Feasibility Study of Applying Modified Nano-SiO2 Hyperbranched Copolymers for Enhanced Oil Recovery in Low-Mid Permeability Reservoirs
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental Materials
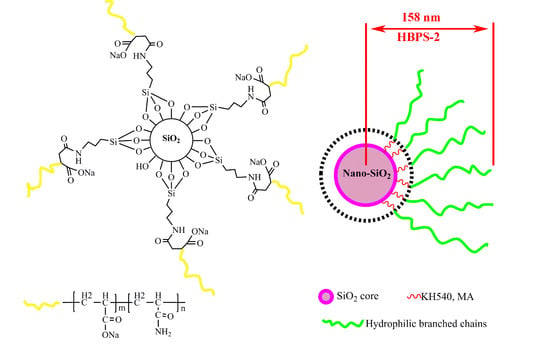
2.2. Preparation of Hyperbranched Copolymers (HBPSs)
2.3. Characterization
2.4. Stability Experiments
2.5. Viscoelasticity Tests
2.6. Sand Pack Model Flooding Tests
3. Results and Discussion
3.1. Structure and Morphology of Hyperbranched Copolymer
3.1.1. IR Characterization
3.1.2. 1H-NMR Characterization
3.1.3. Diameter Distribution.
3.1.4. Morphology
3.2. Temperature Resistance
3.3. Salt Resistance
3.4. Mechanical Shearing Resistance
3.5. Viscoelasticity of HBPS Copolymer
3.6. Feasibility of HBPS in Low-Mid Permeability Reservoirs
3.6.1. Injectivity and Mobility Control Ability
3.6.2. EOR Ability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Liao, X.; Dou, X. Potential Evaluation of CO2 Sequestration and Enhanced Oil Recovery of Low Permeability Reservoir in the Junggar Basin, China. Energy Fuels 2014, 28, 3281–3291. [Google Scholar] [CrossRef]
- Dai, C.; Chen, W.; You, Q.; Wang, H.; Yang, Z.; He Jiao, B.; Wu, Y. A novel strengthened dispersed particle gel for enhanced oil recovery application. J. Ind. Eng. Chem. 2016, 41, 175–182. [Google Scholar] [CrossRef]
- Sabhapondit, A.; Borthakur, A.; Haque, I. Water Soluble Acrylamidomethyl Propane Sulfonate (AMPS) Copolymer as an Enhanced Oil Recovery Chemical. Energy Fuels 2003, 17, 683–688. [Google Scholar] [CrossRef]
- Tiab, D.; Donaldson, E.C. Petrophysics: Theory and Practice of Mesauring Reservoir Rock and Fluid Transport Properties, 3rd ed.; Gulf Professional Publishing: Oxford, UK, 2012. [Google Scholar]
- Yekeen, N.; Padmanabhan, E.; Idris, A. A review of recent advances in foam-based fracturing fluid application in unconventional reservoirs. J. Ind. Eng. Chem. 2018, 66, 45–71. [Google Scholar] [CrossRef]
- Lai, J.; Wang, G.; Chen, M.; Wang, S.; Chai, Y.; Cai, C.; Zhang, Y.; Li, J. Pore structures evaluation of low permeability clastic reservoirs based on petrophysical facies: A case study on Chang 8 reservoir in the Jiyuan region, Ordos Basin. Pet. Explor. Dev. 2013, 40, 606–614. [Google Scholar] [CrossRef]
- Han, H.; Zhao, L.; Xu, X.; Lu, W. Characteristics and Genesis of Low to Ultra-Low Permeability Carbonate Rocks: A Case Study in Late Cretaceous Sadi Formation in H Oilfield, Southeast of Iraq. In Proceedings of the SPE Reservoir Characterization and Simulation Conference and Exhibition, Abu Dhabi, UAE, 14–16 September 2015. [Google Scholar]
- Jakobsson, N.; Christian, T. Historical Performance of Gas Injection of Ekofisk. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 25–28 September 1994. [Google Scholar]
- Liu, W. Case Study Development of Low-Permeability and Low-Pressure Reservoir Block Bao14 by Fracturing and Water Injection. In Proceedings of the SPE Europec/EAGE Annual Conference and Exhibition, London, UK, 11–14 June 2007. [Google Scholar]
- Lai, N.; Zhang, Y.; Xu, Q.; Zhou, N.; Wang, H.; Ye, Z. A water-soluble hyperbranched copolymer based on a dendritic structure for low-to-moderate permeability reservoirs. RSC Adv. 2016, 6, 32586–32597. [Google Scholar] [CrossRef]
- Song, Z.; Li, Z.; Lai, F.; Liu, G.; Gan, H. Derivation of water flooding characteristic curve for high water-cut oilfields. Pet. Explor. Dev. 2013, 40, 216–223. [Google Scholar] [CrossRef]
- Song, Z.; Li, Z.; Yu, C.; Hou, J.; Wei, M.; Bai, B.; Hu, Y. D-optimal design for rapid assessment model of CO2 flooding in high water cut oil reservoirs. J. Nat. Gas. Sci. Eng. 2014, 21, 764–771. [Google Scholar] [CrossRef]
- Wang, L.; Long, Y.; Ding, H.; Geng, J.; Bai, B. Mechanically robust re-crosslinkable polymeric hydrogels for water management of void space conduits containing reservoirs. Chem. Eng. J. 2017, 317, 952–960. [Google Scholar] [CrossRef] [Green Version]
- Riahinezhad, M.; Zerón, L.; McManus, N.; Penlidis, A. Evaluating the performance of tailor-made water-soluble copolymers for enhanced oil recovery polymer flooding applications. Fuel 2017, 203, 269–278. [Google Scholar] [CrossRef]
- Maurya, N.K.; Kushwaha, P.; Mandal, A. Studies on interfacial and rheological properties of water soluble polymer grafted nanoparticle for application in enhanced oil recovery. J. Taiwan Inst. Chem. Eng. 2017, 70, 319–330. [Google Scholar]
- Ye, Z.; Gou, G.; Gou, S.; Jiang, W.; Liu, T. Synthesis and characterization of a water-soluble sulfonates copolymer of acrylamide and N-Allylbenzamide as enhanced oil recovery. J. Appl. Polym. Sci. 2013, 128, 2003–2011. [Google Scholar] [CrossRef]
- Yamamoto, H.; Hashidzume, A.; Morishima, Y.; Tomatsu, I. Associative properties in water of copolymers of sodium 2-(acrylamido)-2-methylpropanesulfonate and methacrylamides substituted with alkyl groups of varying lengths. Macromolecules 2000, 33, 7852–7861. [Google Scholar] [CrossRef]
- Cao, R.; Zhi, H.; Liu, H.; Hou, W.; Liu, Y.; Bu, X. Experimental research on permeability limits and displacement characteristics of polymer flooding in Low Permeability Oil Layers. Pet. Geol. Oilfield Dev. 2005, 24, 71–73. [Google Scholar]
- Dai, C.; Liu, Y.; Zou, C.; You, Q.; Yang, S.; Zhao, M.; Zhao, G.; Wu, Y.; Sun, Y. Investigation on matching relationship between dispersed particle gel (DPG) and reservoir pore-throats for in-depth profile control. Fuel 2017, 207, 109–120. [Google Scholar] [CrossRef]
- Jang, H.Y.; Zhang, K.; Chon, B.H.; Choi, H.J. Enhanced oil recovery performance and viscosity characteristics of polysaccharide xanthan gum solution. J. Ind. Eng. Chem. 2015, 21, 741–745. [Google Scholar] [CrossRef]
- Qiao, R.; Zhang, R.; Zhu, W.; Gong, P. Lab simulation of profile modification and enhanced oil recovery with a quaternary ammonium cationic polymer. J. Ind. Eng. Chem. 2012, 18, 111–115. [Google Scholar] [CrossRef]
- Akuanyionwu, O.; Norris, M. Evaluating Possible Solutions to Enhancing Injectivity in Low Permeability Reservoirs—A Modeling Perspective. In Proceedings of the SPE Reservoir Characterization and Simulation Conference and Exhibition, Abu Dhabi, UAE, 16–18 September 2013. [Google Scholar]
- Xue, L.; Agarwal, U.; Lemstra, P. Shear degradation resistance of star polymers during elongational flow. Macromolecules 2005, 38, 8825–8832. [Google Scholar] [CrossRef]
- Xin, H.; Ao, D.; Wang, X.; Zhu, Y.; Zhang, J.; Tan, Y. Synthesis, characterization, and properties of copolymers of acrylamide with sodium 2-acrylamido-2-methylpropane sulfonate with nano silica structure. Colloid Polym. Sci. 2015, 293, 1307–1316. [Google Scholar] [CrossRef]
- Pu, W.; Liu, R.; Li, B.; Jin, F.; Peng, Q.; Sun, L.; Du, D.; Yao, F. Amphoteric hyperbranched polymers with multistimuli-responsive behavior in the application of polymer flooding. RSC Adv. 2015, 5, 88002–88013. [Google Scholar] [CrossRef]
- Li, B.; He, C.; Lu, W.; Wang, J.; Zeng, Y.; Gao, B. Synthesis of highly branched polymethylphenylsiloxane grafted epoxy resin copolymer for high efficiency ablation thermal protection coating. Prog. Org. Coat. 2019, 126, 178–186. [Google Scholar] [CrossRef]
- Siljanovska Petreska, G.; Petreska, A.; Arbe, C.; Auschra, M. Mechanical and morphological properties of waterborne ABA hard-soft-hard block copolymers synthesized by means of RAFT miniemulsion polymerization. Polymers 2019, 11, 1259. [Google Scholar] [CrossRef] [PubMed]
- Linul, E.; Linul, C.; Vălean, P. Compressive behavior of aluminum microfibers reinforced semi-rigid polyurethane foams. Polymers 2018, 10, 1298. [Google Scholar] [CrossRef] [PubMed]
- Şerban, D.A.; Weissenborn, O.; Geller, S.; Marşavina, L.; Gude, M. Evaluation of the mechanical and morphological properties of long fibre reinforced polyurethane rigid foams. Polym. Test. 2016, 49, 121–127. [Google Scholar] [CrossRef]
- Werne, T.; Patten, T. Atom transfer radical polymerization from nanoparticles: A tool for the preparation of well-defined hybrid nano structures and for understanding the chemistry of controlled/living radical polymerizations from surfaces. J. Am. Chem. Soc. 2001, 123, 7497–7505. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Pu, W.; Sheng, J.; Du, D. Star-like hydrophobically associative polyacrylamide for enhanced oil recovery: Comprehensive properties in harsh reservoir conditions. J. Taiwan Inst. Chem. E 2017, 80, 639–649. [Google Scholar] [CrossRef]
- Lai, N.; Guo, X.; Zhou, N.; Xu, Q. Shear Resistance Properties of Modified Nano-SiO2/AA/AM Copolymer Oil Displacement Agent. Energies 2016, 9, 1037. [Google Scholar] [CrossRef]
- Veerabhadrappa, S.; Trivedi, J.; Kuru, E. Visual confirmation of the elasticity dependence of unstable secondary polymer floods. Ind. Eng. Chem. Res. 2013, 52, 6234−6241. [Google Scholar] [CrossRef]
- Hua, Z.; Lin, M.; Dong, Z.; Li, M.; Zhang, G.; Yang, J. Study of deep profile control and oil displacement technologies with nanoscale polymer microspheres. J. Colloid Interface Sci. 2014, 424, 67–74. [Google Scholar] [CrossRef]
- Sang, Q.; Li, Y.; Yu, L.; Li, Z.; Dong, M. Enhanced oil recovery by branched-preformed particle gel injection in parallel-sandpack models. Fuel 2014, 136, 295–306. [Google Scholar] [CrossRef]
- Xue, Z.; Foster, E.; Wang, Y.; Nayak, S.; Cheng, V.; Ngo, V.; Johnston, K. Effect of Grafted Copolymer Composition on Iron Oxide Nanoparticle Stability and Transport in Porous Media at High Salinity. Energy Fuels 2014, 28, 3655–3665. [Google Scholar] [CrossRef]
- Seright, R.; Campbell, A.; Mozley, P.; Han, P. Stability of partially hydrolyzed polyacrylamides at elevated temperatures in the absence of divalent cations. J. SPE 2010, 15, 341–348. [Google Scholar] [CrossRef]
- Francois, J.; Sarazin, D.; Schwartz, T.; Weill, G. Polyacrylamide in water: Molecular weight dependence of <R2> and [η] and the problem of the excluded volume exponent. Polymer 1979, 20, 969–975. [Google Scholar] [CrossRef]
- Hatzignatiou, D.G.; Giske, N.H.; Stavland, A. Polymers and polymer-based gelants for improved oil recovery and water control in naturally fractured chalk formations. Chem. Eng. Sci. 2018, 187, 302–317. [Google Scholar] [CrossRef]
- Gumpenberger, T.; Kornberger, M.; Deckers, M.; Clemens, T. Polymer Viscosity in Porous Media and Near Wellbore Behaviour of A Polymer Pilot in The Matzen Field, Austria. In Proceedings of the Offshore Mediterranean Conference, Ravenna, Italy, 20–22 March 2013. [Google Scholar]









| Level of Reservoirs | Permeability (10−3 µm2) | Permeability Category |
|---|---|---|
| Poor | <1 | Tight |
| Fair | 1~10 | Low |
| Moderate | 10~50 | Medium |
| Good | 50~250 | Medium |
| Very good | >250 | High |
| Total * | AA | AM | NSIO | Initiator | |
|---|---|---|---|---|---|
| Concentration (wt.%) | 26.0 | 7.6 | 17.7 | 0.5 | 0.2 |
| Ions | [Na++K+] | [Ca2+] | [Mg2+] | [HCO3−] | [SO42−] | [Cl−] |
|---|---|---|---|---|---|---|
| Content (mg/L) | 2049 | 80 | 28 | 400 | 90 | 3080 |
| Polymer | MW (107 g/mol) | Rg (nm) | Rh (nm) | A (nm (mol/g) 1/2) |
|---|---|---|---|---|
| HPAM | 1.24 | 129 | 315 | 0.090 |
| HBPS-1 | 1.10 | 117 | 196 | 0.086 |
| HBPS-2 | 1.02 | 96 | 158 | 0.074 |
| Polymer * | (mPa·s) | 3600 RPM tshearing = 20 s | 7200 RPM tshearing = 20 s | 11500 RPM tshearing = 20 s | |||
|---|---|---|---|---|---|---|---|
| HPAM | 6.5 | 4.2 | 64.62 | 3.5 | 53.85 | 2.8 | 43.08 |
| HBPS-1 | 8.1 | 7.1 | 87.65 | 6.8 | 83.95 | 5.9 | 72.84 |
| HBPS-2 | 7.7 | 6.9 | 89.61 | 6.5 | 84.42 | 6.0 | 77.92 |
| Polymer | Permeability (× 10−3 μm2) | Stable Pressure (MPa) | Porosity (%) | RF | RRF |
|---|---|---|---|---|---|
| HPAM | 47.76 | / | 20.88 | / | / |
| HBPS-1 | 49.89 | 0.0851 | 21.61 | 80.19 | 31.12 |
| HBPS-2 | 52.62 | 0.0807 | 21.85 | 90.72 | 39.11 |
| HPAM | 87.55 | 0.0481 | 23.79 | 47.40 | 18.88 |
| HBPS-1 | 93.12 | 0.0456 | 24.67 | 76.45 | 27.67 |
| HBPS-2 | 91.32 | 0.0465 | 24.31 | 85.73 | 32.73 |
| HPAM | 135.98 | 0.0110 | 28.23 | 41.74 | 17.41 |
| HBPS-1 | 132.92 | 0.0129 | 27.98 | 66.01 | 23.47 |
| HBPS-2 | 128.12 | 0.0151 | 27.42 | 72.30 | 28.07 |
| Samples | Polymer | Permeability (× 10−3 μm2) | ET (%) | EW (%) | EOR (%) |
|---|---|---|---|---|---|
| #1 | HPAM | 74.89 | 54.30 | 46.18 | 8.12 |
| #2 | HBPS-1 | 78.49 | 64.11 | 47.86 | 16.25 |
| #3 | HBPS-2 | 81.18 | 68.36 | 49.62 | 18.74 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, N.; Tang, L.; Jia, N.; Qiao, D.; Chen, J.; Wang, Y.; Zhao, X. Feasibility Study of Applying Modified Nano-SiO2 Hyperbranched Copolymers for Enhanced Oil Recovery in Low-Mid Permeability Reservoirs. Polymers 2019, 11, 1483. https://doi.org/10.3390/polym11091483
Lai N, Tang L, Jia N, Qiao D, Chen J, Wang Y, Zhao X. Feasibility Study of Applying Modified Nano-SiO2 Hyperbranched Copolymers for Enhanced Oil Recovery in Low-Mid Permeability Reservoirs. Polymers. 2019; 11(9):1483. https://doi.org/10.3390/polym11091483
Chicago/Turabian StyleLai, Nanjun, Lei Tang, Na Jia, Dongyu Qiao, Jianlin Chen, Yong Wang, and Xubin Zhao. 2019. "Feasibility Study of Applying Modified Nano-SiO2 Hyperbranched Copolymers for Enhanced Oil Recovery in Low-Mid Permeability Reservoirs" Polymers 11, no. 9: 1483. https://doi.org/10.3390/polym11091483
APA StyleLai, N., Tang, L., Jia, N., Qiao, D., Chen, J., Wang, Y., & Zhao, X. (2019). Feasibility Study of Applying Modified Nano-SiO2 Hyperbranched Copolymers for Enhanced Oil Recovery in Low-Mid Permeability Reservoirs. Polymers, 11(9), 1483. https://doi.org/10.3390/polym11091483