Influence of Organo-Sepiolite on the Morphological, Mechanical, and Rheological Properties of PP/ABS Blends
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification of Sep
2.3. Preparation of PP /ABS/O-Sep Blends
2.4. Measurements and Characterizations
3. Results and Discussions
3.1. Modification of Sep
3.2. Morphology of Blends
3.3. Mechanical Properties
3.4. Thermal Properties
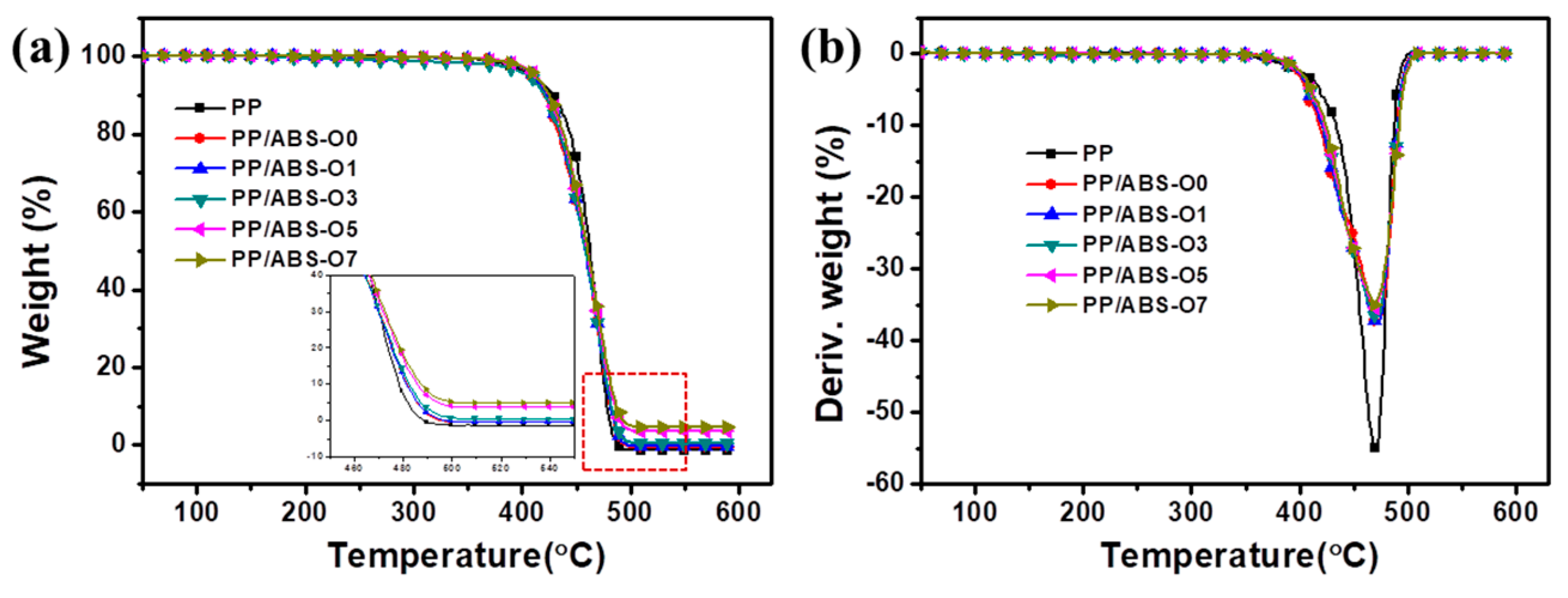
3.4.1. Thermogravimetric Analysis
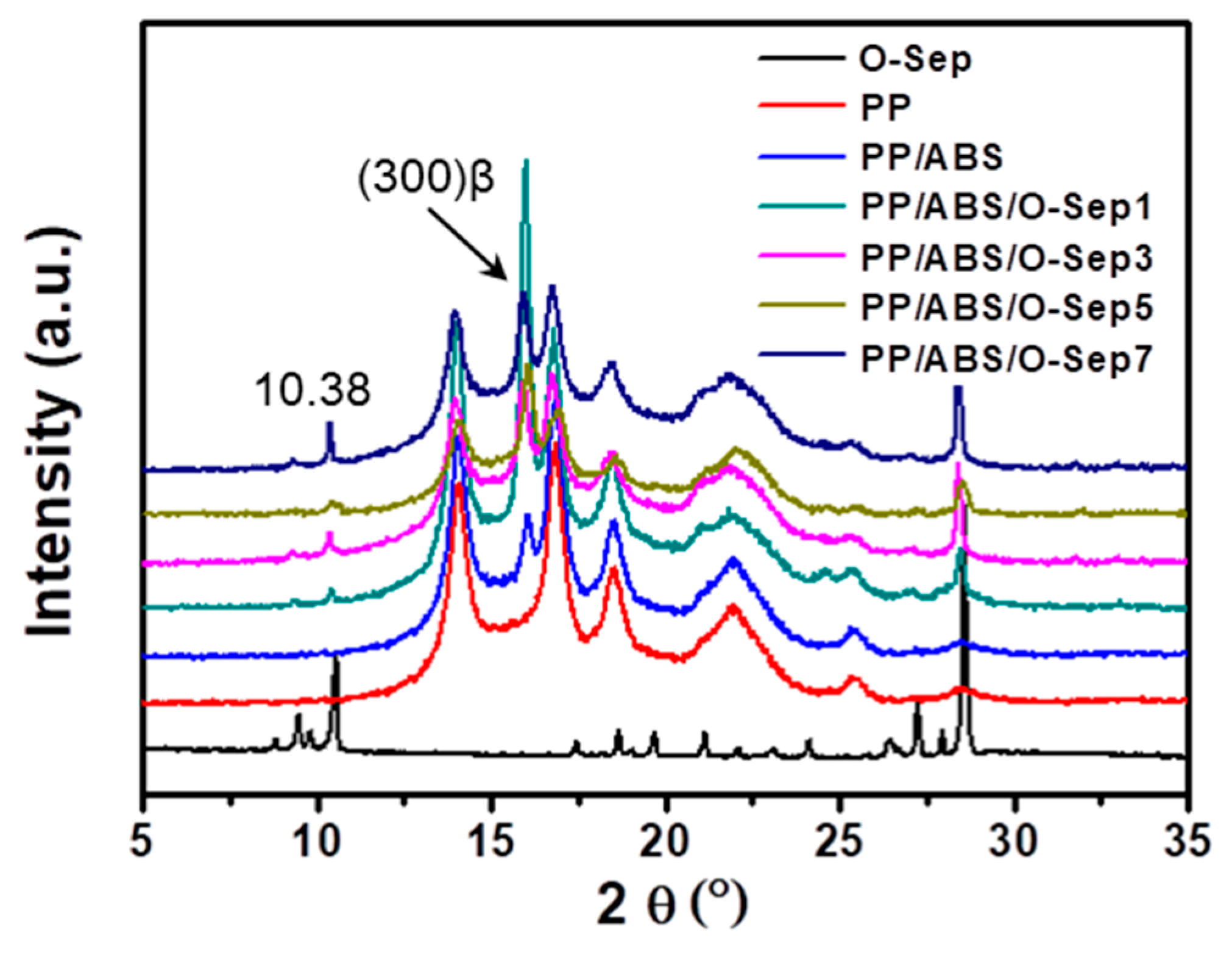
3.4.2. Differential Scanning Calorimetry (DSC) and X-Ray Diffraction (XRD)
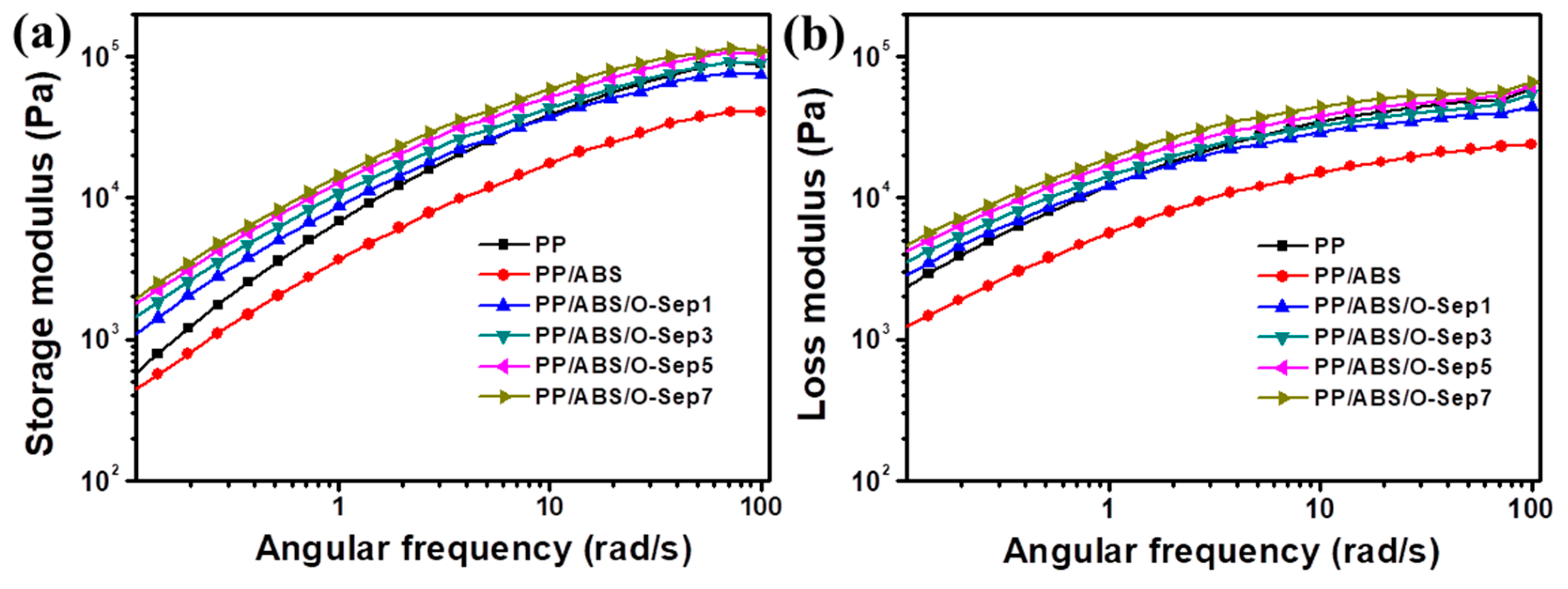
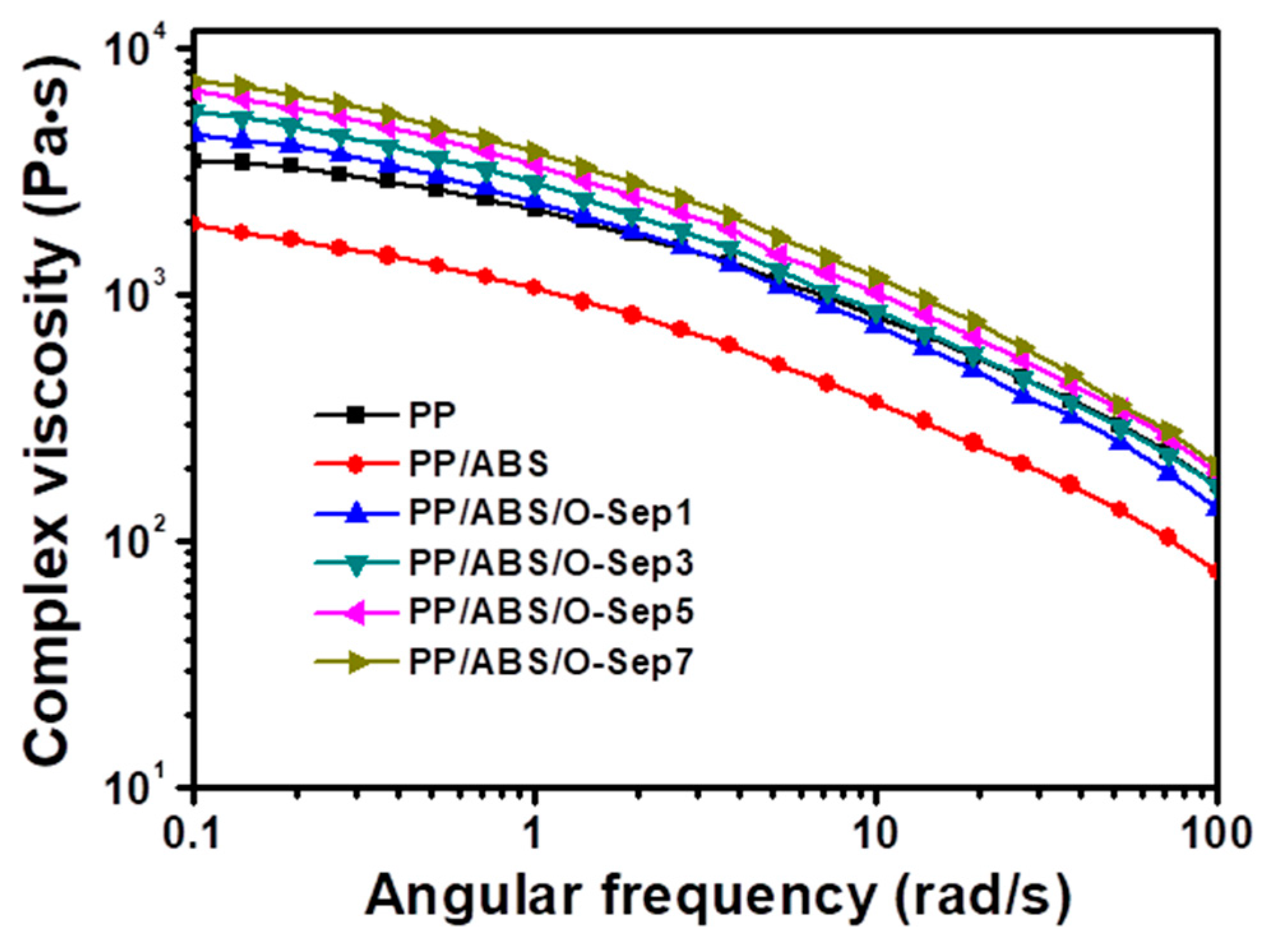
3.5. Rheological Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Khare, R.A.; Bhattacharyya, A.R.; Kulkarni, A.R.; Saroop, M.; Biswas, A. Influence of multiwall carbon nanotubes on morphology and electrical conductivity of PP/ABS blends. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 2286–2295. [Google Scholar] [CrossRef]
- Weng, J.; Gong, Z.; Liao, L.; Lv, G.; Tan, J. Comparison of organo-sepiolite modified by different surfactants and their rheological behavior in oil-based drilling fluids. Appl. Clay Sci. 2018, 159, 94–101. [Google Scholar] [CrossRef]
- Hao, Z.; Li, L.; Yang, B.; Sheng, X.; Liao, X.; He, L.; Liu, P. Influences of hyperbranched polyester modification on the crystallization kinetics of isotactic polypropylene/graphene oxide composites. Polymers 2019, 11, 433. [Google Scholar] [CrossRef]
- Huang, C.-W.; Yang, T.-C.; Hung, K.-C.; Xu, J.-W.; Wu, J.-H. The effect of maleated polypropylene on the non-isothermal crystallization kinetics of wood fiber-reinforced polypropylene composites. Polymers 2018, 10, 382. [Google Scholar] [CrossRef] [PubMed]
- Sangroniz, L.; Palacios, J.K.; Fernández, M.; Eguiazabal, J.I.; Santamaria, A.; Müller, A.J. Linear and non-linear rheological behavior of polypropylene/polyamide blends modified with a compatibilizer agent and nanosilica and its relationship with the morphology. Eur. Polym. J. 2016, 83, 10–21. [Google Scholar] [CrossRef]
- Hernandez, Y.; Lozano, T.; Morales, A.B.; Navarro-Pardo, F.; Lafleur, P.G.; Sanchez-Valdes, S.; Martinez-Colunga, G.; Morales-Zamudio, L.; de Lira-Gomez, P. Improvement of toughness properties of polypropylene filled with nanobentonite using stearic acid as interface modifier. J. Compos. Mater. 2016, 51, 373–380. [Google Scholar] [CrossRef]
- Jhu, Y.-S.; Yang, T.-C.; Hung, K.-C.; Xu, J.-W.; Wu, T.-L.; Wu, J.-H. Nonisothermal crystallization kinetics of acetylated bamboo fiber-reinforced polypropylene composites. Polymers 2019, 11, 1078. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Zhou, X.; Chen, X.; Zhao, H.; Wang, Y.; Zhu, H.; Shan, X.; Sha, J.; Ma, Y.; Xie, L. Effects of solid-state stretching on microstructure evolution and physical properties of isotactic polypropylene sheets. Polymers 2019, 11, 618. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zhao, S.; Xin, Z. Influence of a novel β-nucleating agent on the structure, mechanical properties, and crystallization behavior of isotactic polypropylene. J. Thermoplast. Compos. Mater. 2013, 28, 610–629. [Google Scholar] [CrossRef]
- Sun, J.; Hu, J.-S.; Guo, Z.-X.; Qi, Y. Study on side-chain liquid-crystalline copolymer as a new β-nucleating agent to induce phase behavior of isotactic polypropylene. Colloid Polym. Sci. 2012, 291, 735–742. [Google Scholar] [CrossRef]
- Yang, R.; Ding, L.; Chen, W.; Chen, L.; Zhang, X.; Li, J. Chain folding in main-chain liquid crystalline polyester with strong π–π interaction: An efficient β-nucleating agent for isotactic polypropylene. Macromolecules 2017, 50, 1610–1617. [Google Scholar] [CrossRef]
- Karger-Kocsis, J. How does “phase transformation toughening” work in semicrystalline polymers? Polym. Eng. Sci. 1996, 36, 203–210. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Varga, J. Effects off β-α transformation on the static and dynamic tensile behavior of isotactic polypropylene. J. Appl. Polym. Sci. 1996, 62, 291–300. [Google Scholar] [CrossRef]
- Wang, C.; Chu, Y.-L.; Wu, Y.-J. Electrospun isotactic polystyrene nanofibers as a novel β-nucleating agent for isotactic polypropylene. Polymer 2012, 53, 5404–5412. [Google Scholar] [CrossRef]
- Phillips, A.; Zhu, P.W.; Edward, G. Polystyrene as a versatile nucleating agent for polypropylene. Polymer 2010, 51, 1599–1607. [Google Scholar] [CrossRef]
- Phillips, A.W.; Bhatia, A.; Zhu, P.W.; Edward, G. Shish formation and relaxation in sheared isotactic polypropylene containing nucleating particles. Macromolecules 2011, 44, 3517–3528. [Google Scholar] [CrossRef]
- Liu, J.; Li, C.; Hu, F. Effect of polystyrenes with different architectures on the β-nucleating efficiency and toughening of isotactic polypropylene. Polym. Int. 2018, 67, 506–514. [Google Scholar] [CrossRef]
- Shu, Q.; Zou, X.; Dai, W.; Fu, Z. Formation of β-iPP in isotactic polypropylene/acrylonitrile-butadiene-styrene blends: Effect of resin type, phase composition, and thermal condition. J. Macromol. Sci. B 2011, 51, 756–766. [Google Scholar] [CrossRef]
- Lee, H.G.; Sung, Y.-T.; Lee, Y.K.; Kim, W.N.; Yoon, H.G.; Lee, H.S. Effects of PP-g-MAH on the mechanical, morphological and rheological properties of polypropylene and poly(acrylonitrile-butadiene-styrene) blends. Macromol. Res. 2009, 17, 417–423. [Google Scholar] [CrossRef]
- Kodgire, P.; Kalgaonkar, R.; Hambir, S.; Bulakh, N.; Jog, J.P. PP/clay nanocomposites: Effect of clay treatment on morphology and dynamic mechanical properties. J. Appl. Polym. Sci. 2001, 81, 1786–1792. [Google Scholar] [CrossRef]
- Wang, L.; Okada, K.; Sodenaga, M.; Hikima, Y.; Ohshima, M.; Sekiguchi, T.; Yano, H. Effect of surface modification on the dispersion, rheological behavior, crystallization kinetics, and foaming ability of polypropylene/cellulose nanofiber nanocomposites. Compos. Sci. Technol. 2018, 168, 412–419. [Google Scholar] [CrossRef]
- Laoutid, F.; Persenaire, O.; Bonnaud, L.; Dubois, P. Flame retardant polypropylene through the joint action of sepiolite and polyamide 6. Polym. Degrad. Stab. 2013, 98, 1972–1980. [Google Scholar] [CrossRef]
- Zeng, A.; Zheng, Y.; Guo, Y.; Qiu, S.; Cheng, L. Effect of tetra-needle-shaped zinc oxide whisker (T-ZnOw) on mechanical properties and crystallization behavior of isotactic polypropylene. Mater. Des. 2012, 34, 691–698. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, X. Isotactic polypropylene toughened with poly(acrylonitrile-butadiene-styrene): Compatibilizing role of nano-ZnO. Polym.-Plast. Technol. Mater. 2019. [Google Scholar] [CrossRef]
- Gupta, A.K.; Jain, A.K.; Maiti, S.N. Studies on binary and ternary blends of polypropylene with ABS and LDPE. I. melt rheological behavior. J. Appl. Polym. Sci. 1989, 38, 1699–1717. [Google Scholar] [CrossRef]
- Bonda, S.; Mohanty, S.; Nayak, S.K. Influence of compatibilizer on mechanical, morphological and rheological properties of PP/ABS blends. Iran. Polym. J. 2014, 23, 415–425. [Google Scholar] [CrossRef]
- Kubade, P.; Tambe, P. Influence of halloysite nanotubes (HNTs) on morphology, crystallization, mechanical and thermal behaviour of PP/ABS blends and its composites in presence and absence of dual compatibilizer. Compos. Interfaces 2016, 23, 433–451. [Google Scholar] [CrossRef]
- Gao, M.; Yang, J.; Zhao, H.; He, H.; Hu, M.; Xie, S. Preparation methods of polypropylene/nano-silica/styrene-ethylene-butylene-styrene composite and its effect on electrical properties. Polymers 2019, 11, 797. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Xin, Z.; Pan, C.; Sun, S.; Jiang, X.; Zhao, S. In situ formation of zinc phthalate as a highly dispersed β-nucleating agent for mechanically strengthened isotactic polypropylene. Chem. Eng. J. 2019, 358, 1243–1252. [Google Scholar] [CrossRef]
- Krause, B.; Rzeczkowski, P.; Pötschke, P. Thermal conductivity and electrical resistivity of melt-mixed polypropylene composites containing mixtures of carbon-based fillers. Polymers 2019, 11, 1073. [Google Scholar] [CrossRef]
- Baudouin, A.-C.; Auhl, D.; Tao, F.; Devaux, J.; Bailly, C. Polymer blend emulsion stabilization using carbon nanotubes interfacial confinement. Polymer 2011, 52, 149–156. [Google Scholar] [CrossRef]
- Mao, H.; He, B.; Guo, W.; Hua, L.; Yang, Q. Effects of nano-CaCO3 content on the crystallization, mechanical properties, and cell structure of PP nanocomposites in microcellular injection molding. Polymers 2018, 10, 1160. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Qin, W.; Xin, Z.; Zhou, S.; Gong, H.; Ni, Y.; Zhang, K. In situ generation of a self-dispersed β-nucleating agent with increased nucleation efficiency in isotactic polypropylene. Polymer 2018, 151, 84–91. [Google Scholar] [CrossRef]
- Sun, F.; Li, T.T.; Ren, H.; Jiang, Q.; Peng, H.K.; Lin, Q.; Lou, C.W.; Lin, J.H. PP/TiO2 melt-blown membranes for oil/water separation and photocatalysis: Manufacturing techniques and property evaluations. Polymers 2019, 11, 775. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Meng, M.; Yu, Y.; Wang, X.; Ding, T. Photoluminescence and photocatalysis of the flower-like nano-ZnO photocatalysts prepared by a facile hydrothermal method with or without ultrasonic assistance. Appl. Catal. B 2011, 105, 335–345. [Google Scholar] [CrossRef]
- Wang, L.; Okada, K.; Hikima, Y.; Ohshima, M.; Sekiguchi, T.; Yano, H. Effect of cellulose nanofiber (CNF) surface treatment on cellular structures and mechanical properties of polypropylene/CNF nanocomposite foams via core-back foam injection molding. Polymers 2019, 11, 249. [Google Scholar] [CrossRef] [PubMed]
- Hapuarachchi, T.D.; Peijs, T.; Bilotti, E. Thermal degradation and flammability behavior of polypropylene/clay/carbon nanotube composite systems. Polym. Adv. Technol. 2013, 24, 331–338. [Google Scholar] [CrossRef]
- Bilotti, E.; Deng, H.; Zhang, R.; Lu, D.; Bras, W.; Fischer, H.R.; Peijs, T. Synergistic reinforcement of highly oriented poly(propylene) tapes by sepiolite nanoclay. Macromol. Mater. Eng. 2010, 295, 37–47. [Google Scholar] [CrossRef]
- Singh, V.P.; Kapur, G.S.; Choudhary, V. High-density polyethylene/needle-like sepiolite clay nanocomposites: Effect of functionalized polymers on the dispersion of nanofiller, melt extensional and mechanical properties. RSC Advance 2016, 6, 59762–59774. [Google Scholar] [CrossRef]
- La Tegola, S.; Terenzi, A.; Martini, R.; Barbosa, S.; Torre, L.; Kenny, J. Processing and final properties improvement of polyolefin-sepiolite and carbon nanofibre nanocomposites. Macromol. Symp. 2011, 301, 128–135. [Google Scholar] [CrossRef]
- Bilotti, E.; Fischer, H.R.; Peijs, T. Polymer nanocomposites based on needle-like sepiolite clays: Effect of functionalized polymers on the dispersion of nanofiller, crystallinity, and mechanical properties. J. Appl. Polym. Sci. 2008, 107, 1116–1123. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, Z.; Wang, C.; Ding, Q.; Jiang, J.; Mai, K. A novel montmorillonite with β-nucleating surface for enhancing β-crystallization of isotactic polypropylene. Composites Part A 2013, 49, 1–8. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Q.; Wang, K.; Deng, H.; Fu, Q. Combined effect of β-nucleating agent and multi-walled carbon nanotubes on polymorphic composition and morphology of isotactic polypropylene. J. Therm. Anal. Calorim. 2011, 107, 733–743. [Google Scholar] [CrossRef]
- Wang, M.; Lin, L.; Peng, Q.; Qu, W.; Li, H. Crystallization and mechanical properties of isotactic polypropylene/calcium carbonate nanocomposites with a stratified distribution of calcium carbonate. J. Appl. Polym. Sci. 2014, 131, 39632. [Google Scholar] [CrossRef]
- Karami, Z.; Youssefi, M.; Borhani, S. The effects of UV irradiation exposure on the structure and properties of polypropylene/ZnO nanocamposite fibers. Fiber. Polym. 2013, 14, 1627–1634. [Google Scholar] [CrossRef]
- Liu, M.; Guo, B.; Du, M.; Chen, F.; Jia, D. Halloysite nanotubes as a novel β-nucleating agent for isotactic polypropylene. Polymer 2009, 50, 3022–3030. [Google Scholar] [CrossRef]
- Mohd Zaini, N.A.; Ismail, H.; Rusli, A. Short review on sepiolite-filled polymer nanocomposites. Polym.-Plast. Technol. Eng. 2017, 56, 1665–1679. [Google Scholar] [CrossRef]
- Manchanda, B.; Vimal, K.K.; Kapur, G.S.; Kant, S.; Choudhary, V. Effect of sepiolite on nonisothermal crystallization kinetics of polypropylene. J. Mater. Sci. 2016, 51, 9535–9550. [Google Scholar] [CrossRef]
- Manchanda, B.; Kakkarakkal Kottiyath, V.; Kapur, G.S.; Kant, S.; Choudhary, V. Morphological studies and thermo-mechanical behavior of polypropylene/sepiolite nanocomposites. Polym. Compos. 2017, 38, E285–E294. [Google Scholar] [CrossRef]
- Ma, J.; Bilotti, E.; Peijs, T.; Darr, J.A. Preparation of polypropylene/sepiolite nanocomposites using supercritical CO2 assisted mixing. Eur. Polym. J. 2007, 43, 4931–4939. [Google Scholar] [CrossRef]
- He, M.; Cao, W.C.; Wang, L.J.; Wilkie, C.A. Synergistic effects of organo-sepiolite and zinc borate on the fire retardancy of polypropylene. Polym. Adv. Technol. 2013, 24, 1081–1088. [Google Scholar] [CrossRef]
- Chang, Y.; Liu, Z.; Fu, Z.; Wang, C.; Dai, Y.; Peng, R.; Hu, X. Preparation and characterization of one-dimensional core−shell sepiolite/polypyrrole nanocomposites and effect of organic modification on the electrochemical properties. Ind. Eng. Chem. Res. 2014, 53, 38–47. [Google Scholar] [CrossRef]
- Xie, S.; Zhang, S.; Wang, F.; Yang, M.; Séguéla, R.; Lefebvre, J.-M. Preparation, structure and thermomechanical properties of nylon-6 nanocomposites with lamella-type and fiber-type sepiolite. Compos. Sci. Technol. 2007, 67, 2334–2341. [Google Scholar] [CrossRef]
- Liao, J.; Brosse, N.; Pizzi, A.; Hoppe, S.; Xi, X.; Zhou, X. Polypropylene blend with polyphenols through dynamic vulcanization: Mechanical, rheological, crystalline, thermal, and UV protective property. Polymers 2019, 11, 1108. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Fang, J.; Zheng, M.; Luo, Y.; Wang, X.; Xu, L.; Zhang, C. Morphology evolution and rheological behaviors of PP/SR thermoplastic vulcanizate. Polymers 2019, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Rezanavaz, R.; Razavi Aghjeh, M.K.; Babaluo, A.A. Rheology, morphology, and thermal behavior of HDPE/clay nanocomposites. Polym. Compos. 2010, 31, 1028–1036. [Google Scholar] [CrossRef]
- Zhang, M.; Sundararaj, U. Thermal, rheological, and mechanical behaviors of LLDPE/PEMA/clay nanocomposites: Effect of interaction between polymer, compatibilizer, and nanofiller. Macromol. Mater. Eng. 2006, 291, 697–706. [Google Scholar] [CrossRef]
- Hoffmann, B.; Dietricha, C.; Thomann, R.; Friedrich, C.; Mülhaupt, R. Morphology and rheology of polystyrene nanocomposites based upon organoclay. Macromol. Rapid Commun. 2000, 21, 57–61. [Google Scholar] [CrossRef]
- Galgali, G.; Ramesh, C.; Lele, A. A rheological study on the kinetics of hybrid formation in polypropylene nanocomposites. Macromolecules 2001, 34, 852–858. [Google Scholar] [CrossRef]
- Lim, Y.T.; Park, O.O. Phase morphology and rheological behavior of polymer/layered silicate nanocomposites. Rheol. Acta. 2001, 40, 220–229. [Google Scholar] [CrossRef]



| Sample Designation | PP (g) | ABS (g) | O-Sep (g) |
|---|---|---|---|
| PP | 60 | — | — |
| PP/ABS | 48.00 | 12.00 | — |
| PP/ABS/O-Sep1 | 47.52 | 11.88 | 0.60 |
| PP/ABS/O-Sep3 | 46.56 | 11.64 | 1.80 |
| PP/ABS/O-Sep5 | 45.60 | 11.40 | 3.00 |
| PP/ABS/O-Sep7 | 44.64 | 11.16 | 4.20 |
| Sample | Tensile Strength (MPa) | Tensile Modulus (MPa) | Flexural Strength (MPa) | Flexural Modulus (MPa) | Impact Strength (kJ/m2) |
|---|---|---|---|---|---|
| PP | 36.3 ± 0.6 | 1186 ± 17 | 42.6 ± 0.6 | 1839 ± 45 | 4.535 ± 0.233 |
| PP/ABS | 38.6 ± 0.5 | 1214 ± 21 | 41.4 ± 1.2 | 1723 ± 38 | 5.497 ± 0.285 |
| PP/ABS/O-Sep1 | 40.8 ± 0.9 | 1229 ± 16 | 44.3 ± 1.5 | 1828 ± 42 | 5.834 ± 0.205 |
| PP/ABS/O-Sep3 | 41.3 ± 0.8 | 1291 ± 14 | 47.2 ± 1.4 | 1739 ± 35 | 6.347 ± 0.267 |
| PP/ABS/O-Sep5 | 42.0 ± 1.1 | 1331 ± 13 | 45.4 ± 0.8 | 1919 ± 47 | 6.392 ± 0.306 |
| PP/ABS/O-Sep7 | 41.3 ± 0.9 | 1371 ± 23 | 45.8 ± 1.1 | 1612 ± 28 | 6.152 ± 0.254 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Li, T.; Xie, S.; Wu, X.; Huang, W.; Tian, Q.; Tu, C.; Yan, W. Influence of Organo-Sepiolite on the Morphological, Mechanical, and Rheological Properties of PP/ABS Blends. Polymers 2019, 11, 1493. https://doi.org/10.3390/polym11091493
Wang K, Li T, Xie S, Wu X, Huang W, Tian Q, Tu C, Yan W. Influence of Organo-Sepiolite on the Morphological, Mechanical, and Rheological Properties of PP/ABS Blends. Polymers. 2019; 11(9):1493. https://doi.org/10.3390/polym11091493
Chicago/Turabian StyleWang, Kui, Tiantian Li, Sen Xie, Xianshun Wu, Weijiang Huang, Qin Tian, Chunyun Tu, and Wei Yan. 2019. "Influence of Organo-Sepiolite on the Morphological, Mechanical, and Rheological Properties of PP/ABS Blends" Polymers 11, no. 9: 1493. https://doi.org/10.3390/polym11091493





