A Quantitative Spectroscopic Study of the Bleaching Phenomena in Plasticized Formulations Containing PVC Exposed to Outdoor Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Degradation Process
2.4. Characterization of Degradation
3. Results and Discussion
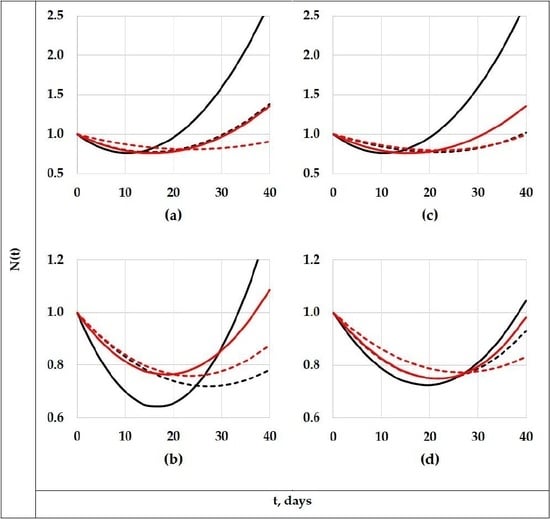
3.1. Evolution of the Concentration of the Different Polyenes (PAB)
3.2. The Degradation Period Effect
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yousif, E.; Hasan, A. Photostabilization of Poly(vinyl Chloride)—Still on the Run. J. Taibah Univ. Sci. 2015, 9, 421–448. [Google Scholar] [CrossRef]
- Global demand for PVC to rise by about 3.2%/year to 2021. In Additives for Polymers; Elsevier: Amsterdam, The Netherlands, 2014; Volume 2014, pp. 10–11. Available online: https://www.sciencedirect.com/science/article/pii/S0306374714701757 (accessed on 9 September 2019).
- Jakubowicz, I.; Yarahmadi, N.; Gevert, T. Effects of Accelerated and Natural Ageing on Plasticized Polyvinyl Chloride (PVC). Polym. Degrad. Stab. 1999, 66, 415–421. [Google Scholar] [CrossRef]
- Jakubowicz, I. Effects of Artificial and Natural Ageing on Impact-Modified Poly(vinyl Chloride) (PVC). Polym. Test. 2001, 20, 545–551. [Google Scholar] [CrossRef]
- Summers, J.W.; Rabinovitch, E.B. The Chemical Mechanisms of Outdoor Weathering in Polyvinyl Chloride. J. Vinyl Technol. 1983, 5, 91–95. [Google Scholar] [CrossRef]
- Gumargalieva, K.Z.; Ivanov, V.B.; Zaikov, G.E.; Moiseev, J.V.; Pokholok, T.V. Problems of Ageing and Stabilization of Poly(vinyl Chloride). Polym. Degrad. Stab. 1996, 52, 73–79. [Google Scholar] [CrossRef]
- D’Aquino, C.A.; Balmant, W.; Ribeiro, R.L.L.; Munaro, M.; Vargas, J.V.C.; Amico, S.C. A Simplified Mathematical Model to Predict PVC Photodegradation in Photobioreactors. Polym. Test. 2012, 31, 638–644. [Google Scholar] [CrossRef]
- Bakar, A.A.; Hassan, A.; Fuad, A.; Yusof, M. Effect of Accelerated Weathering on the Mechanical Properties of Oil Palm Empty Fruit Bunch Filled UPVC Composites. Polym. J. 2005, 14, 627–635. [Google Scholar] [CrossRef]
- Starnes, W.H. Structural and Mechanistic Aspects of the Thermal Degradation of Poly(vinyl Chloride). Prog. Polym. Sci. 2002, 27, 2133–2170. [Google Scholar] [CrossRef]
- Braun, D. Thermal Degradation of Polyvinyl Chloride. Pure Appl. Chem. 1971, 26, 173–192. [Google Scholar] [CrossRef]
- González-Ortiz, L.J.; Arellano, M.; Jasso, C.F.; Mendizábal, E.; Sánchez-Peña, M.J. Thermal Stability of Plasticized Poly(vinyl Chloride) Compounds Stabilized with Pre-Heated Mixtures of Calcium And/or Zinc Stearates. Polym. Degrad. Stab. 2005, 90, 154–161. [Google Scholar] [CrossRef]
- González-Ortiz, L.J.; Arellano, M.; Sánchez-Peña, M.J.; Mendizábal, E.; Jasso-Gastinel, C.F. Effect of Stearate Preheating on the Thermal Stability of Plasticized PVC Compounds. Polym. Degrad. Stab. 2006, 91, 2715–2722. [Google Scholar] [CrossRef]
- Wang, M.; Xu, J.; Wu, H.; Guo, S. Effect of Pentaerythritol and Organic Tin with Calcium/zinc Stearates on the Stabilization of Poly(vinyl Chloride). Polym. Degrad. Stab. 2006, 91, 2101–2109. [Google Scholar] [CrossRef]
- Lévai, G.; Ocskay, G.; Nyitrai, Z. The Effect of Pentaerythritol on the Kinetics of the Heat Stabilization of PVC with Cadmium Stearate. Polym. Degrad. Stab. 1992, 35, 261–265. [Google Scholar] [CrossRef]
- Gao, J.G.; Liu, X.Q.; Yang, J.B.; Zhu, F.L. Influence of Polyols as a Co-Stabilizer on Stabilization Efficiency of Calcium/Zinc Stabilizers to PVC. Adv. Mater. Res. 2012, 549, 251–254. [Google Scholar] [CrossRef]
- Steenwijk, J.; Langerock, R.; van Es, D.S.; van Haveren, J.; Geus, J.W.; Jenneskens, L.W. Long-Term Heat Stabilisation by (Natural) Polyols in Heavy Metal- and Zinc-Free Poly(vinyl Chloride). Polym. Degrad. Stab. 2006, 91, 52–59. [Google Scholar] [CrossRef]
- Benavides, R.; Edge, M.; Allen, N.S.; Téllez, M.M. Stabilization of Poly(vinyl Chloride) with Preheated Metal Stearates and Costabilizers. II. Use of a Polyol. J. Appl. Polym. Sci. 1998, 68, 11–27. [Google Scholar] [CrossRef]
- Li, D.; Zhou, M.; Xie, L.; Yu, X.; Yu, Y.; Ai, H.; Tang, S. Synergism of Pentaerythritol-Zinc with β-Diketone and Calcium Stearate in Poly(vinyl Chloride) Thermal Stability. Polym. J. 2012, 45, 775. [Google Scholar] [CrossRef]
- Wang, M.; Li, H.; Tang, X.; Huang, X. Effect of Calcium Stearates and Zinc Stearates on Polyene Formation of Poly(vinyl Chloride) under Degradation. J. Elastom. Plast. 2013, 45, 173–186. [Google Scholar] [CrossRef]
- Abbås, K.B.; Sörvik, E.M. Heat Stabilizers for Poly (Vinyl Chloride). I. Synergistic Systems Based on Calcium/zinc Stearate. J. Vinyl Technol. 1980, 2, 87–94. [Google Scholar] [CrossRef]
- Baltacioğlu, H.; Balköse, D. Effect of Zinc Stearate And/or Epoxidized Soybean Oil on Gelation and Thermal Stability of PVC-DOP Plastigels. J. Appl. Polym. Sci. 1999, 74, 2488–2498. [Google Scholar] [CrossRef]
- Gardette, J.L.; Gaumet, S.; Lemaire, J. Photooxidation of Poly (Vinyl Chloride). 1. A Reexamination of the Mechanism. Macromolecules 1989, 22, 2576–2581. [Google Scholar] [CrossRef]
- Decker, C. Degradation of Poly(vinyl Chloride) by U.v. radiation—II: Mechanism. Eur. Polym. J. 1984, 20, 149–155. [Google Scholar] [CrossRef]
- Gardette, J.-L.; Lemaire, J. Prediction of the Long-Term Outdoor Weathering of Poly(vinyl Chloride). J. Vinyl Technol. 1993, 15, 113–117. [Google Scholar] [CrossRef]
- Decker, C.; Balandier, M. Photo-Oxidation of Poly(vinyl Chloride). Polym. Photochem. 1981, 1, 221–232. [Google Scholar] [CrossRef]
- Geddes, W.C. Mechanism of PVC Degradation. Rubber Chem. Technol. 1967, 40, 177–216. [Google Scholar] [CrossRef]
- Feldman, D. Polymer Weathering: Photo-Oxidation. J. Polym. Environ. 2002, 10, 163–173. [Google Scholar] [CrossRef]
- Rabek, J.F.; Rånby, B.; Östensson, B.; Flodin, P. Oxidation of Polyene Structures in Poly(vinyl Chloride) by Molecular Oxygen and Singlet Oxygen. J. Appl. Polym. Sci. 1979, 24, 2407–2413. [Google Scholar] [CrossRef]
- Gardette, J.-L.; Lemaire, J. Reversible Discoloration Effects in the Photoaging of Poly(vinyl Chloride). J. Vinyl Addit. Technol. 2004, 3, 107–111. [Google Scholar] [CrossRef]
- Veronelli, M.; Mauro, M.; Bresadola, S. Influence of Thermal Dehydrochlorination on the Photooxidation Kinetics of PVC Samples. Polym. Degrad. Stab. 1999, 66, 349–357. [Google Scholar] [CrossRef]
- Lemaire, J.; Siampiringue, N.; Chaigneau, R.; Delprat, P.; Parmeland, G.; Dabin, P.; Spriet, C. Towards the Prediction of Pinking of PVC Profiles in Mild Climatic Conditions. J. Vinyl Addit. Technol. 2000, 6, 69–79. [Google Scholar] [CrossRef]
- Bacaloglu, R.; Fisch, M. Degradation and Stabilization of Poly(vinyl Chloride). I. Kinetics of the Thermal Degradation of Poly(vinyl Chloride). Polym. Degrad. Stab. 1994, 45, 301–313. [Google Scholar] [CrossRef]
- Benavides, R.; Arias, G.; Castillo, E.L.; Téllez, M.M. Crosslinking of PVC Formulations Treated with UV Light. II. Color Formation and Viscoelastic Properties. J. Vinyl Addit. Technol. 2007, 13, 189–194. [Google Scholar] [CrossRef]
- Pimentel Real, L.E.; Ferraria, A.M.; Botelho do Rego, A.M. Comparison of Different Photo-Oxidation Conditions of Poly(vinyl Chloride) for Outdoor Applications. Polym. Test. 2008, 27, 743–751. [Google Scholar] [CrossRef]
- Real, L.P.; Gardette, J.-L. Ageing and Characterisation of PVC-Based Compounds Utilised for Exterior Applications in the Building Construction Field: 1: Thermal Ageing. Polym. Test. 2001, 20, 779–787. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/uv/faq/whatisuv/en/index3.html (accessed on 20 May 2019).






| Reactions | PVC | PVCOOH | PVC• | PVC•(frac) | PVCOO• | P1(frac) | P1 | Pa | Pb | Pc | Pn | Pm | Ps | Pt | Pcn-2 | Pn+1(frac) | Pn+1 | PnOOH | P1• | Pn• | Pn+1• | PnOO• | O2 | HCl | Cl• | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PF | a) | R | P | P | ||||||||||||||||||||||
| b)* | R | P | P | |||||||||||||||||||||||
| PI | c) | P | R | P | R | |||||||||||||||||||||
| d) | P | R | P | |||||||||||||||||||||||
| e) | P | P | R | |||||||||||||||||||||||
| PD | f)+ | P | P | P | R | R | ||||||||||||||||||||
| g)* | R | P | P | |||||||||||||||||||||||
| h) | R | P | P | R | ||||||||||||||||||||||
| OR | i) | R | P | P | ||||||||||||||||||||||
| j) | R | P | P | |||||||||||||||||||||||
| k) | R | P | P | R | ||||||||||||||||||||||
| l) | R | P | P | R | ||||||||||||||||||||||
| m) | R | P | P | R | ||||||||||||||||||||||
| n) | R | P | R | |||||||||||||||||||||||
| o) | R | P | P | R | ||||||||||||||||||||||
| p) | R | P | R | |||||||||||||||||||||||
| q) | R | P | P | R | ||||||||||||||||||||||
| r) | R | P | P | R | ||||||||||||||||||||||
PVC 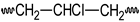 | PVCOOH 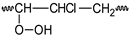 | PVC• 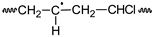 | PVCOO• 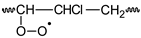 | |||||||||||||||||||||||
Pn 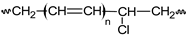 n ∈ {a,b,c,n,m,s,t,1} | 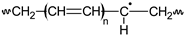 Pn• n ∈ {a,b,c,n,m,s,t,1} | PnOOH 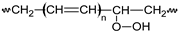 |
PnOO• 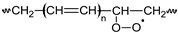 | |||||||||||||||||||||||
| Reactions | PVC• | PVC•(frac) | P1(frac) | Pa,b,cbran | Pn | Pm | Ps,tbran | Pn+1(frac) | Pn• | |
|---|---|---|---|---|---|---|---|---|---|---|
| PF | (a) | R | P | P | ||||||
| PI | (b) | P | P | R | ||||||
| PD | (c) + | P | R | R | ||||||
| (d) * | R | P | ||||||||
PVC• 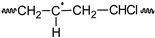 | Pn  n ∈ {n,m,1} | Pn• 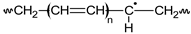 n ∈ {n,m,1} | ||||||||
Ps,tbran  | Pa,b,cbran  | |||||||||
| Formulations | Degradation Periods | ||
|---|---|---|---|
| Formulation Code | Relative Content of Components, g/100 g of PVC (PVC/DEHP/ESO/CaSt2/ZnSt2) | Period Code | Initial Date–Final Date |
| F1 | 100/45/3/1.0/0.0 | P1 | 16 April–25 May |
| F2 | 100/45/3/0.8/0.2 | P2 | 27 May–5 July |
| F3 | 100/45/3/0.6/0.4 | P3 | 7 July–15 August |
| F4 | 100/45/3/0.4/0.6 | P4 | 17 August–25 September |
| F5 | 100/45/3/0.2/0.8 | P5 | 27 September–5 November |
| F6 | 100/45/3/0.0/1.0 | P6 | 7 November–16 December |
| P7 | 18 December–26 January | ||
| P8 | 28 January–8 March | ||
| P9 | 10 March–18 April | ||
| submerged | 7 November–16 December | ||
| not submerged | 7 November–16 December | ||
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | |
|---|---|---|---|---|---|---|---|---|---|
| Days with rain | 0 | 18 | 23 | 27 | 13 | 2 | 0 | 0 | 7 |
| L/m2 collected in period | 0 | 151 | 244 | 324 | 126 | 12 | 0 | 0 | 117 |
| F1-P1 | F1-P2 | F1-P3 | F1-P4 | F1-P7 | F1-P8 | F1-Psubmerged | F1-Pnot submerged | |
| m | 567.79 | 487.46 | 261.41 | 144.15 | 156.81 | 198.40 | 103.08 | 340.00 |
| A | 551.64 | 472.66 | 250.63 | 135.74 | 148.53 | 188.11 | 95.655 | 324.63 |
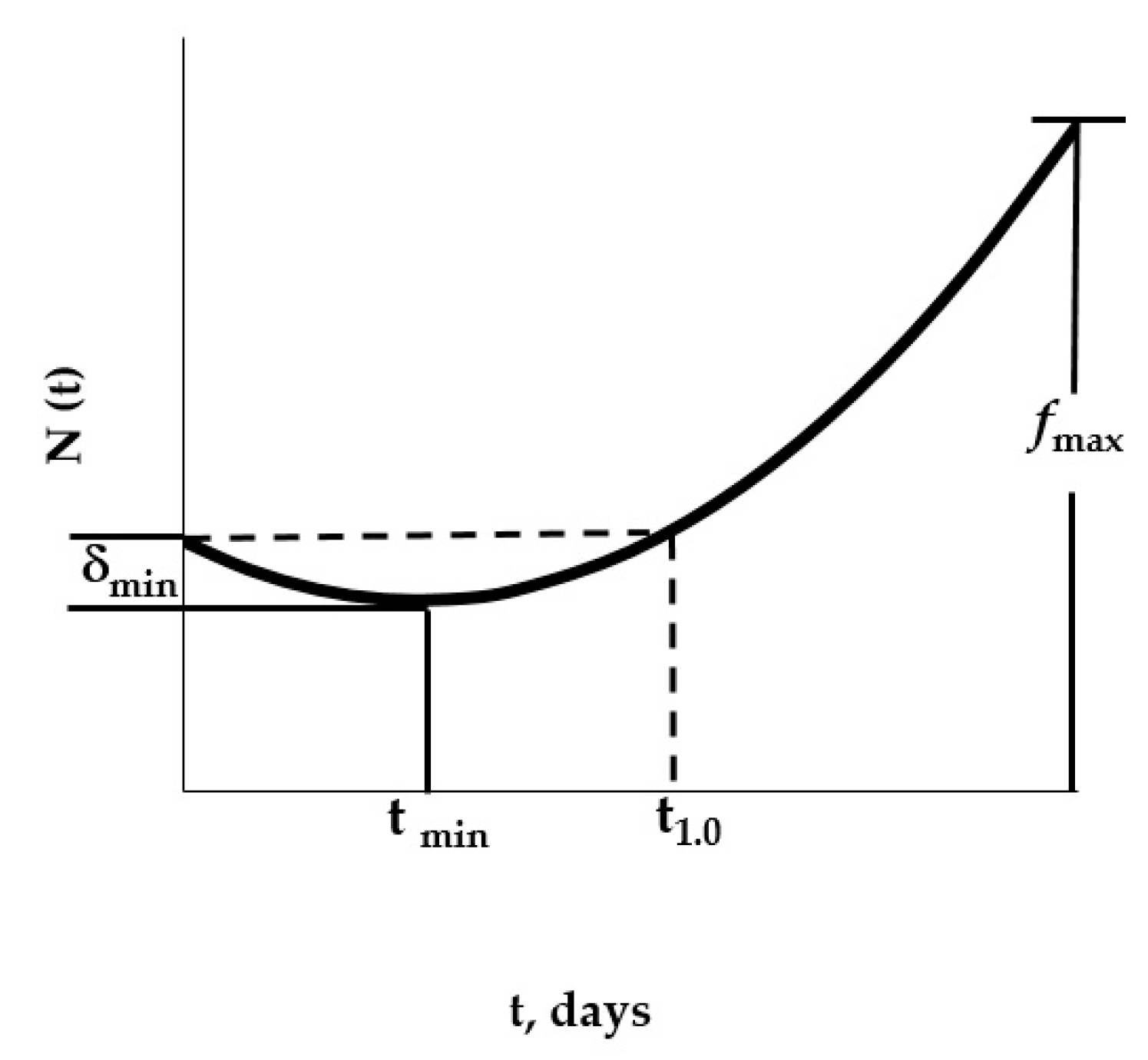
| δmin | 0.23 | 0.23 | 0.23 | 0.26 | 0.23 | 0.28 | 0.28 | 0.36 |
| tmin | 11 | 11 | 15 | 22 | 20 | 19 | 27 | 17 |
| fmax | 2.67 | 2.32 | 1.38 | 0.92 | 1.02 | 1.04 | 0.78 | 1.34 |
| t1.0 | 21 | 22 | 30 | >40 | 39 | 38 | >40 | 33 |
| R | 27.34 | 29.94 | 16.89 | 31.58 | 30.82 | 28.39 | 25.27 | 24.05 |
| S | −0.1328 | −0.1254 | −0.1074 | −0.1336 | −0.1351 | −0.1289 | −0.1215 | −0.1203 |
| U | 700.5 | 1645 | 417.0 | 687.2 | 1856 | 1709 | 939.1 | 702.2 |
| V | −0.7002 | −0.8151 | −0.5441 | −0.7095 | −0.8593 | −0.8349 | −0.7305 | −0.6727 |
| F2-P1 | F2-P2 | F2-P3 | F2-P4 | F2-P7 | F2-P8 | F2-Psubmerged | F2-Pnot submerged | |
| m | 263.41 | 90.681 | 95.031 | 87.472 | 133.49 | 160.32 | 81.943 | 184.40 |
| A | 252.31 | 83.886 | 89.109 | 81.303 | 126.24 | 151.53 | 76.465 | 175.22 |
| δmin | 0.24 | 0.27 | 0.19 | 0.23 | 0.20 | 0.25 | 0.19 | 0.24 |
| tmin | 16 | 28 | 23 | 27 | 20 | 21 | 25 | 19 |
| fmax | 1.36 | 0.78 | 0.91 | 0.83 | 0.99 | 0.98 | 0.88 | 1.09 |
| t1.0 | 31 | >40 | >40 | >40 | >40 | >40 | >40 | 37 |
| R | 21.35 | 15.68 | 16.67 | 15.39 | 21.99 | 20.83 | 17.37 | 20.83 |
| S | −0.1204 | −0.1034 | −0.1070 | −0.1026 | −0.1192 | −0.1184 | −0.1072 | −0.1184 |
| U | 484.0 | 262.4 | 274.2 | 236.1 | 1087 | 525.9 | 277.6 | 525.4 |
| V | −0.6189 | −0.5005 | −0.5114 | −0.4865 | −0.7448 | −0.6245 | −0.5143 | −0.6284 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Falcón, E.; Arellano, M.; Sanchez-Peña, M.J.; González-Ortiz, L.J. A Quantitative Spectroscopic Study of the Bleaching Phenomena in Plasticized Formulations Containing PVC Exposed to Outdoor Conditions. Polymers 2019, 11, 1481. https://doi.org/10.3390/polym11091481
González-Falcón E, Arellano M, Sanchez-Peña MJ, González-Ortiz LJ. A Quantitative Spectroscopic Study of the Bleaching Phenomena in Plasticized Formulations Containing PVC Exposed to Outdoor Conditions. Polymers. 2019; 11(9):1481. https://doi.org/10.3390/polym11091481
Chicago/Turabian StyleGonzález-Falcón, Elizabeth, Martin Arellano, M. Judith Sanchez-Peña, and L. Javier González-Ortiz. 2019. "A Quantitative Spectroscopic Study of the Bleaching Phenomena in Plasticized Formulations Containing PVC Exposed to Outdoor Conditions" Polymers 11, no. 9: 1481. https://doi.org/10.3390/polym11091481
APA StyleGonzález-Falcón, E., Arellano, M., Sanchez-Peña, M. J., & González-Ortiz, L. J. (2019). A Quantitative Spectroscopic Study of the Bleaching Phenomena in Plasticized Formulations Containing PVC Exposed to Outdoor Conditions. Polymers, 11(9), 1481. https://doi.org/10.3390/polym11091481