, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, A.E.; Butler, L.G. Condensed tannin purification and characterization of tannin-associated proteins. J. Agric. Food Chem. 1980, 28, 947–952. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.D.; Wu, J.P. Thermal-aided phosvitin extraction from egg yolk. J. Sci. Food Agric. 2015, 95, 2595–2600. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; He, L.; Liang, Y.; Yue, L.; Peng, W.; Jin, G.; Ma, M. Preparation process optimization of pig bone collagen peptide-calcium chelate using response surface methodology and its structural characterization and stability analysis. Food Chem. 2019, 284, 80–89. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, X.; Wang, L.; Lv, X.; Li, D.; Liu, C.; Feng, Z. Two-step isolation, purification, and characterization of lectin from zihua snap bean (phaseolus vulgaris) seeds. Polymers 2019, 11, 785. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, Y.; Wang, S. Purification of algal calcium-chelating peptide and its physical chemical properties. J. Aquat. Food Prod. T. 2018, 27, 518–530. [Google Scholar] [CrossRef]
- Shang, L.; Wang, Y.; Jiang, J.; Dong, S. pH-dependent protein conformational changes in albumin: Gold nanoparticle bioconjugates: A spectroscopic study. Langmuir 2007, 23, 2714–2721. [Google Scholar] [CrossRef]
- Zhang, F.; Qu, J.; Thakur, K.; Zhang, J.G.; Mocan, A.; Wei, Z.J. Purification and identification of an antioxidative peptide from peony (paeonia suffruticosa andr.) seed dreg. Food Chem. 2019, 285, 266–274. [Google Scholar] [CrossRef]
- Jiang, B.; Na, J.; Wang, L.; Li, D.; Liu, C.; Feng, Z. Reutilization of food waste: One-step extration, purification and characterization of ovalbumin from salted egg white by aqueous two-phase flotation. Foods 2019, 8, 286. [Google Scholar] [CrossRef] [PubMed]
- Shevkani, K.; Singh, N.; Rana, J.C.; Kaur, A. Relationship between physicochemical and functional properties of amaranth (amaranthus hypochondriacus) protein isolates. Int. J. Food Sci. Technol. 2014, 49, 541–550. [Google Scholar] [CrossRef]
- Feng, Z.; Li, L.; Zhang, Y.; Li, X.; Liu, C.; Jiang, B.; Xu, J.; Sun, Z. Formation of whey protein isolate nanofibrils by endoproteinase gluc and their emulsifying properties. Food Hydrocolloids 2019, 94, 71–79. [Google Scholar] [CrossRef]
- Lopes-da-Silva, J.A.; Monteiro, S.R. Gelling and emulsifying properties of soy protein hydrolysates in the presence of a neutral polysaccharide. Food Chem. 2019, 294, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, J.; Ren, J.; Zhao, M. Effects of ultrasound pretreatment on the enzymatic hydrolysis of soy protein isolates and on the emulsifying properties of hydrolysates. J. Agric. Food Chem. 2011, 59, 2600–2609. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, D.; Ji, B.; Chen, J. Characterization of the binding of nevadensin to bovine serum albumin by optical spectroscopic technique. J. Mol. Struct. 2008, 889, 422–428. [Google Scholar] [CrossRef]
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. J. Am. Chem. Soc. 1981, 20, 3096–3102. [Google Scholar]








| Samples | α-helix | β-sheet | β-turn | Unordered |
|---|---|---|---|---|
| Pv | 17.11 ± 0.16a | 27.32 ± 0.14b | 38.91 ± 0.17f | 16.66 ± 0.17a |
| Za | 18.01 ± 0.14c | 26.32 ± 0.13a | 38.31 ± 0.19e | 17.27 ± 0.17c |
| Zb | 18.02 ± 0.12c | 27.71 ± 0.12c | 37.31 ± 0.14d | 16.96 ± 0.17b |
| Zc | 18.00 ± 0.12c | 28.23 ± 0.15d | 36.90 ± 0.15c | 16.87 ± 0.17ab |
| Zd | 17.92 ± 0.14ac | 28.71 ± 0.13e | 36.51 ± 0.18b | 16.86 ± 0.17ab |
| Ze | 17.41 ± 0.15b | 30.62 ± 0.17f | 35.20 ± 0.21a | 16.77 ± 0.17ab |
| T/K | Ksv (L/mol) | Kq (L/mol·s) | R2 |
|---|---|---|---|
| 298 | 3.403 × 106 | 3.403 × 1014 | 0.9976 |
| 304 | 1.455 × 106 | 1.455 × 1014 | 0.9953 |
| 310 | 1.045 × 106 | 1.045 × 1014 | 0.9938 |
| T/K | Ka (L/mol) | n | R2 |
|---|---|---|---|
| 298 | 3.09 × 106 | 0.9948 | 0.9978 |
| 304 | 1.53 × 106 | 0.9956 | 0.9985 |
| 310 | 1.31 × 106 | 1.037 | 0.9999 |
| T/K | ΔH (kJ/mol) | ΔG (kJ/mol) | ΔS (J/mol·K) |
|---|---|---|---|
| 298 | −54.34 | −37.02 | −58.33 |
| 304 | −35.99 | ||
| 310 | −35.60 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, B.; Wang, X.; Wang, L.; Wu, S.; Li, D.; Liu, C.; Feng, Z. Study on the Preparation and Conjugation Mechanism of the Phosvitin-Gallic Acid Complex with an Antioxidant and Emulsifying Capability. Polymers 2019, 11, 1464. https://doi.org/10.3390/polym11091464
Jiang B, Wang X, Wang L, Wu S, Li D, Liu C, Feng Z. Study on the Preparation and Conjugation Mechanism of the Phosvitin-Gallic Acid Complex with an Antioxidant and Emulsifying Capability. Polymers. 2019; 11(9):1464. https://doi.org/10.3390/polym11091464
Chicago/Turabian StyleJiang, Bin, Xiaojing Wang, Linlin Wang, Shuang Wu, Dongmei Li, Chunhong Liu, and Zhibiao Feng. 2019. "Study on the Preparation and Conjugation Mechanism of the Phosvitin-Gallic Acid Complex with an Antioxidant and Emulsifying Capability" Polymers 11, no. 9: 1464. https://doi.org/10.3390/polym11091464
APA StyleJiang, B., Wang, X., Wang, L., Wu, S., Li, D., Liu, C., & Feng, Z. (2019). Study on the Preparation and Conjugation Mechanism of the Phosvitin-Gallic Acid Complex with an Antioxidant and Emulsifying Capability. Polymers, 11(9), 1464. https://doi.org/10.3390/polym11091464









