New Strategy to Cope with Common Fishery Policy Landing Obligation: Collagen Extraction from Skins and Bones of Undersized Hake (Merluccius merluccius)
Abstract
:1. Introduction
2. Material and Methods
2.1. Biological Samples
2.1.1. Proximate Composition of SBF
2.1.2. Hydroxyproline Content
2.2. Collagen Extraction and Characterization
2.2.1. Molecular Weight Determination of Collagen by GPC
2.2.2. Circular Dichroism
2.2.3. X-Ray Diffraction (XRD)
2.2.4. Fourier-Transform Infrared (FTIR) Spectroscopy
2.2.5. Amino Acid Composition
2.2.6. SDS-PAGE
3. Results and Discussion
3.1. Characterization of Hake Specimens
3.2. Mechanical Separation of Skin and Bone Fraction (SBF): Yields and Chemical Characterization
3.3. Collagen Extraction: Protein and Collagen Yields
3.4. Characterization of Collagen
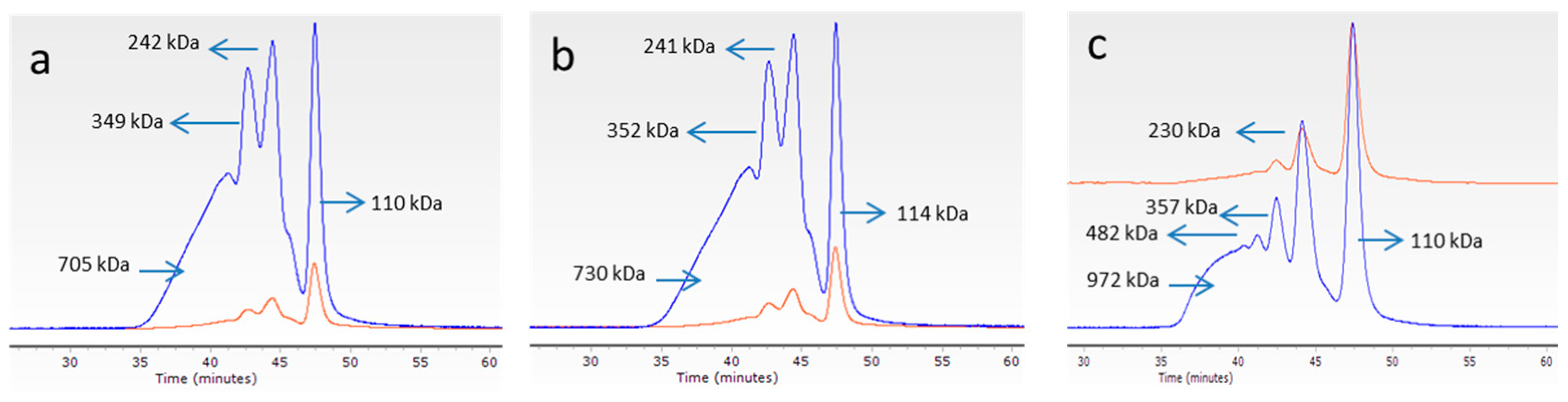
3.4.1. Molecular Weight of Collagen
3.4.2. Amino Acid Content
3.4.3. FTIR
3.4.4. Circular Dichroism
3.4.5. X-ray Diffraction
3.4.6. SDS-PAGE
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- European Union. Reglamento (UE) N° 1380/2013 Del Parlamento Europeo y Del Consejo De 11 De Diciembre De 2013; Sobre la Política Pesquera Común, por el que se modifican los Reglamentos (CE) n° 1954/2003 y (CE) n° 1224/2009 del Consejo, y se derogan los Reglamentos (CE) n° 2371/2002 y (CE) n° 639/2004 del Consejo y la Decisión 2004/585/CE del Consejo; European Union: Brussels, Belgium, 2013. [Google Scholar]
- Blanco, M.; Sotelo, C.G.; Pérez-Martín, R.I. Hydrolysis as a valorization strategy for unused marine food biomass: Boarfish and small-spotted catshark discards and by-products. J. Food Biochem. 2015, 39, 368–376. [Google Scholar] [CrossRef]
- Blanco, M.; Vázquez, J.A.; Pérez-Martín, R.I.; Sotelo, C.G. Hydrolysates of Fish Skin Collagen: An Opportunity for Valorizing Fish Industry Byproducts. Mar. Drugs 2017, 15, 131. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Blanco, M.; Massa, A.E.; Amado, I.R.; Pérez-Martín, R.I. Production of Fish Protein Hydrolysates from Scyliorhinus canicula Discards with Antihypertensive and Antioxidant Activities by Enzymatic Hydrolysis and Mathematical Optimization Using Response Surface Methodology. Mar. Drugs 2017, 15, 306. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, J.A.; Fernández-Compás, A.; Blanco, M.; Rodríguez-Amado, I.; Moreno, H.; Borderías, J.; Pérez-Martín, R.I. Development of bioprocesses for the integral valorisation of fish discards. Biochem. Eng. J. 2019, 144, 198–208. [Google Scholar] [CrossRef]
- Alves, A.L.; Marques, A.L.P.; Martins, E.; Silva, T.H.; Reis, R.L. Cosmetic potential of marine fish skin collagen. Cosmetics 2017, 4, 39. [Google Scholar] [CrossRef]
- Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M.S. Marine Fish Proteins and Peptides for Cosmeceuticals: A Review. Mar. Drugs 2017, 15, 143. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillén, M.C.; Turnay, J.; Fernández-Díaz, M.D.; Ulmo, N.; Lizarbe, M.A.; Montero, P. Structural and physical propoerties of gelatin extracted from different marine species: A comparative study. Food Hydrocoll. 2002, 16, 25–34. [Google Scholar] [CrossRef]
- Karayannakidis, P.D.; Chatziantoniou, S.E.; Zotos, A. Effects of selected process parameters on physical and sensorial properties of yellowfin tuna (Thunnus albacares) skin gelatin. J. Food Process Eng. 2014, 37, 461–473. [Google Scholar] [CrossRef]
- Sotelo, C.G.; Blanco, M.; Ramos-Ariza, P.; Pérez-Martín, R.I. Characterization of collagen from different discarded fish species of the west coast of the Iberian Peninsula. J. Aquat. Food Prod. Technol. 2016, 25, 388–399. [Google Scholar] [CrossRef]
- Blanco, M.; Vázquez, J.A.; Pérez-Martín, R.I.; Sotelo, C.G. Collagen extraction optimization from teh skin of the small-spotted catshark (S. canicula) by Response Surface Methodology. Mar. Drugs 2019, 17, 40. [Google Scholar] [CrossRef]
- Zhu, B.W.; Dong, X.P.; Zhou, D.Y.; Gao, Y.; Yang, J.F.; Li, D.M.; Zhao, X.K.; Ren, T.T.; Ye, W.X.; Tan, H.; et al. Physicochemical properties and radical scavenging capacities of pepsin-solubilized collagen from sea cucumber Stichopus japonicus. Food Hydrocoll. 2012, 28, 182–188. [Google Scholar] [CrossRef]
- Woo, J.W.; Yu, S.J.; Cho, S.M.; Lee, Y.B.; Kim, S.B. Extraction optimization and properties of collagen from yellowfin tuna (Thunnus albacares) dorsal skin. Food Hydrocoll. 2008, 22, 879–887. [Google Scholar] [CrossRef]
- Benjakul, S.; Thiansilakul, Y.; Visessanguan, W.; Roytrakul, S.; Kishimura, H.; Prodpran, T.; Meesane, J. Extraction and characterisation of pepsin-solubilised collagen from the skin of bigeye snapper (Priacanthus tayenus and Priacanthus macracanthus). J. Sci. Food Agric. 2010, 90, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, N.; Shakila, R.J.; Sukumar, D.; Jeyasekaran, G. Skin, bone and muscle collagen extraction from the trash fish, leather jacket (Odonus niger) and their characterization. J. Food Sci. Technol. 2013, 50, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Mahboob, S. Isolation and characterization of collagen from fish waste material-skin, scales and fins of Catla catla and Cirrhinus mrigala. J. Food Sci. Technol. 2015, 52, 4296–4305. [Google Scholar] [CrossRef] [PubMed]
- EU, DG MARE. The EU Fish Market 2018; EU, DG MARE: Brussels, Belgium, 2018. [Google Scholar]
- European Commission. Impact assessment of discard reducing policies. In Studies in the Field of the Common Fisheries Policy and Maritime Affairs; EU Discard Annex; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- Valeiras, J.; Pérez, N.; Araujo, H.; Salinas, I.; Bellido, J.M. Atlas De Los Descartes De La Flota De Arrastre u Enmalle En El Caladero Nacional Cantábrico-Noroeste; Instituto Español de Oceanografía: Madrid, Spain, 2014; p. 120. [Google Scholar]
- AOAC. Official Methods of Analysis of the AOAC; Association of Official Analytical Chemists Inc.: Arlington, VA, USA, 2000. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Meyer, M.; Morgenstern, B. Characterization of gelatin and acid soluble collagen by size exclusion chromatography coupled with multi angle light scattering. Biomacromolecules 2003, 4, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Agricultura, Pesca y Alimentación. Real Decreto 560/1995, De 7 De Abril, Por El Que Se Establece Las Tallas Mínimas De Determinadas Especies Pesqueras; BOE-A-1995-8639; Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 1995. [Google Scholar]
- Borges, L. One Year on: The Landing Obligation in Europe. News ICES 2017, 10, 1–3. [Google Scholar]
- Chef’s Resources. Available online: http://www.chefs-resources.com/seafood/seafood-yields/ (accessed on 10 September 2019).
- Edwards, C.A.; O’Brien, W.D., Jr. Modified assay for determination of hydroxyproline in a tissue hydrolysate. Clin. Chim. Acta 1980, 104, 161–167. [Google Scholar] [CrossRef]
- Carbalho, A.M.; Marques, A.P.; Silva, T.H.; Reis, R.L. Evaluation of the potential of collagen from codfish skin as a biomaterial for biomedical applications. Mar. Drugs 2018, 16, 495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, A.; Li, Z.; He, S.; Shao, L. Preparation and characterization of collagen from freshwater fish scales. Food Nutr. Sci. 2011, 2, 818–823. [Google Scholar]
- Ahmad, M.; Benjakul, S.; Nalinalon, S. Composition and physicochemical characteristics of acid solubilized collagen extracted from the skin of unicorn leatherjacket (Aluterus Monoceros). Food Hydrocoll. 2010, 24, 588–594. [Google Scholar] [CrossRef]
- De Sousa, R.A.O. Valorization of Cod (Gadus morhua) by-Products by Extraction of Collagen with Potential Application in Cosmetic and Biomedical Field. Ph.D. Thesis, Escola superior de Turismo e Tecnologia do Mar e o Instituto Politécnico de Leiria, Peniche, Portugal, 2017. [Google Scholar]
- Habermehl, J.; Skopinska, J.; Boccafoschi, F.; Sionkowska, A.; Kaczmarek, H.; Laroche, G.; Mantovani, D.; Skopiǹska, J. Preparation of Ready-to-use, Stockable and Reconstituted Collagen. Macromol. Biosci. 2005, 5, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Veeruraj, A.; Arumugam, M.; Balasubramanian, T. Isolation and characterization of thermostable collagen from the marine eel-fish (Evenchelys macrura). Process Biochem. 2013, 48, 1592–1602. [Google Scholar] [CrossRef]
- Chen, J.; Ruizao, L.L.; Xu, N.; Gao, R.; Hong, B. Extraction and characterization of acid-soluble collagen from scales and skin of tilapia (Oreochromis niloticus). LWT Food Sci. Technol. 2016, 66, 453–459. [Google Scholar] [CrossRef]
- Lee, J.K.; Kang, S.I.; Kim, Y.J.; Kim, M.J.; Heu, M.S.; Choi, B.D. Comparison of collagen characteristics of sea-and freshwater rainbowtrout skin. Food Sci. Biotechnol. 2016, 25, 131–136. [Google Scholar] [CrossRef]
- Ramanathan, G.; Singarevelu, S.; Raja, M.; Sobhana, S.; Sivagranam, U.T. Extraction and characterization of collagen from the skin of Arotron stellatus fish—A novel source of collagen for tissue engineering. J. Biomater. Tissue Eng. 2014, 4, 203–209. [Google Scholar] [CrossRef]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Nawshad, M.; Safi, S.Z.; Rahim, A.; Rizvi, S.A.A.; Rehman, I.U. FTIR analysis of natural and synthetic collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, L.; Gu, Z.; Dan, W.; Dan, N.; Yu, X. Modification of collagen with a natural derived cross-linker, alginate dialdehyde. Carbohyd. Polym. 2014, 102, 324–332. [Google Scholar] [CrossRef]
- Drzewieccki, K.E.; Grisham, D.R.; Parmar, A.S.; Nanda, V.; Shreiber, D.I. Circular dichroism spectroscopy of collagen fibrillogenesis: A new use for an old technique. Biophys. J. 2016, 111, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2006, 1, 2876–2890. [Google Scholar] [CrossRef] [PubMed]
- Fiorani, A.; Gualandi, C.; Panseri, S.; Montesi, M.; Marcacci, M.; Focarete, M.; Bigi, A. Comparative performance of collagen nanofibers electrospun from different solvents and stabilized by different crosslinkers. J. Mater. Sci. Mater. Med. 2014, 25, 2313–2321. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, B.; Song, W.; Si, L.; Hou, H. Characterization of Pacific cod (Gadus macrocephalus) skin collagen and fabrication of collagen sponge as a good biocompatible biomedical material. Process Biochem. 2017, 63, 229–235. [Google Scholar] [CrossRef]










| Amino Acid | Collagen Fractions (nmols/mg) | ||
|---|---|---|---|
| F1 | F2 | F3 | |
| OHpro | 416.31 ± 53 | 421.03 ± 148 | 440.01 ± 18 |
| Asp | 397.96 ± 22 | 455.76 ± 54 | 413.03 ± 40 |
| Thr | 225.60 ± 15 | 246.02 ± 36 | 232.79 ± 18 |
| Ser | 414.09 ± 26 | 463.12 ± 96 | 439.29 ± 45 |
| Glu | 577.27 ± 34 | 672.81 ± 79 | 604.96 ± 50 |
| Pro | 713.18 ± 47 | 750.22 ± 216 | 740.38 ± 31 |
| Gly | 2402.60 ± 211 | 2469.78 ± 701 | 2480.66 ± 27 |
| Ala | 920.25 ± 52 | 972.22 ± 242 | 925.32 ± 56 |
| Cys | 20.95 ± 3 | 24.87 ± 7 | 20.72 ± 4 |
| Val | 163.85 ± 16 | 177.08 ± 11 | 169.62 ± 10 |
| Met | 130.92 ± 6 | 138.62 ± 15 | 129.39 ± 14 |
| Ile | 79.78 ± 8 | 92.88 ± 10 | 84.71 ± 5 |
| Leu | 174.65 ± 7 | 216.24 ± 40 | 190.23 ± 26 |
| Tyr | 43.71 ± 9 | 62.04 ± 28 | 46.93 ± 24 |
| Phe | 123.56 ± 10 | 155.46 ± 36 | 130.66 ± 31 |
| Ohlys | 37.95 ± 5 | 56.61 ± 25 | 52.12 ± 17 |
| His | 63.14 ± 2 | 74.60 ± 14 | 67.69 ± 11 |
| Lys | 228.61 ± 6 | 262.17 ± 30 | 225.91 ± 25 |
| Arg | 368.93 ± 30 | 386.26 ± 92 | 380.43 ± 22 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco, M.; G. Sotelo, C.; I. Pérez-Martín, R. New Strategy to Cope with Common Fishery Policy Landing Obligation: Collagen Extraction from Skins and Bones of Undersized Hake (Merluccius merluccius). Polymers 2019, 11, 1485. https://doi.org/10.3390/polym11091485
Blanco M, G. Sotelo C, I. Pérez-Martín R. New Strategy to Cope with Common Fishery Policy Landing Obligation: Collagen Extraction from Skins and Bones of Undersized Hake (Merluccius merluccius). Polymers. 2019; 11(9):1485. https://doi.org/10.3390/polym11091485
Chicago/Turabian StyleBlanco, María, Carmen G. Sotelo, and Ricardo I. Pérez-Martín. 2019. "New Strategy to Cope with Common Fishery Policy Landing Obligation: Collagen Extraction from Skins and Bones of Undersized Hake (Merluccius merluccius)" Polymers 11, no. 9: 1485. https://doi.org/10.3390/polym11091485
APA StyleBlanco, M., G. Sotelo, C., & I. Pérez-Martín, R. (2019). New Strategy to Cope with Common Fishery Policy Landing Obligation: Collagen Extraction from Skins and Bones of Undersized Hake (Merluccius merluccius). Polymers, 11(9), 1485. https://doi.org/10.3390/polym11091485






