Synthesis, Crystal Structures, and Spectroscopic Characterization of Bis-aldehyde Monomers and Their Electrically Conductive Pristine Polyazomethines
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Physical Characterizations
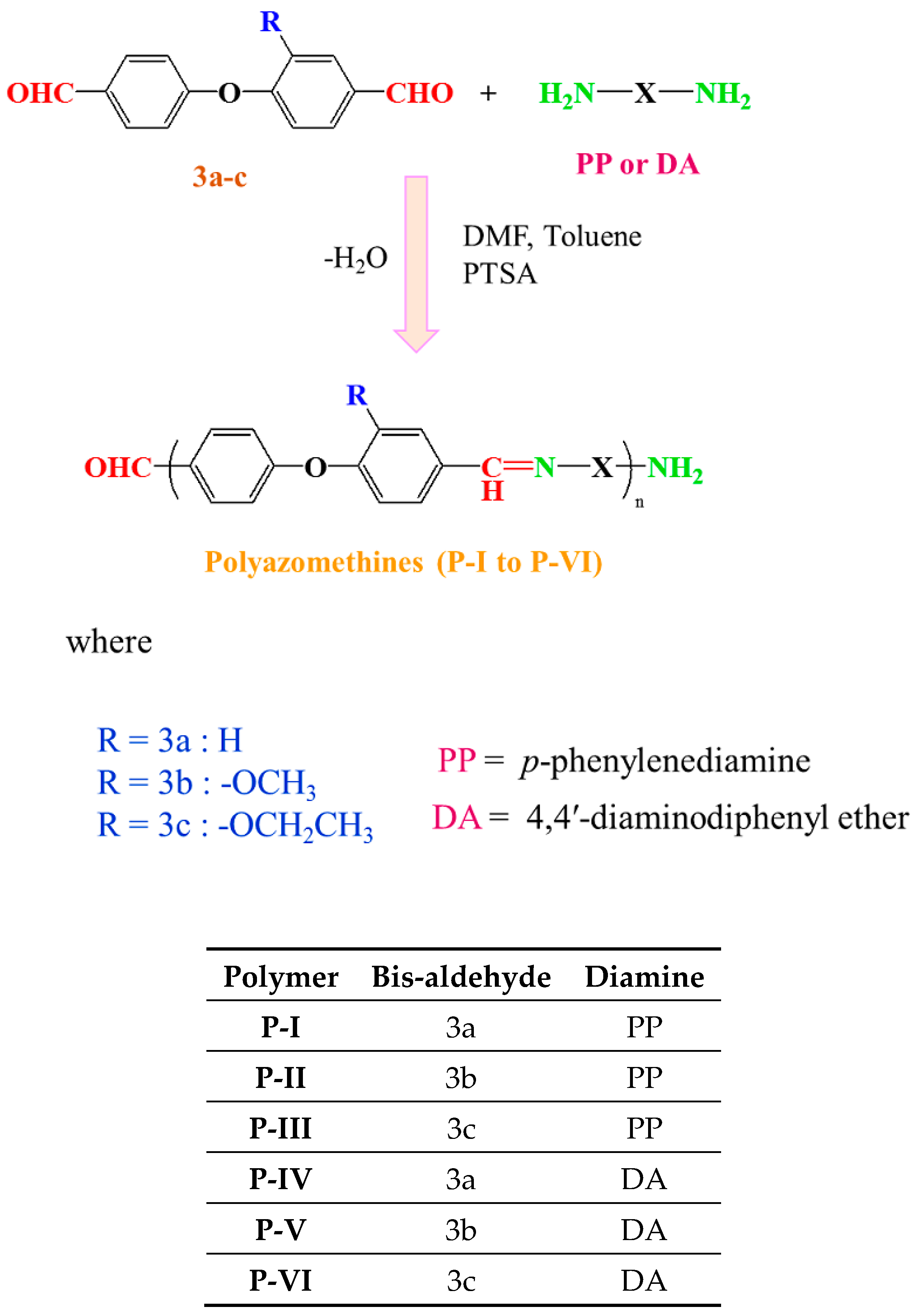
2.3. Syntheses of Monomers (3a to 3c)
4-(4′-.Formyl-phenoxy)benzaldehyde (3a)
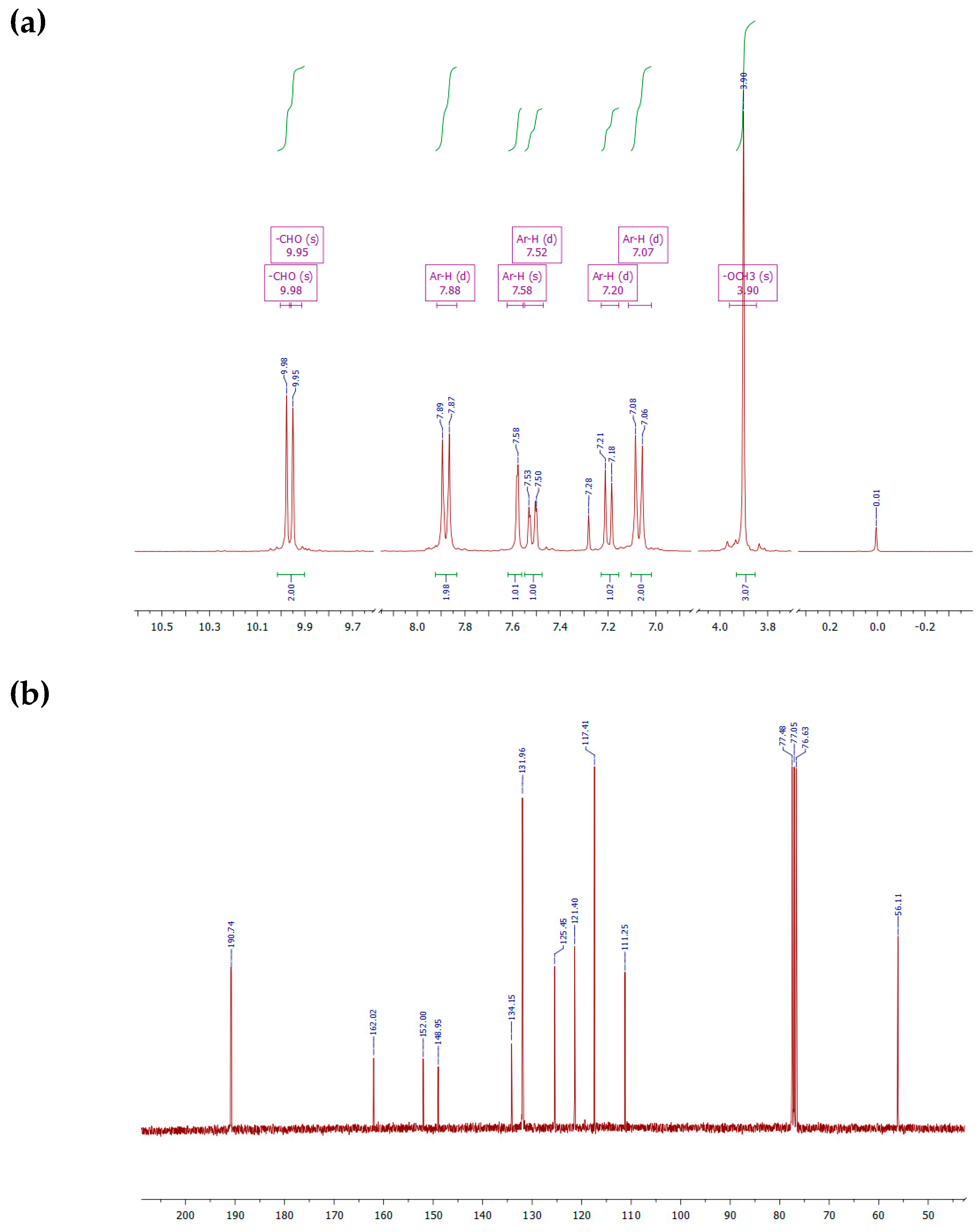
3-Met.hoxy-4-(4′-formyl-phenoxy)benzaldehyde (3b)
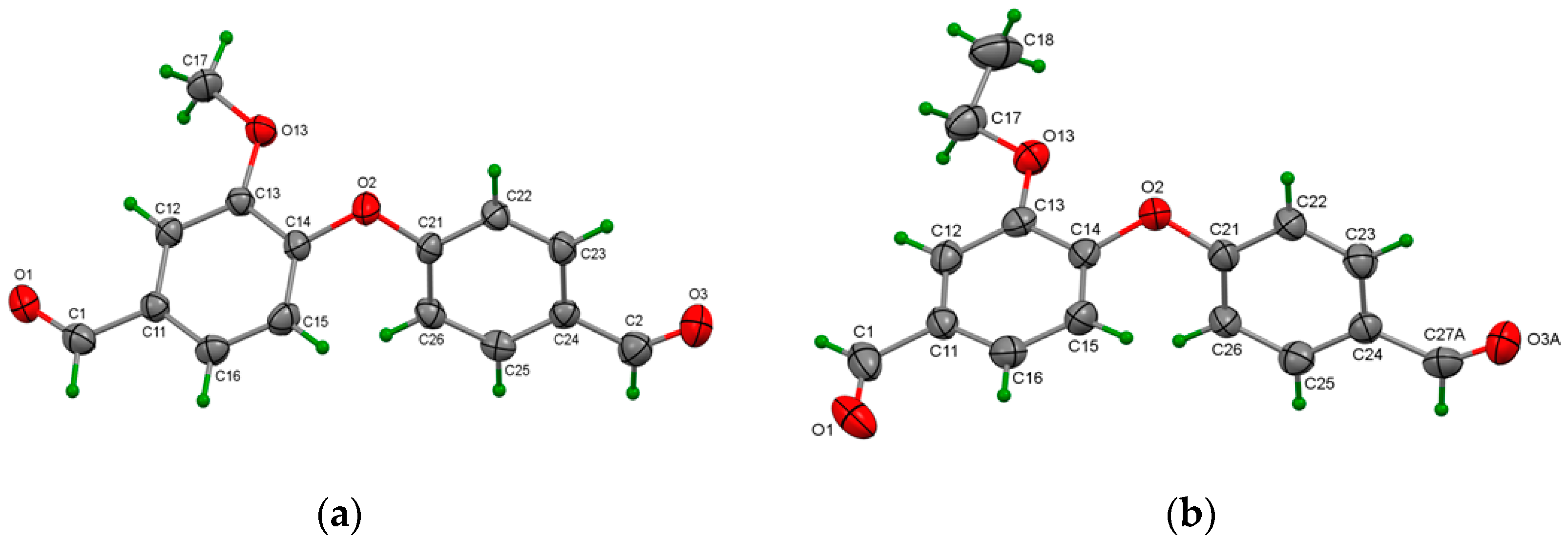
X-ray Crystallographic Data of 3b
3-Eth.oxy-4-(4′-formyl-phenoxy)benzaldehyde (3c)
X-ray Crystallographic Data of 3c
2.4. Syntheses of Polyazomethine Polymers (P-I to P-VI)
3. Results and Discussion
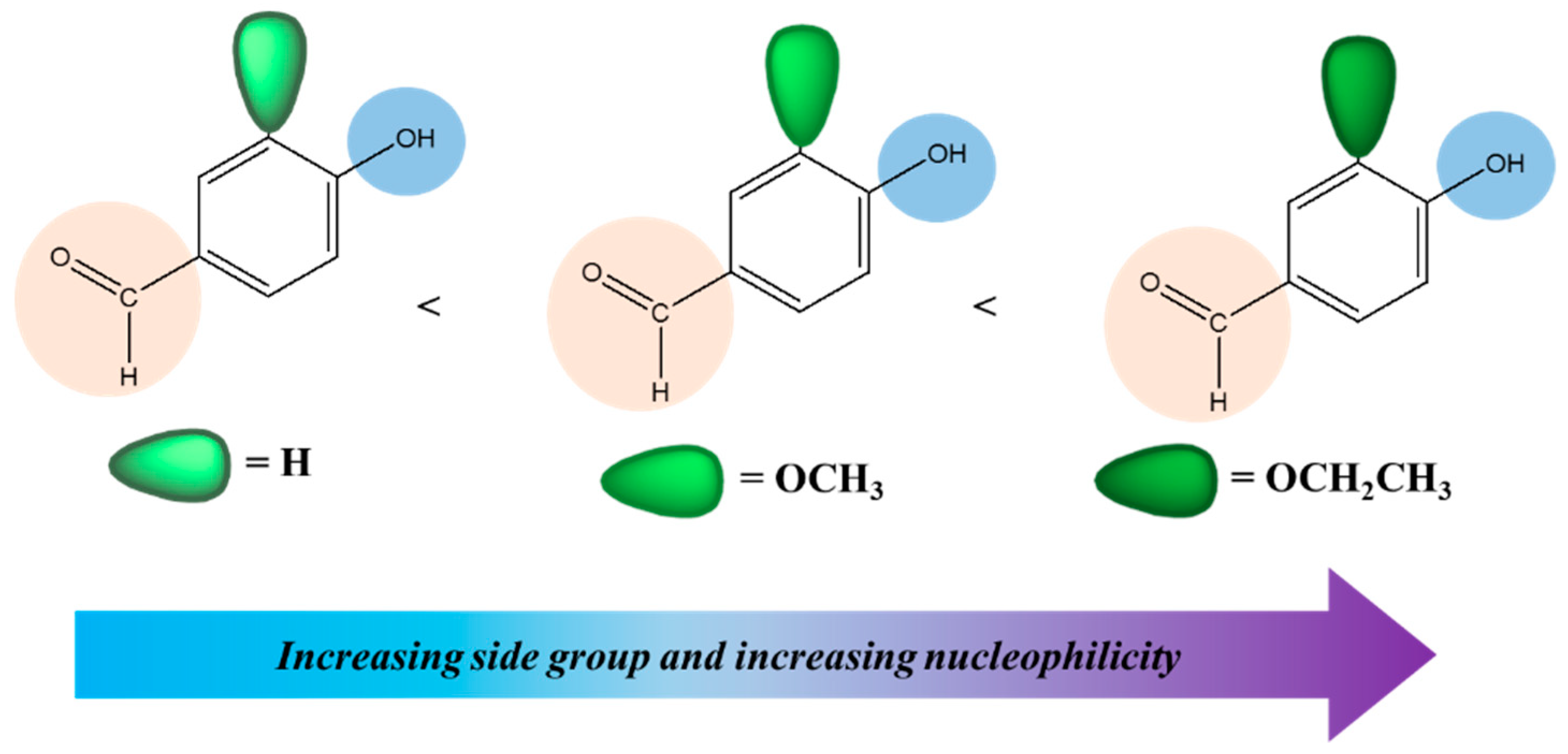
3.1. Syntheses and Characterization of Monomers (3a, 3b, and 3c)
3.2. Syntheses of Polyazomethines
3.3. Powder X-ray Diffraction
3.4. UV–Vis and Photoluminescence Measurements
3.5. Thermal Properties
3.6. Electrical Conductivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yen, H.J.; Liou, G.S. Recent advances in triphenylamine-based electrochromic derivatives and polymers. Polym. Chem. 2018, 9, 3001–3018. [Google Scholar] [CrossRef]
- Metzger, R.M. Unimolecular electronics. Chem. Rev. 2015, 115, 5056–5115. [Google Scholar] [CrossRef] [PubMed]
- Yıldırım, M.; Avcı, A. Synthesis and characterization of fluorescent polyphenol species derived from methyl substituted aminopyridine based Schiff bases: The effect of substituent position on optical, electrical, electrochemical, and fluorescence properties. Synth. Met. 2010, 160, 911–920. [Google Scholar]
- Yang, C.J.; Jenekhe, S.A. Conjugated aromatic polyimines. 2. synthesis, structure, and properties of new aromatic polyazomethines. Macromolecules 1995, 28, 1180–1196. [Google Scholar] [CrossRef]
- Jenekhe, S.A.; Yang, C.J.; Vanherzeele, H.; Meth, J.S. Cubic nonlinear optics of polymer thin films. Effects of structure and dispersion on the nonlinear optical properties of aromatic Schiff base polymers. Chem. Mater. 1991, 3, 985–987. [Google Scholar] [CrossRef]
- Dineshkumar, S.; Muthusamy, A.; Chandrasekaran, J. Temperature and frequency dependent dielectric properties of electrically conducting oxidatively synthesized polyazomethines and their structural, optical, and thermal characterizations. J. Mol. Struct. 2017, 1128, 730–740. [Google Scholar] [CrossRef]
- More, A.S.; Sane, P.S.; Patil, A.S.; Wadgaonkar, P.P. Synthesis and characterization of aromatic polyazomethines bearing pendant pentadecyl chains. Polym. Degrad. Stab. 2010, 95, 1727–1735. [Google Scholar] [CrossRef]
- Baran, Y.N.; Saçak, M. Preparation of highly thermally stable and conductive Schiff base polymer: Molecular weight monitoring and investigation of antimicrobial properties. J. Mol. Struct. 2018, 1163, 22–32. [Google Scholar] [CrossRef]
- Jarząbek, B.; Kaczmarczyk, B.; Jurusik, J.; Siwy, M.; Weszka, J. Optical properties of thin films of polyazomethine with flexible side chains. J. Non-Cryst. Solids 2013, 375, 13–18. [Google Scholar] [CrossRef]
- Jarzabek, B.; Weszka, J.; Hajduk, B.; Jurusik, J.; Domanski, M.; Cisowski, J. A study of optical properties and annealing effect on the absorption edge of pristine- and iodine-doped polyazomethine thin films. Synth. Met. 2011, 161, 969–975. [Google Scholar] [CrossRef]
- Constantin, C.P.; Damaceanu, M.D. In-depth investigation of the optical effects in rationally designed phenoxazine-based polyazomethines with activated quenched fluorescence. J. Phys. Chem. C 2017, 121, 6300–6313. [Google Scholar] [CrossRef]
- Khalid, N.; Bibi, A.; Akhtar, K.; Mustafa, K.; Khan, M.; Saeed, N. New blue light emissive polyazomethine(s) containing bromo-triphenyl units: Synthesis and photophysics. Polym. Plast. Technol. Eng. 2019, 58, 419–426. [Google Scholar] [CrossRef]
- Chen, X.; Gong, F.; Cao, Z.; Zou, W.; Gu, T. Highly cysteine-selective fluorescent nanoprobes based on ultrabright and directly synthesized carbon quantum dots. Anal. Bioanal. Chem. 2018, 410, 2961–2970. [Google Scholar] [CrossRef] [PubMed]
- Sakai, N.; Matile, S. Conjugated polyimine dynamers as phase-sensitive membrane probes. J. Am. Chem. Soc. 2018, 140, 11438–11443. [Google Scholar] [CrossRef] [PubMed]
- Mallet, C.; Le Borgne, M.; Starck, M.; Skene, W.G. Unparalleled fluorescence of a polyazomethine prepared from the self-condensation of an automer and its potential use as a fluorimetric sensor for explosive detection. Polym. Chem. 2013, 4, 250–254. [Google Scholar] [CrossRef]
- Şenol, D.; Kolcu, F.; Kaya, İ. Synthesis, characterization, electrical conductivity and fluorescence properties of polyimine bearing phenylacetylene units. J. Fluoresc. 2016, 26, 1579–1590. [Google Scholar] [CrossRef] [PubMed]
- Kaya, I.; Yildirim, M.; Aydin, A.; Enol, D. Synthesis and characterization of fluorescent graft fluorene-co-polyphenol derivatives: The effect of substituent on solubility, thermal stability, conductivity, optical and electrochemical properties. React. Funct. Polym. 2010, 70, 815–826. [Google Scholar] [CrossRef]
- Salunkhe, P.H.; Patil, Y.S.; Patil, V.B.; Navale, Y.H.; Dhole, I.A.; Ubale, V.P.; Maldar, N.N.; Ghanwat, A.A. Synthesis and characterization of conjugated porous polyazomethines with excellent electrochemical energy storage performance. J. Polym. Res. 2018, 25, 147. [Google Scholar] [CrossRef]
- Bolduc, A.; Al Ouahabi, A.; Mallet, C.; Skene, W.G. Insight into the isoelectronic character of azomethines and vinylenes using representative models: A spectroscopic and electrochemical Study. J. Org. Chem. 2013, 78, 9258–9269. [Google Scholar] [CrossRef]
- Schlu, A.D. The tenth anniversary of Suzuki polycondensation (SPC). J. Polym. Sci. A 2001, 39, 1533–1556. [Google Scholar]
- Pinto, M.R.; Hu, B.; Karasz, F.E.; Akcelrud, L. Emitting polymers containing cyano groups. Synthesis and photophysical properties of a fully conjugated polymer obtained by Wittig reaction. Polymer 2000, 41, 8095–8102. [Google Scholar] [CrossRef]
- Carsten, B.; He, F.; Son, H.J.; Xu, T.; Yu, L. Stille polycondensation for synthesis of functional materials. Chem. Rev. 2011, 111, 1493–1528. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, X.; Tian, H.; Zhang, J.; Yan, D.; Geng, Y.; Wang, F. High mobility ambipolar diketopyrrolopyrrole-based conjugated polymer synthesized via direct arylation polycondensation. Adv. Mater. 2015, 27, 6753–6759. [Google Scholar] [CrossRef] [PubMed]
- Facchetti, A.; Vaccaro, L.; Marrocchi, A. Semiconducting polymers prepared by direct arylation polycondensation. Angew. Chem. Int. Ed. 2012, 51, 3520–3523. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, S.; Allard, S.; Zilberberg, K.; Riedl, T.; Scherf, U. Direct arylation polycondensation as simplified alternative for the synthesis of conjugated (co)polymers. Prog. Polym. Sci. 2013, 38, 1805–1814. [Google Scholar] [CrossRef]
- Scherf, U.; List, E.J.W. Semiconducting polyfluorenes—Towards reliable structure–property relationships. Adv. Mater. 2002, 14, 477–487. [Google Scholar] [CrossRef]
- Yang, B.; Zhao, Y.; Wei, Y.; Fu, C.; Tao, L. The Ugi reaction in polymer chemistry: Syntheses, applications and perspectives. Polym. Chem. 2015, 6, 8233–8239. [Google Scholar] [CrossRef]
- Kim, H.C.; Kim, J.S.; Kim, K.S.; Park, H.K.; Baek, S.; Ree, M. Synthesis and characterization of new, soluble polyazomethines bearing fluorene and carbazole units in the backbone and solubility-improving moieties in the side group. J. Polym. Sci. A 2004, 42, 825–834. [Google Scholar] [CrossRef]
- Iwan, A.; Boharewicz, B.; Parafiniuk, K.; Tazbir, I.; Gorecki, L.; Sikora, A.; Filapek, M.; Schab-Balcerzak, E. New air-stable aromatic polyazomethines with triphenylamine or phenylenevinylene moieties towards photovoltaic application. Synth. Met. 2014, 195, 341–349. [Google Scholar] [CrossRef]
- Wojtkiewicz, J.; Iwan, A.; Pilch, M.; Boharewicz, B.; Wójcik, K.; Tazbir, I.; Kaminska, M. Towards designing polymers for photovoltaic applications: A DFT and experimental study of polyazomethines with various chemical structures. Spectrochim. Acta A 2017, 181, 208–217. [Google Scholar] [CrossRef]
- Kaya, I.; Kilavuz, E.; Temizkan, K. Synthesis, characterization and quantum yields of multichromic poly(azomethine)s containing carbazole unit. Arab. J. Chem. 2017. [Google Scholar] [CrossRef]
- Kaya, İ.; Yılmaz, T. Preparation and characterization of poly(azomethines) containing ether and methylene bridges: Photophysical, electrochemical, conductivity and thermal properties. J. Fluoresc. 2017, 27, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Baran, Y.N.; Karakişla, M.; Demir, H.Ö.; Saçak, M. Synthesis, characterization, conductivity and antimicrobial study of a novel thermally stable polyphenol containing azomethine group. J. Mol. Struct. 2016, 1123, 153–161. [Google Scholar] [CrossRef]
- Bronnikov, S.; Kostromin, S.; Saprykina, N.; Asandulesa, M.; Podshivalov, A.; Cozan, V. Morphology and electrical conductivity of polyazomethine/hybrid carbon nanofillers composites. Fuller. Nanotub. Carbon Nanostruct. 2018, 26, 709–714. [Google Scholar] [CrossRef]
- Dineshkumar, S.; Muthusamy, A.; Chitra, P.; Anand, S. Synthesis, characterization, optical and electrical properties of thermally stable polyazomethines derived from 4,4′-oxydianiline. J. Adhes. Sci. Technol. 2015, 29, 2605–2621. [Google Scholar] [CrossRef]
- Diaz, F.R.; Moreno, J.; Tagle, L.H.; East, G.A.; Radic, D. Synthesis, characterization and electrical properties of polyimines derived from selenophene. Synth. Met. 1999, 100, 187–193. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; Van De Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Thomas, I.R.; Bruno, I.J.; Cole, J.C.; MacRae, C.F.; Pidcock, E.; Wood, P.A. WebCSD: The online portal to the Cambridge structural database. J. Appl. Crystallogr. 2010, 43, 362–366. [Google Scholar] [CrossRef]
- Allen, F.H. The Cambridge structural database: A quarter of a million crystal structures and rising. Acta Crystallogr. Sect. B 2002, 58, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Goyal, K.M.; Chauhan, P. GC-MS technique and its analytical applications in science and technology. J. Anal. Bioanal. Tech. 2014, 5, 222. [Google Scholar] [CrossRef]
- Hafeez, A.; Akhter, Z.; Gallagher, J.F.; Siddiqui, H.M. Liquid phase synthesis of aromatic poly(azomethine)s, their physicochemical properties, and measurement of ex situ electrical conductivity of pelletized powdered samples. Des. Monomers Polym. 2017, 20, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Kaya, İ.; Ayten, B.; Şenol, D. Syntheses of poly(phenoxy-imine)s anchored with carboxyl group: Characterization and photovoltaic studies. Opt. Mater. 2018, 78, 421–431. [Google Scholar] [CrossRef]
- Şenol, D.; Kaya, İ. Synthesis and characterization of azomethine polymers containing ether and ester groups. J. Saudi Chem. Soc. 2017, 21, 505–516. [Google Scholar] [CrossRef] [Green Version]
- Gul, A.; Akhter, Z.; Siddiq, M.; Qureshi, R.; Bhatti, A.S. Synthesis and physicochemical characterization of poly(azomethine)esters containing aliphatic/aromatic moieties: Electrical studies complemented by DFT calculation. J. Appl. Polym. Sci. 2014, 131, 1–8. [Google Scholar] [CrossRef]

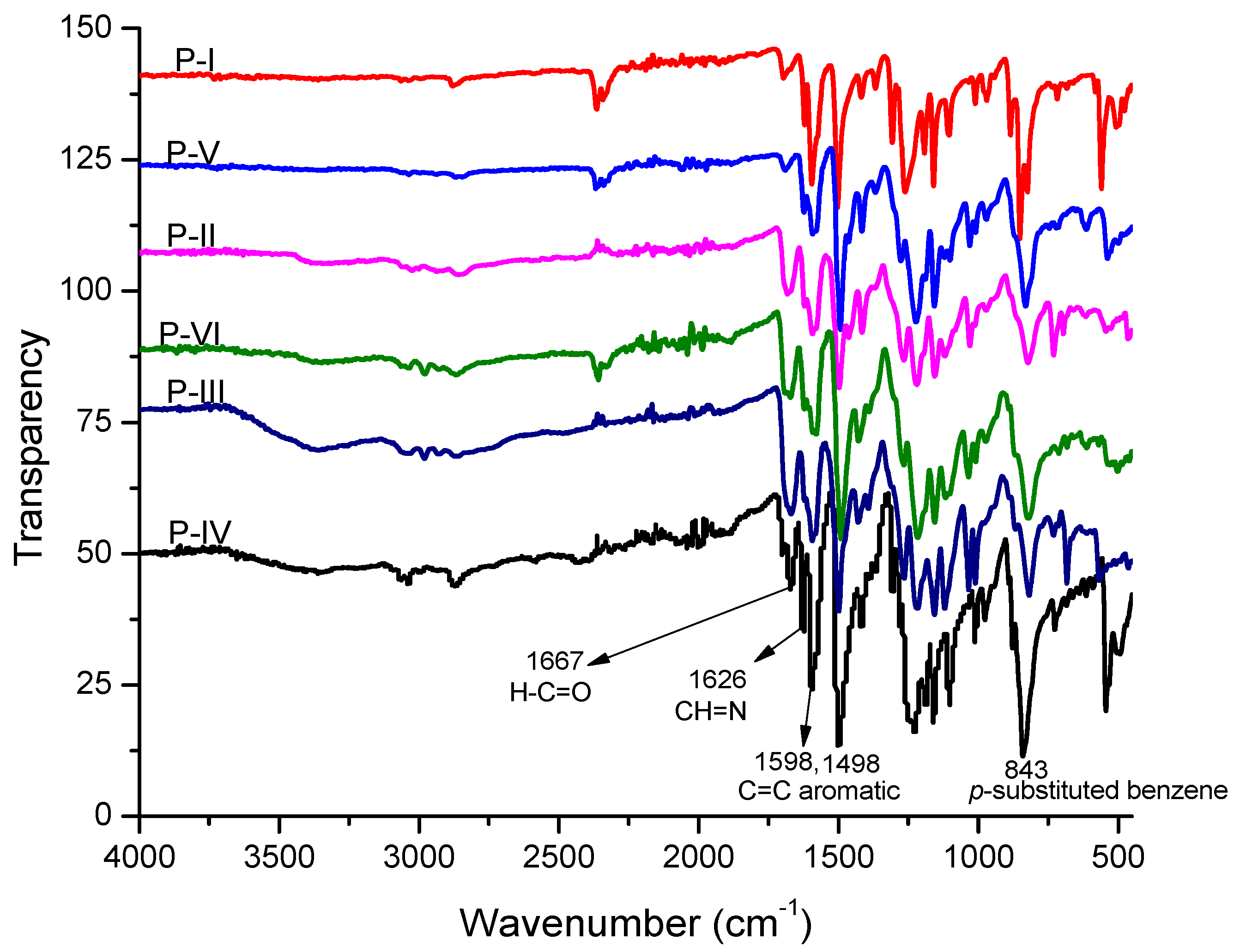
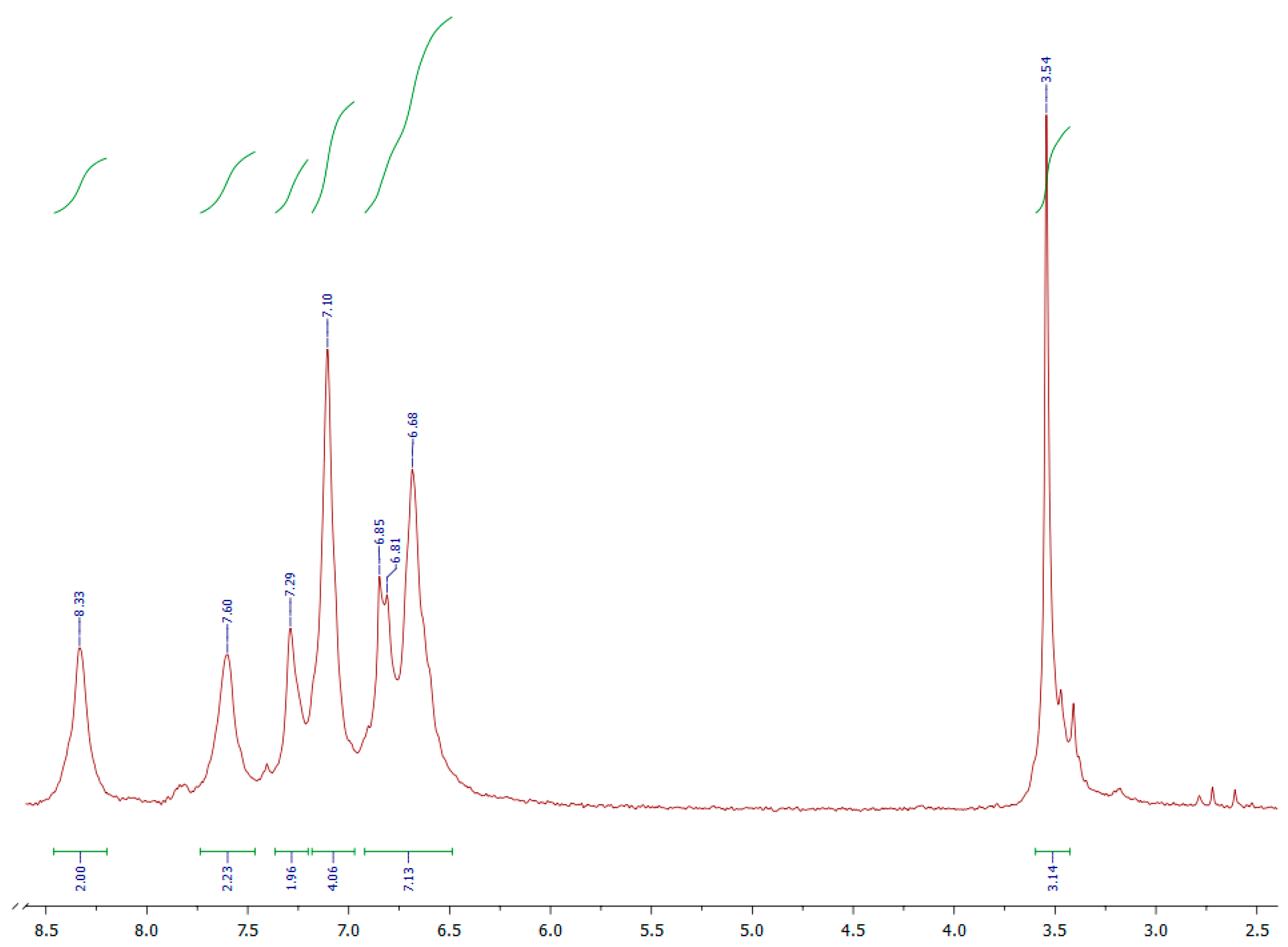

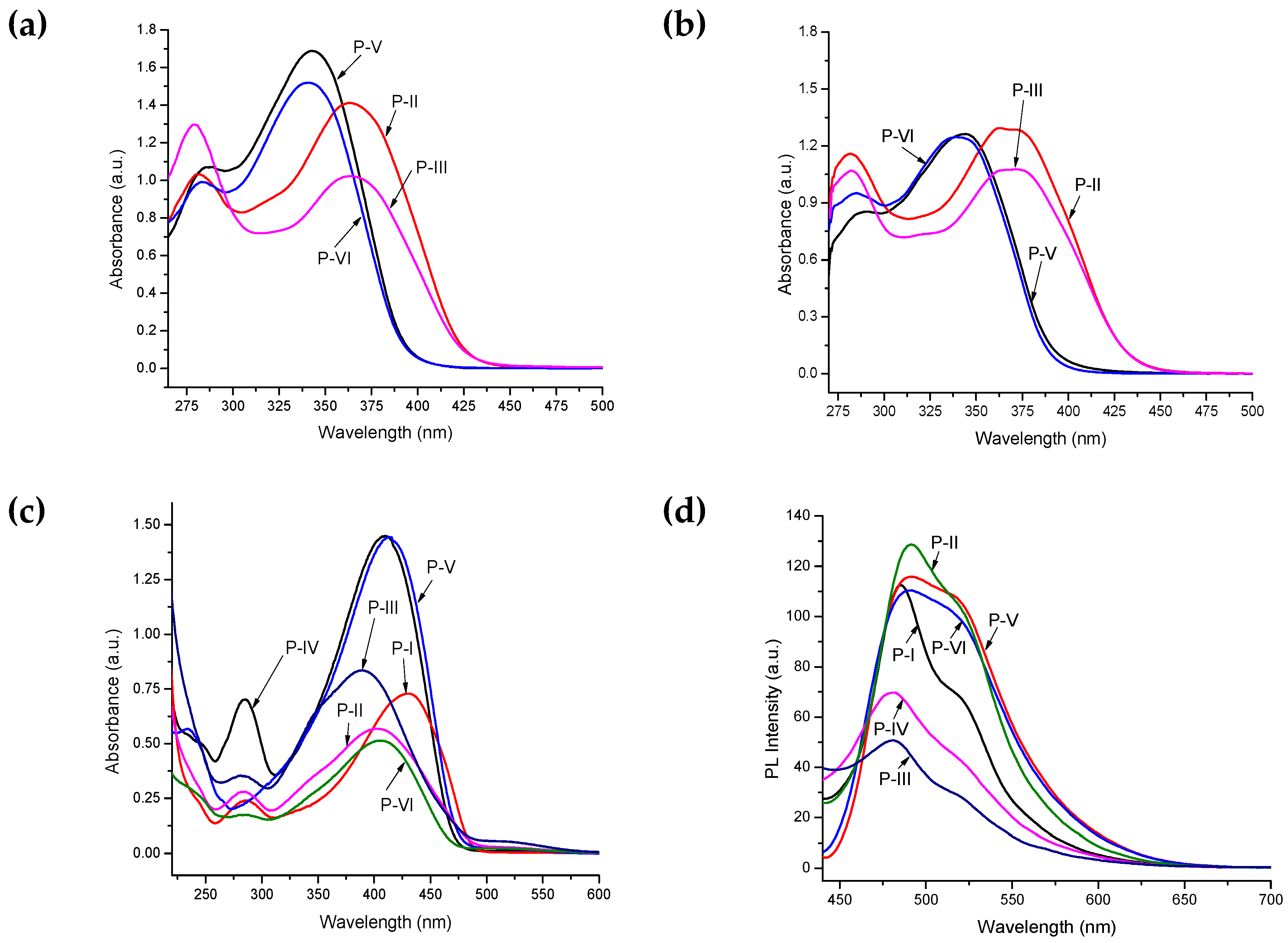
| Polymer | λmax (nm) a | λmax (nm) b | λmax (nm) c | λonset (nm) c | Eg (eV) c,d | PL λmax (nm) c | Stoke’s Shift (nm) |
|---|---|---|---|---|---|---|---|
| P-I | – | – | 432, 286 | 488 | 2.54 | 486 | 54 |
| P-II | 365, 281 | 372, 282 | 404, 283 | 487 | 2.55 | 491 | 87 |
| P-III | 365, 279 | 373, 282 | 391, 283 | 485 | 2.56 | 482 | 91 |
| P-IV | – | – | 411, 285 | 477 | 2.60 | 481 | 70 |
| P-V | 344, 285 | 345, 287 | 415 | 480 | 2.59 | 492 | 77 |
| P-VI | 341, 282 | 343, 284 | 408, 285 | 471 | 2.64 | 492 | 84 |
| Polymer | Ton (°C) | T20% (°C) | Tmax (°C) | TF (°C) | %Char Residue |
|---|---|---|---|---|---|
| P-I | 300 | 525 | 525 | 650 | 53.3 |
| P-II | 300 | 490 | 650 | 950 | 38.5 |
| P-III | 150 | 510 | 550 | 950 | 32 |
| P-IV | 450 | 520 | 530 | 650 | 46.6 |
| P-V | 360 | 430 | 475 | 630 | 57 |
| P-VI | 125 | 450 | 575 | 600 | 60 |
| Polymer | Resistance (R) × 103 Ω | Conductance (G) × 10−3 Ohm−1 | Conductivity (σ) Scm−1 |
|---|---|---|---|
| P-I | 4.74437 ± 1.07 | 0.210 | 5.1 × 10−5 |
| P-II | 6.08604 ± 1.49 | 0.164 | 4.0 × 10−5 |
| P-III | 3.83071 ± 0.81 | 0.261 | 6.4 × 10−5 |
| P-IV | 5.12437 ± 0.69 | 0.195 | 4.8 × 10−5 |
| P-V | 4.91478 ± 0.82 | 0.203 | 5.0 × 10−5 |
| P-VI | 5.28489 ± 0.63 | 0.198 | 4.8 × 10−5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafeez, A.; Akhter, Z.; Gallagher, J.F.; Khan, N.A.; Gul, A.; Shah, F.U. Synthesis, Crystal Structures, and Spectroscopic Characterization of Bis-aldehyde Monomers and Their Electrically Conductive Pristine Polyazomethines. Polymers 2019, 11, 1498. https://doi.org/10.3390/polym11091498
Hafeez A, Akhter Z, Gallagher JF, Khan NA, Gul A, Shah FU. Synthesis, Crystal Structures, and Spectroscopic Characterization of Bis-aldehyde Monomers and Their Electrically Conductive Pristine Polyazomethines. Polymers. 2019; 11(9):1498. https://doi.org/10.3390/polym11091498
Chicago/Turabian StyleHafeez, Abdul, Zareen Akhter, John F. Gallagher, Nawazish Ali Khan, Asghari Gul, and Faiz Ullah Shah. 2019. "Synthesis, Crystal Structures, and Spectroscopic Characterization of Bis-aldehyde Monomers and Their Electrically Conductive Pristine Polyazomethines" Polymers 11, no. 9: 1498. https://doi.org/10.3390/polym11091498






