Effect of Conductive Carbon Black on Mechanical Properties of Aqueous Polymer Binders for Secondary Battery Electrode
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Samples
2.2. Test Methods
3. Results and Discussion
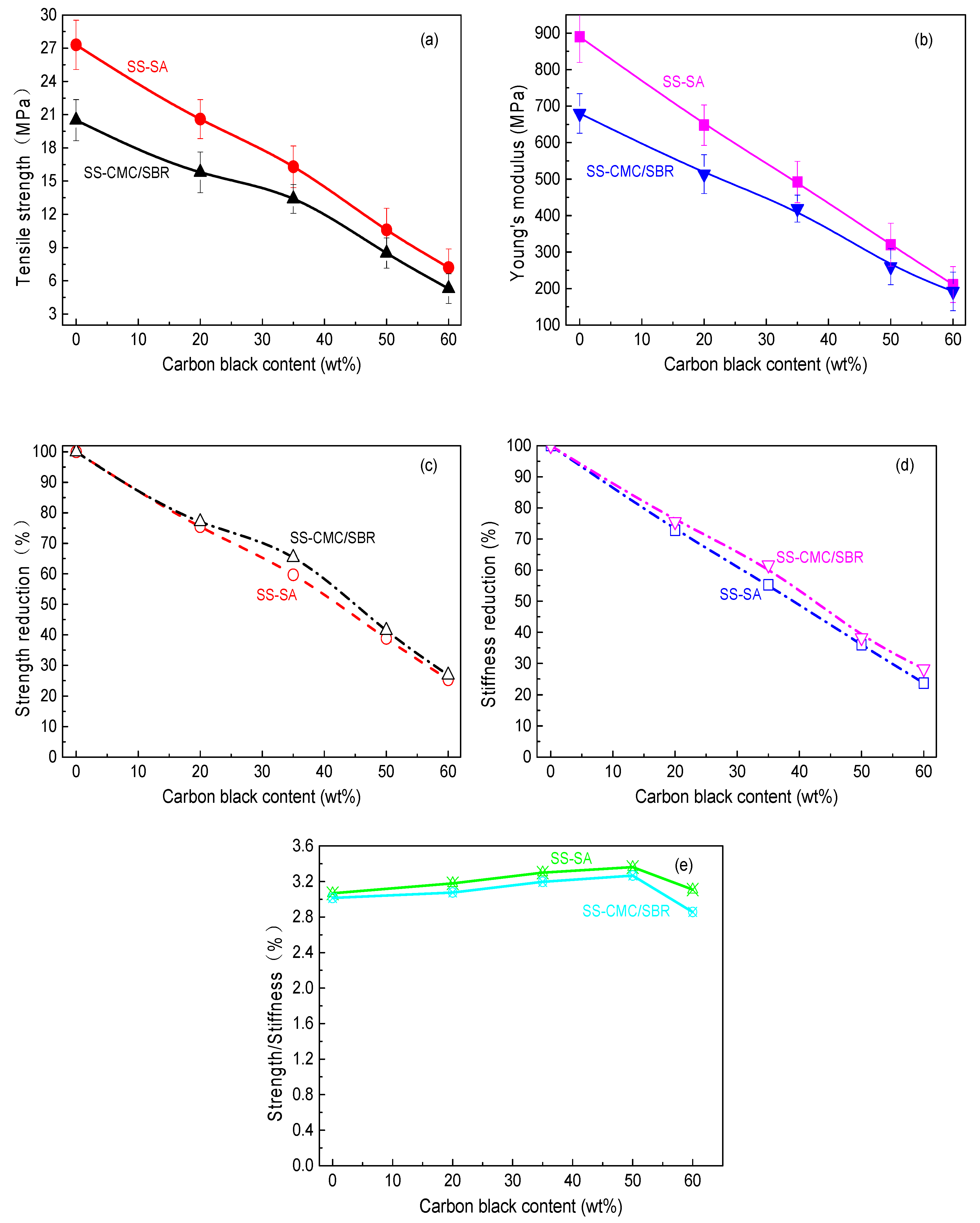
3.1. Uniaxial Tensile Properties of Cured BCC Film
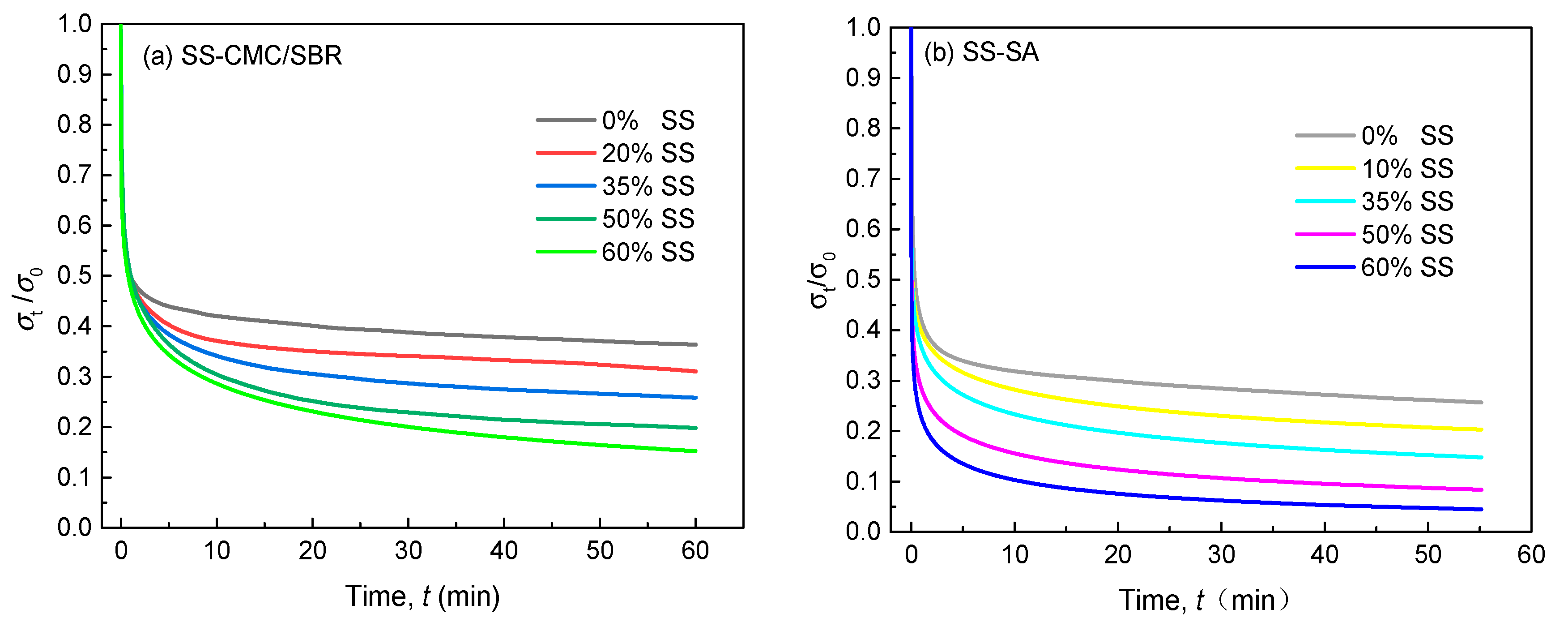
3.2. Tensile Stress Relaxation Behaviors of Cured BCC Film
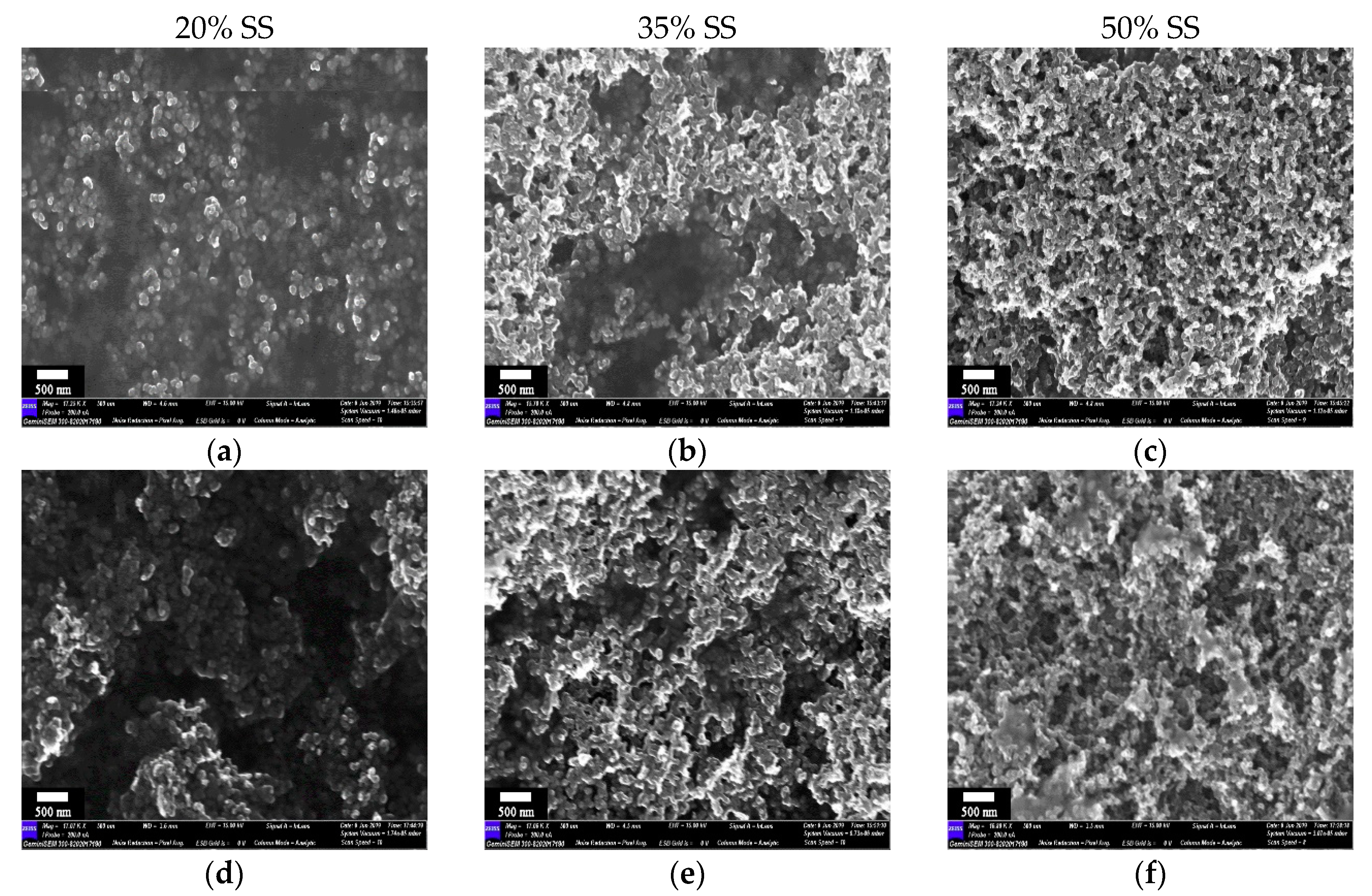
3.3. Microstructure of Cured BCC Films
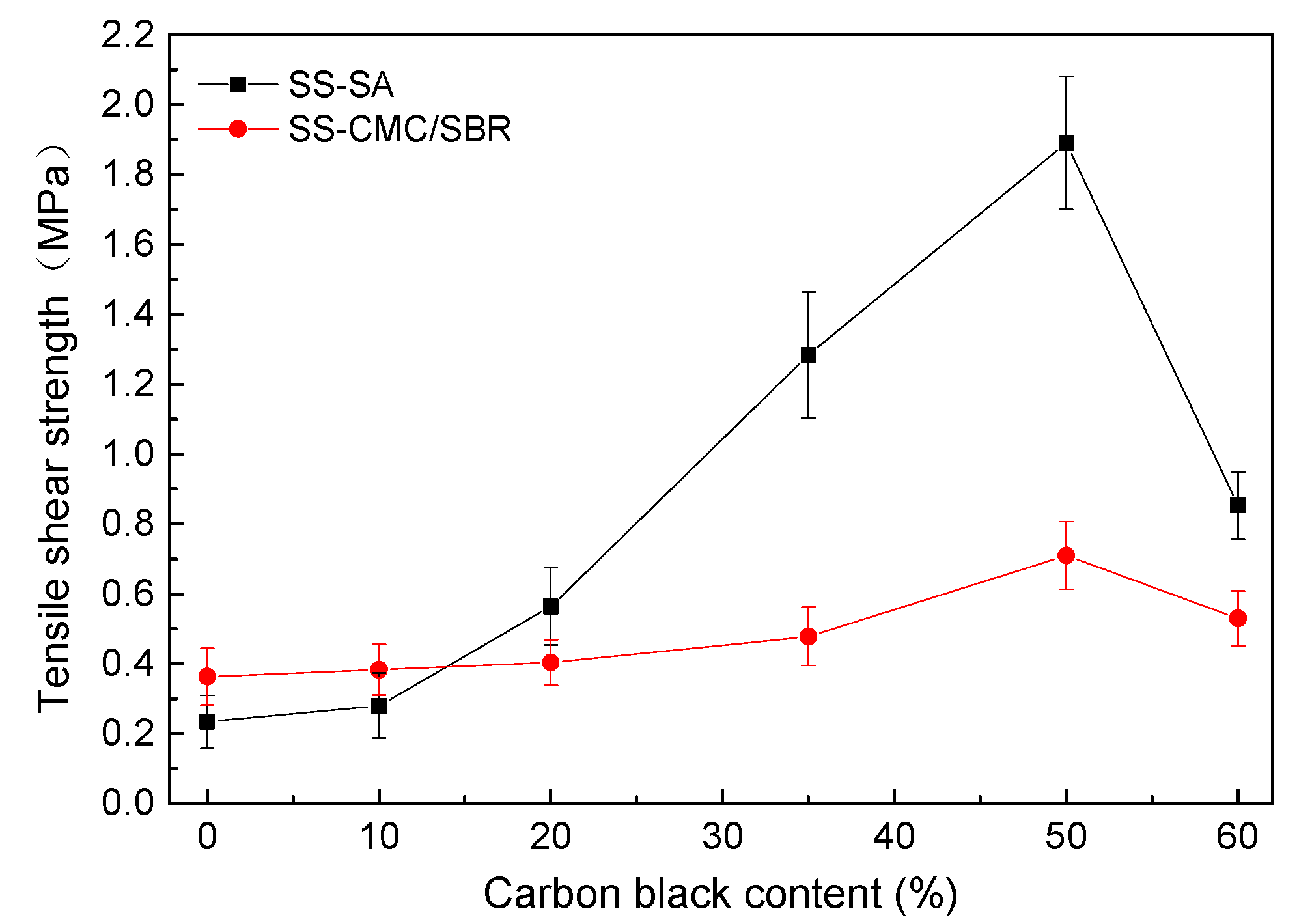
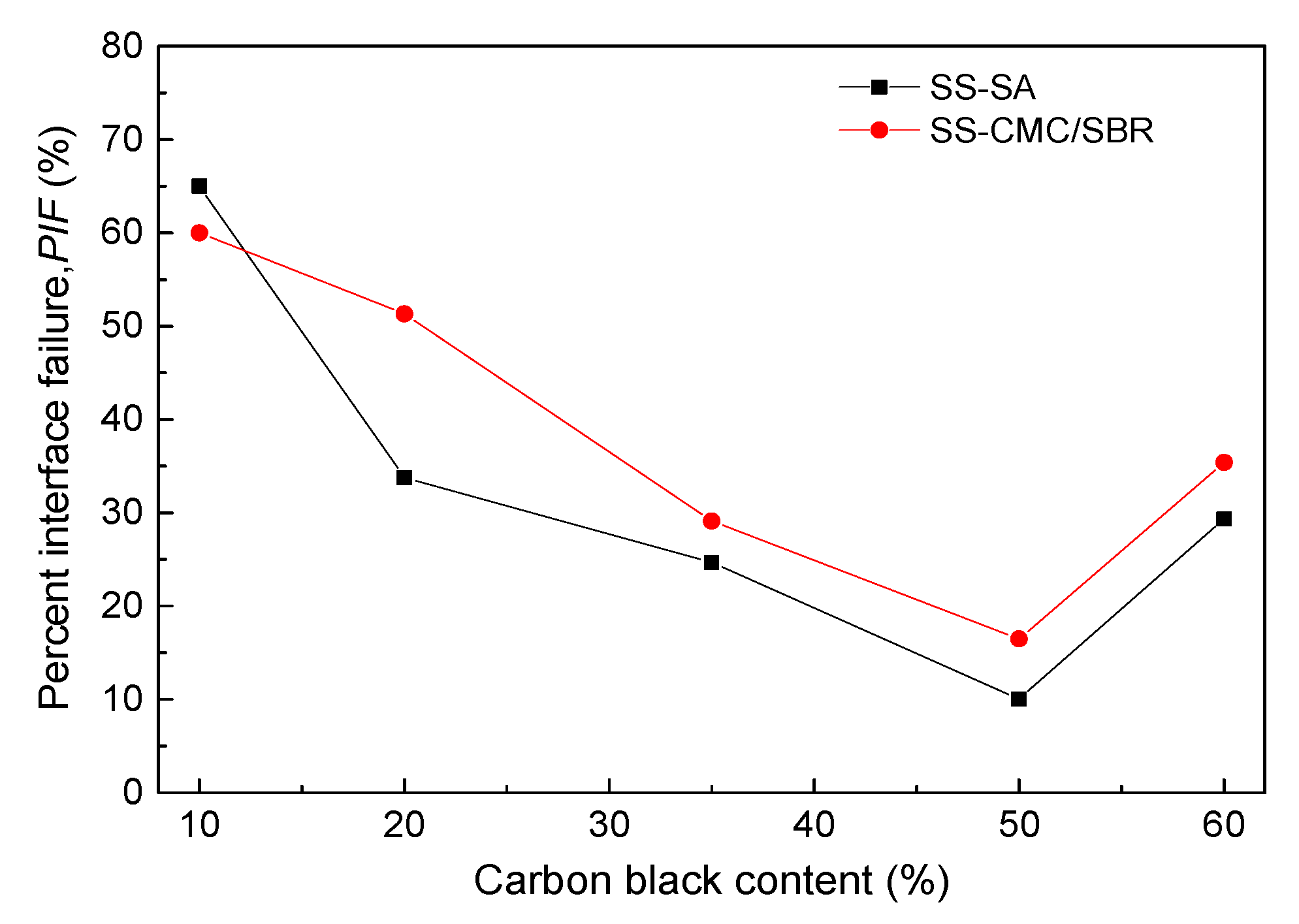
3.4. Bonding Performance of BCC Adhesive
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chou, S.L.; Pan, Y.D.; Wang, J.Z.; Liu, H.K.; Dou, S.X. Small things make a big difference: Binder effects on the performance of Li and Na batteries. Phys. Chem. Chem. Phys. 2014, 16, 20347–20359. [Google Scholar] [CrossRef] [PubMed]
- Nirmale, T.C.; Kale, B.B.; Varma, A.J. A review on cellulose and lignin based binders and electrodes: Small steps towards a sustainable lithium ion battery. Int. J. Biol. Macromol. 2017, 103, 1032–1043. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.F.; Naguib, M.; Du, Z.; Stacy, E.W.; Li, B.; Hong, T.; Xing, K.; Voylov, D.; Li, J.; Wood, D.L.; et al. Effect of binder architecture on the performance of silicon/graphite composite anodes for lithium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 43470–43478. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Paik, U.; Hackley, V.A.; Choi, Y.-M. Effect of poly(acrylic acid) on adhesion strength and electrochemical performance of natural graphite negative electrode for lithium-ion batteries. J. Power Sources 2006, 161, 612–616. [Google Scholar] [CrossRef]
- Shin, D.; Park, H.; Paik, U. Cross-linked poly(acrylic acid)-carboxymethyl cellulose and styrene-butadiene rubber as an efficient binder system and its physicochemical effects on a high energy density graphite anode for Li-ion batteries. Electrochem. Commun. 2017, 77, 103–106. [Google Scholar] [CrossRef]
- Park, H.-K.; Kong, B.-S.; Oh, E.-S. Effect of high adhesive polyvinyl alcohol binder on the anodes of lithium ion batteries. Electrochem. Commun. 2011, 13, 1051–1053. [Google Scholar] [CrossRef]
- Ling, L.M.; Bai, Y.; Wang, Z.H.; Ni, Q.; Chen, G.H.; Zhou, Z.M.; Wu, C. Remarkable effect of sodium alginate aqueous binder on anatase TiO2 as high-performance anode in sodium ion batteries. ACS Appl. Mater. Inter. 2018, 10, 5560–5568. [Google Scholar] [CrossRef]
- Tran, H.Y.; Wohlfahrt-Mehrens, M.; Dsoke, I.S. Influence of the binder nature on the performance and cycle life of activated carbon electrodes in electrolytes containing Li-salt. J. Power Sources 2017, 342, 301–312. [Google Scholar] [CrossRef]
- Kovalenko, I.; Zdyrko, B.; Magasinski, A.; Hertzberg, B.; Milicev, Z.; Burtovyy, R.; Luzinov, I.; Yushin, G. A major constituent of brown algae for use in high-capacity Li-ion batteries. Science 2011, 334, 75. [Google Scholar] [CrossRef]
- Wang, R.; Feng, L.; Yang, W.; Zhang, Y.; Zhang, Y.; Bai, W.; Liu, B.; Zhang, W.; Chuan, Y.; Zheng, Z.; et al. Effect of different binders on the electrochemical performance of metal oxide anode for lithium-ion batteries. Nanoscale Res. Lett. 2017, 12, 575. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kinoshita, Y.; Misaki, K.; Matsuyama, T.; Komaba, S. Electrochemical properties of LiCoO2 electrodes with latex binders on high-voltage exposure. J. Electrochem. Soc. 2015, 162, A538–A544. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Yang, X.; Zhang, D.; Qiu, H.L.; Fu, Q.; Na, H.; Guo, Z.D.; Du, F.; Chen, G.; Wei, Y.J. Water soluble styrene butadiene rubber and sodium carboxyl methyl cellulose binder for ZnFe2O4 anode electrodes in lithium ion batteries. J. Power Sources 2015, 285, 227–234. [Google Scholar] [CrossRef]
- Yen, J.P.; Chang, C.C.; Lin, Y.R.; Shen, S.T.; Hong, J.L. Effects of styrene-butadiene rubber/ carboxymethyl cellulose (SBR/CMC) and polyvinylidene difluoride (PVdF) binders on low temperature lithium ion batteries. J. Electrochem. Soc. 2013, 160, A1811–A1818. [Google Scholar] [CrossRef]
- Buqa, H.; Holzapfel, M.; Krumeich, F.; Veit, C.; Novak, P. Study of styrene butadiene rubber and sodium methyl cellulose as binder for negative electrodes in lithium-ion batteries. J. Power Sources 2006, 161, 617–622. [Google Scholar] [CrossRef]
- He, M.; Yuan, L.X.; Zhang, W.X.; Hu, X.L.; Huang, Y.H. Enhanced cyclability for sulfur cathode achieved by a water-soluble binder. J. Phys. Chem. C 2011, 115, 15703–15709. [Google Scholar] [CrossRef]
- Magasinski, A.; Zdyrko, B.; Kovalenko, I.; Hertzberg, B.; Burtovyy, R.; Huebner, C.F.; Fuller, T.F.; Luzinov, I.; Yushin, G. Toward efficient binders for Li-ion battery Si-based anodes: Polyacrylic acid. ACS Appl. Mater. Interfaces 2010, 11, 3004–3010. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Li, N.; Su, Y.N.; Yang, S.J.; Wang, J.L. Effects of binders on the electrochemical performance of rechargeable magnesium batteries. J. Power Sources 2017, 314, 219–229. [Google Scholar] [CrossRef]
- Li, D.W.; Wang, Y.K.; Hu, J.Z.; Lu, B.; Dang, D.Y.; Zhang, J.Q.; Cheng, Y.T. Role of polymeric binders on mechanical behavior and cracking resistance of silicon composite electrodes during electrochemical cycling. J. Power Sources 2018, 387, 9–15. [Google Scholar] [CrossRef]
- Liu, G.; Zheng, H.; Simens, A.S.; Minor, A.M.; Song, X.; Battaglia, V.S. Optimization of acetylene black conductive additive and PVDF composition for high-power rechargeable lithium-ion cells. J. Electrochem. Soc. 2007, 154, A1129–A1134. [Google Scholar] [CrossRef]
- Liu, G.; Zheng, H.; Kim, S.; Deng, Y.; Minor, A.M.; Song, X.; Battaglia, V.S. Effects of various conductive additive and polymeric binder contents on the performance of a lithium-ion composite cathode. J. Electrochem. Soc. 2008, 155, A887–A892. [Google Scholar] [CrossRef]
- Zheng, H.H.; Yang, R.Z.; Liu, G.; Song, X.Y.; Battaglia, V.S. Cooperation between active material, polymeric binder and conductive carbon additive in lithium ion battery cathode. J. Phys. Chem. C 2012, 116, 4875–4882. [Google Scholar] [CrossRef]
- Chen, Z.H.; Christensen, L.; Dahn, J.R. A study of the mechanical and electrical properties of a polymer/carbon black binder system used in battery electrodes. J. Appl. Polym. Sci. 2003, 90, 1891–1899. [Google Scholar] [CrossRef]
- Takahashi, K.; Higa, K.; Mair, S.; Chintapalli, M.; Balsara, N.; Srinivasan, V. Mechanical degradation of graphite/PVdF composite electrodes: A model-experimental study. J. Electrochem. Soc. 2016, 163, A385–A395. [Google Scholar] [CrossRef]
- Grillet, A.M.; Humplik, T.; Stirrup, E.K.; Roberts, S.A.; Barringer, D.A.; Snyder, C.M.; Janvrin, M.R.; Apblett, C.A. Conductivity degradation of polyvinylidene fluoride composite binder during cycling: Measurements and simulations for lithium-ion batteries. J. Electrochem. Soc. 2016, 163, A1859–A1871. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, H.; Tao, B.; He, Y.; Zhou, S. Effect of Conductive Carbon Black on Mechanical Properties of Aqueous Polymer Binders for Secondary Battery Electrode. Polymers 2019, 11, 1500. https://doi.org/10.3390/polym11091500
Hu H, Tao B, He Y, Zhou S. Effect of Conductive Carbon Black on Mechanical Properties of Aqueous Polymer Binders for Secondary Battery Electrode. Polymers. 2019; 11(9):1500. https://doi.org/10.3390/polym11091500
Chicago/Turabian StyleHu, Hongjiu, Bao Tao, Yaolong He, and Sihao Zhou. 2019. "Effect of Conductive Carbon Black on Mechanical Properties of Aqueous Polymer Binders for Secondary Battery Electrode" Polymers 11, no. 9: 1500. https://doi.org/10.3390/polym11091500
APA StyleHu, H., Tao, B., He, Y., & Zhou, S. (2019). Effect of Conductive Carbon Black on Mechanical Properties of Aqueous Polymer Binders for Secondary Battery Electrode. Polymers, 11(9), 1500. https://doi.org/10.3390/polym11091500






