Numerical Studies of the Viscosity of Reacting Polyurethane Foam with Experimental Validation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment
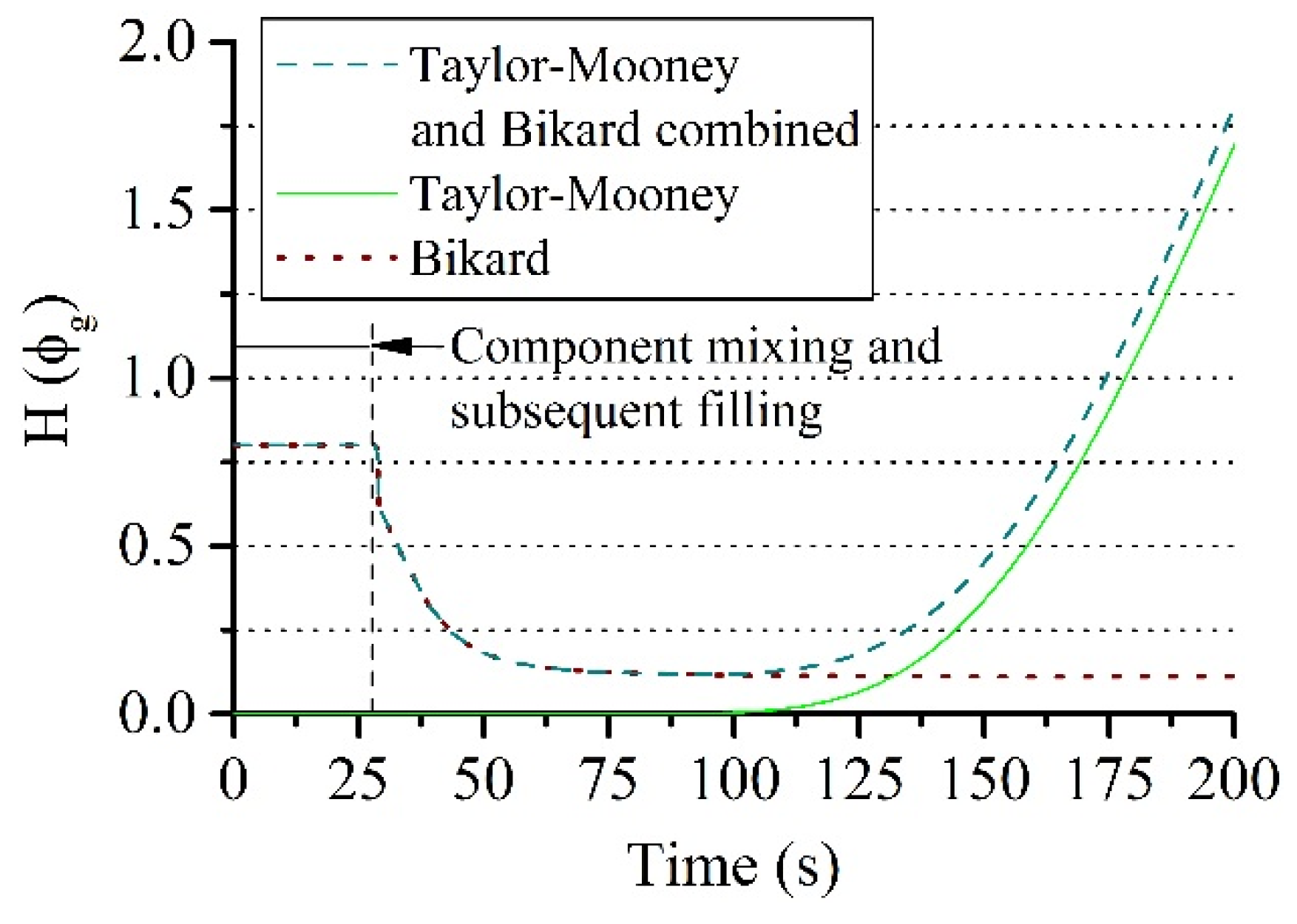
2.2. Mathematical Modeling
3. Results and Discussion
3.1. Experiment
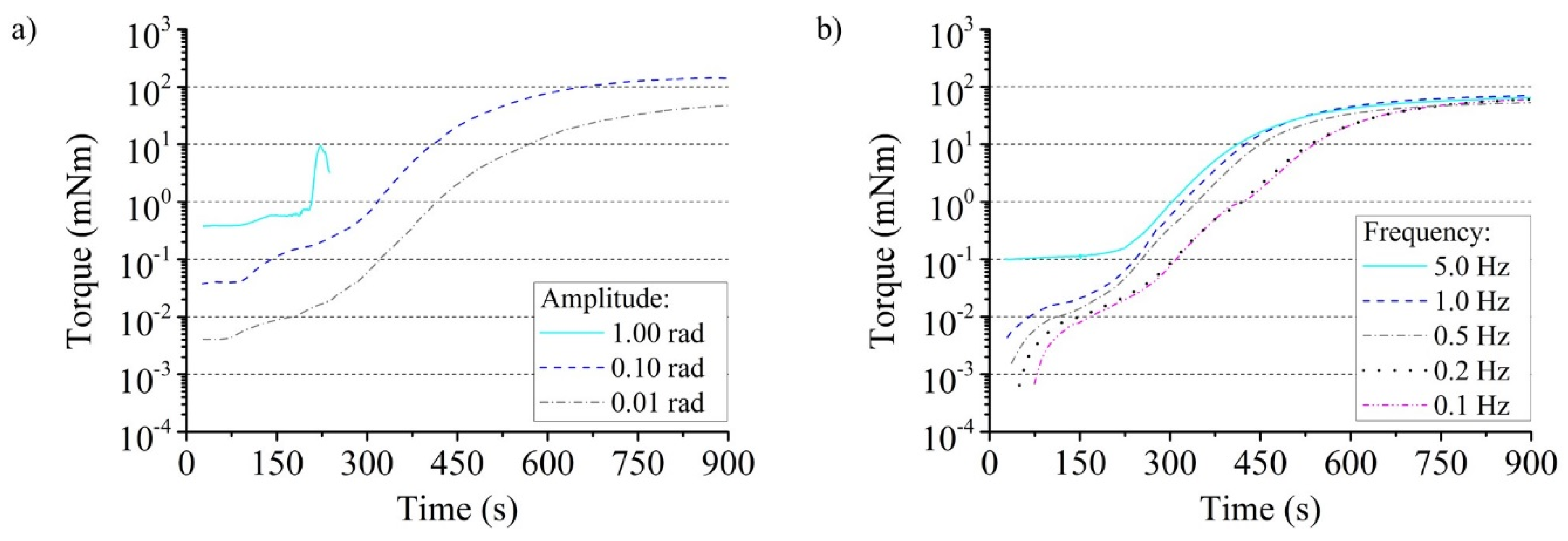
3.1.1. Amplitude and Frequency Dependence of the Torque
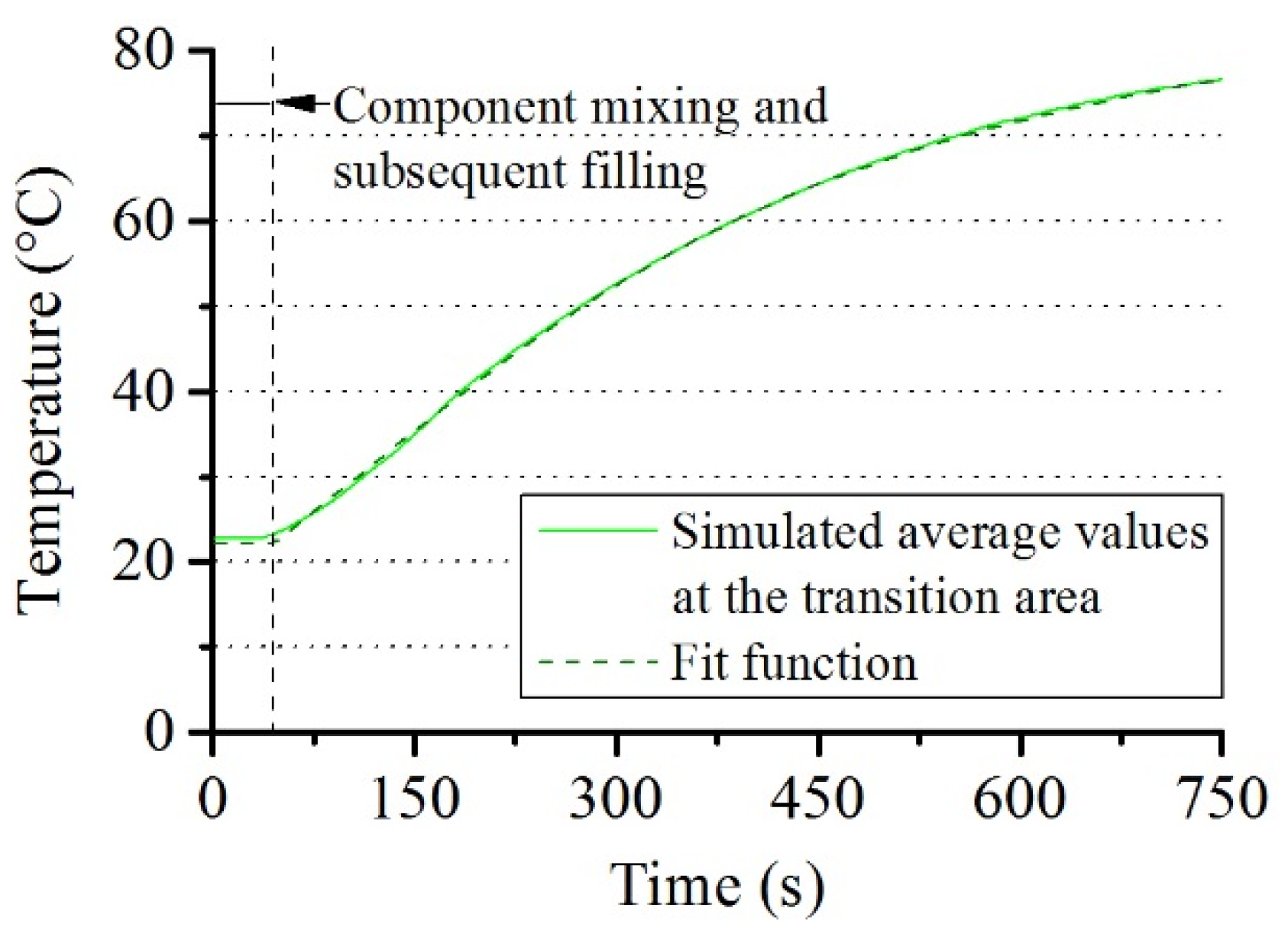
3.1.2. Shear Stress within Reacting PUR Foam and Its Dependence on the Reaction Mass
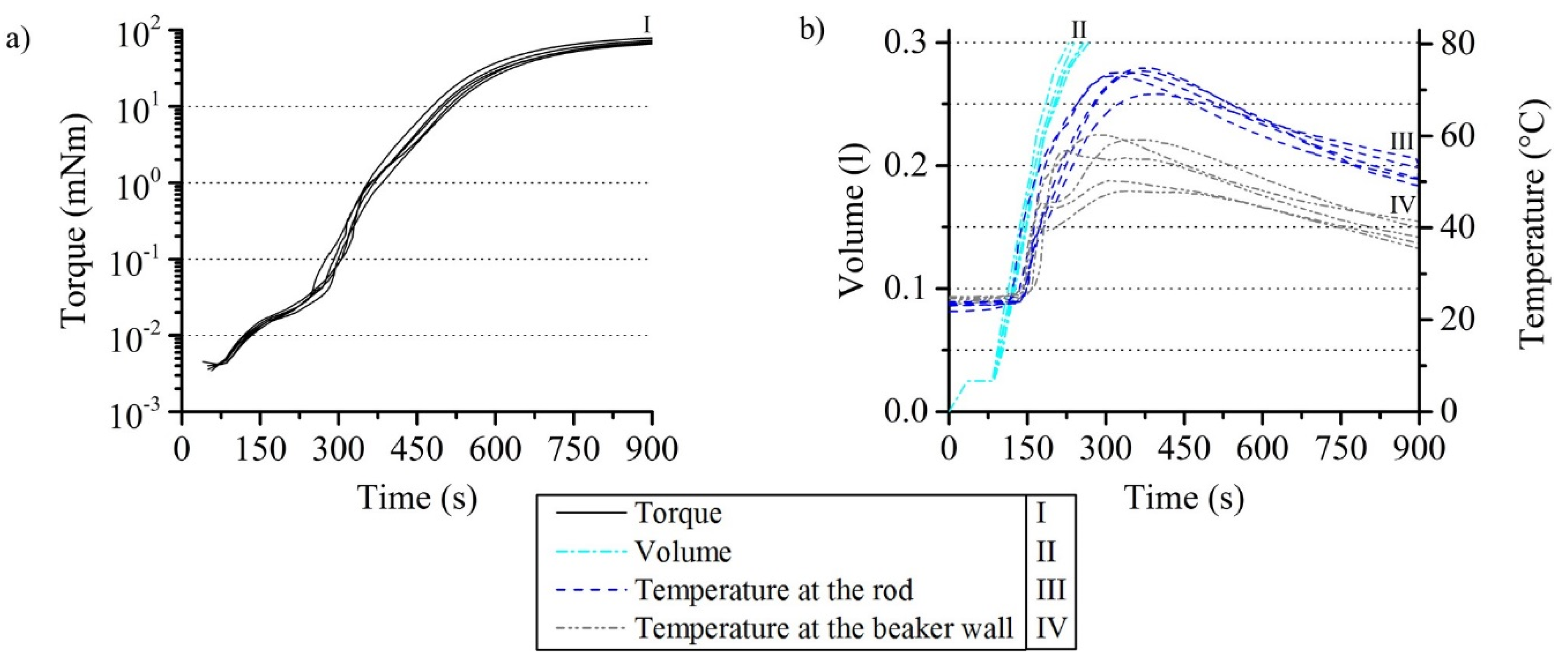
3.1.3. Reproducibility of the Rheometer Test with Reacting PUR Foam
3.2. Simulation of the Torque in the Experiment
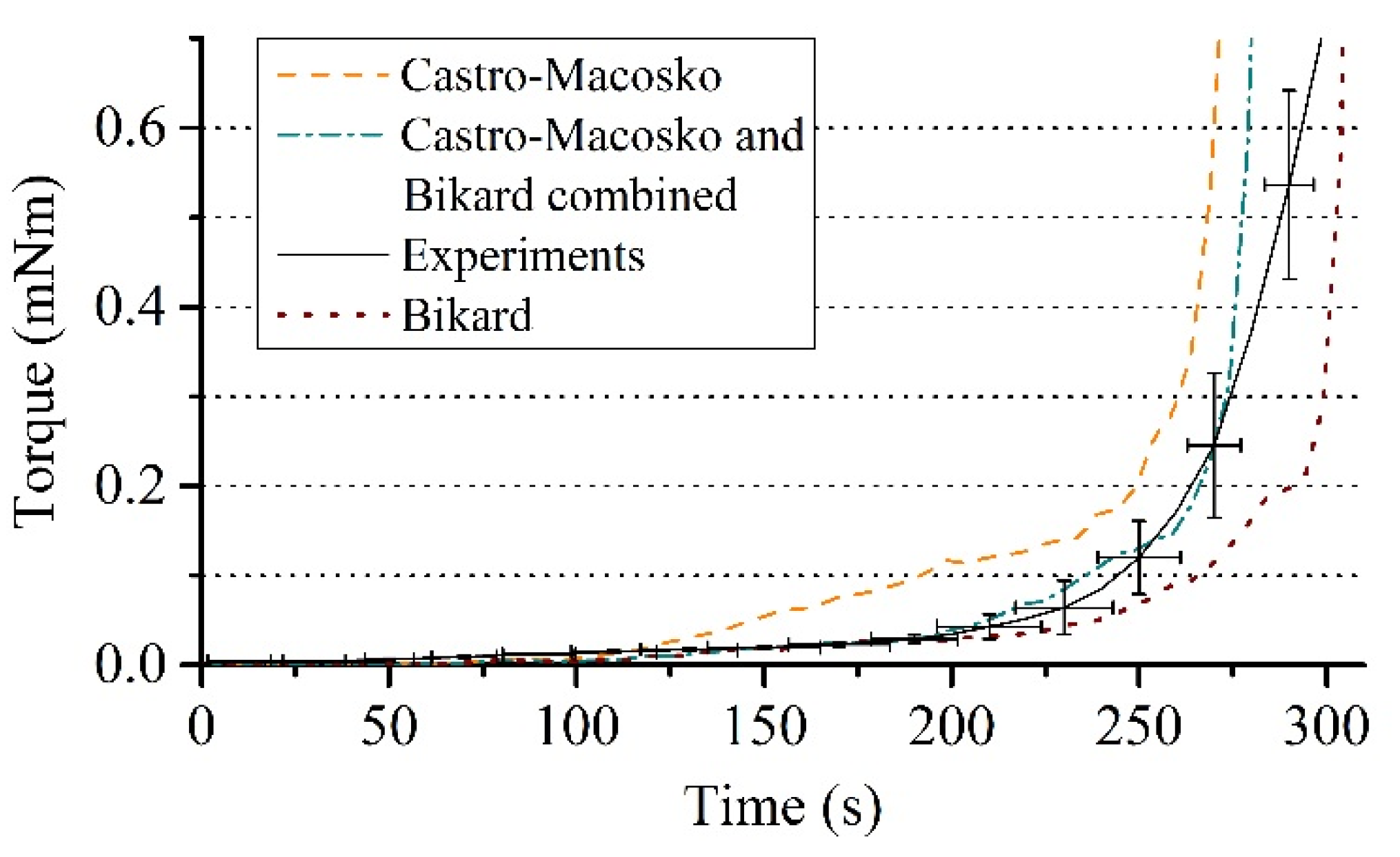
3.3. Comparison of Experiment and Simulation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Szycher, M. Szycher’s Handbook of Polyurethanes, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 2–12, 373–392, 523–545. [Google Scholar]
- Neff, R.A.; Macosko, C.W. Simultaneous measurement of viscoelastic changes and cell opening during processing of flexible polyurethane foam. Rheol. Acta 1996, 35, 656–666. [Google Scholar] [CrossRef]
- Bouayad, R.; Bikard, J.; Agassant, J.-F. Compressible flow in a plate/plate rheometer: Application to the experimental determination of reactive expansion’s models parameters for polyurethane foam. Int. J. Mater. Form. 2009, 2, 243–260. [Google Scholar] [CrossRef]
- Abdessalam, H.; Abbès, B.; Li, Y.; Guo, Y.-Q.; Kwassi, E.; Romain, J.-L. Parameter identification and computational simulation of polyurethane foaming process by finite pointset method. Int. J. Mater. Form. 2016, 9, 85–100. [Google Scholar] [CrossRef]
- Chandan, M.R.; Naskar, N.; Das, A.; Mukherjee, R.; Harikrishnan, G. Deducing Multiple Interfacial Dynamics during Polymeric Foaming. Langmuir 2018, 34, 8024–8030. [Google Scholar] [CrossRef]
- Bakhtiyarov, S.I.; Oxley, J.C.; Smith, J.L.; Baldovi, P.M.; Siginer, D.A. Rheometric studies of functional polyurethane foam composite. IMECE 2017, 7, 133385. [Google Scholar]
- Kostrzewski, W. Foam rheometer: Its principle and applications. J. Cell. Plast. 1985, 21, 424–429. [Google Scholar] [CrossRef]
- Webb, D.D. Urethane systems reactivity measurement. J. Cell. Plast. 1985, 21, 208–212. [Google Scholar] [CrossRef]
- Carriere, C.J.; Bank, D.H.; Christenson, C.P. A pulse rheometer for foams. Polym. Eng. Sci. 1992, 32, 426–430. [Google Scholar] [CrossRef]
- Singh, A.P.; Bhattacharya, M. Viscoelastic changes and cell opening of reacting polyurethane foams from soy oil. Polym. Eng. Sci. 2004, 44, 1977–1986. [Google Scholar] [CrossRef]
- McClusky, J.V.; O′Neill, R.E.; Priester, R.D., Jr.; Ramsey, W.A. Vibrating Rod Viscometer: A Valuable Probe into Polyurethane Chemistry. J. Cell. Plast. 1994, 30, 224–241. [Google Scholar] [CrossRef]
- Falke, P.; Kudoke, C.; Rotermund, I.; Zaschke, B. Some aspects of matrix formation of flexible polyurethane foams. J. Cell. Plast. 1999, 35, 43–67. [Google Scholar] [CrossRef]
- Zhao, Y.; Gordon, M.J.; Tekeei, A.; Hsieh, F.-H.; Suppes, G.J. Modeling reaction kinetics of rigid polyurethane foaming process. J. Appl. Polym. Sci. 2013, 130, 1131–1138. [Google Scholar] [CrossRef]
- Karimi, M.; Marchisio, D.; Laurini, E.; Fermeglia, M.; Pricl, S. Bridging the gap across scales: Coupling CFD and MD / GCMC in polyurethane foam simulation. Chem. Eng. Sci. 2018, 178, 39–47. [Google Scholar] [CrossRef]
- Castro, J.M.; Macosko, C.W. Studies of mold filling and curing in the reaction injection molding process. AIChE J. 1982, 28, 250–260. [Google Scholar] [CrossRef]
- Rao, R.R.; Mondy, L.A.; Noble, D.R.; Moffat, H.K.; Adolf, D.B.; Notz, P.K. A level set method to study foam processing: A validation study. Int. J. Numer. Methods Fluids 2012, 68, 1362–1392. [Google Scholar] [CrossRef]
- Rao, R.; Mondy, L.; Noble, D.; Brunini, V.; Long, K.; Roberts, C.; Wyatt, N.; Celina, M.; Thompson, K.; Tinsly, J. Density predictions using a finite element / level set model of polyurethane foam expansion and polymerization. Comput. Fluids 2018, 175, 20–35. [Google Scholar] [CrossRef]
- Prud′homme, R.K.; Khan, S.A. Foams: Theory, Measurements, and Applications, 1st ed.; Marcel Dekker, Inc.: New York, NY, USA, 1996. [Google Scholar]
- Chong, T.-H.; Ha, Y.-W.; Jeong, D.-J. Effect of Dissolved Gas on the Viscosity of HIPS in the Manufacture of Microcellular Plastics. Polym. Eng. Sci. 2003, 43, 1337–1344. [Google Scholar] [CrossRef]
- Bikard, J.; Bruchon, J.; Coupez, T.; Silva, L. Numerical simulation of 3D polyurethane expansion during manufacturing process. Colloids Surf. A 2007, 309, 49–63. [Google Scholar] [CrossRef]
- Kamal, M.R. Thermoset characterization for modality analysis. Polym. Eng. Sci. 1974, 14, 231–239. [Google Scholar] [CrossRef]
- Ireka, I.E.; Niedziela, D.; Schäfer, K.; Tröltzsch, J.; Steiner, K.; Helbig, F.; Chinyoka, T.; Kroll, L. Computational modelling of the complex dynamics of chemically blown polyurethane foam. Phys. Fluids 2015, 27, 113102. [Google Scholar] [CrossRef]
- Tröltzsch, J.; Schäfer, K.; Niedziela, D.; Ireka, I.E.; Steiner, K.; Kroll, L. Simulation of RIM-process for Polyurethane Foam Expansion in Fiber Reinforced Sandwich Structures. Procedia CIRP 2017, 66, 62–67. [Google Scholar] [CrossRef]
- Niedziela, D.; Ireka, I.E.; Steiner, K. Computational Analysis of Nonuniform Expansion in Polyurethane Foams. Polymers 2019, 11, 100. [Google Scholar] [CrossRef] [PubMed]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schäfer, K.; Nestler, D.; Tröltzsch, J.; Ireka, I.; Niedziela, D.; Steiner, K.; Kroll, L. Numerical Studies of the Viscosity of Reacting Polyurethane Foam with Experimental Validation. Polymers 2020, 12, 105. https://doi.org/10.3390/polym12010105
Schäfer K, Nestler D, Tröltzsch J, Ireka I, Niedziela D, Steiner K, Kroll L. Numerical Studies of the Viscosity of Reacting Polyurethane Foam with Experimental Validation. Polymers. 2020; 12(1):105. https://doi.org/10.3390/polym12010105
Chicago/Turabian StyleSchäfer, Kay, Daisy Nestler, Jürgen Tröltzsch, Ikenna Ireka, Dariusz Niedziela, Konrad Steiner, and Lothar Kroll. 2020. "Numerical Studies of the Viscosity of Reacting Polyurethane Foam with Experimental Validation" Polymers 12, no. 1: 105. https://doi.org/10.3390/polym12010105
APA StyleSchäfer, K., Nestler, D., Tröltzsch, J., Ireka, I., Niedziela, D., Steiner, K., & Kroll, L. (2020). Numerical Studies of the Viscosity of Reacting Polyurethane Foam with Experimental Validation. Polymers, 12(1), 105. https://doi.org/10.3390/polym12010105






