Challenges with Verifying Microbial Degradation of Polyethylene
Abstract
:1. Introduction
2. Abiotic Deterioration of PE
3. Biodeterioration of PE
4. General Overview of Biodegradation Processes
5. Factors Involved in Microbial Degradation Experiments of PE
5.1. Polyethylene Structure and Shape
5.2. Modification of Polyethylene
5.3. Partial Biodegradation versus Complete Degradation
5.4. Interference of Other Carbon Sources in Biodegradation
5.4.1. Carbon Sources Incorporated in Main PE Chain
5.4.2. Accidental Impurities Carbon Sources in PE Chain
5.4.3. Carbon Sources from Culture-Independent Methods
6. Type of Microorganisms Used
6.1. Polyethylene Degradation by Bacterial Consortia
6.2. Fungi versus Bacteria in Biodegradation of Polyethylene
6.3. Using Bacteria that Can Form Biofilms and Secrete Biosurfactants
7. Experimental Conditions
Limitations of the Methods and Techniques Used in Real Biodegradation Assays
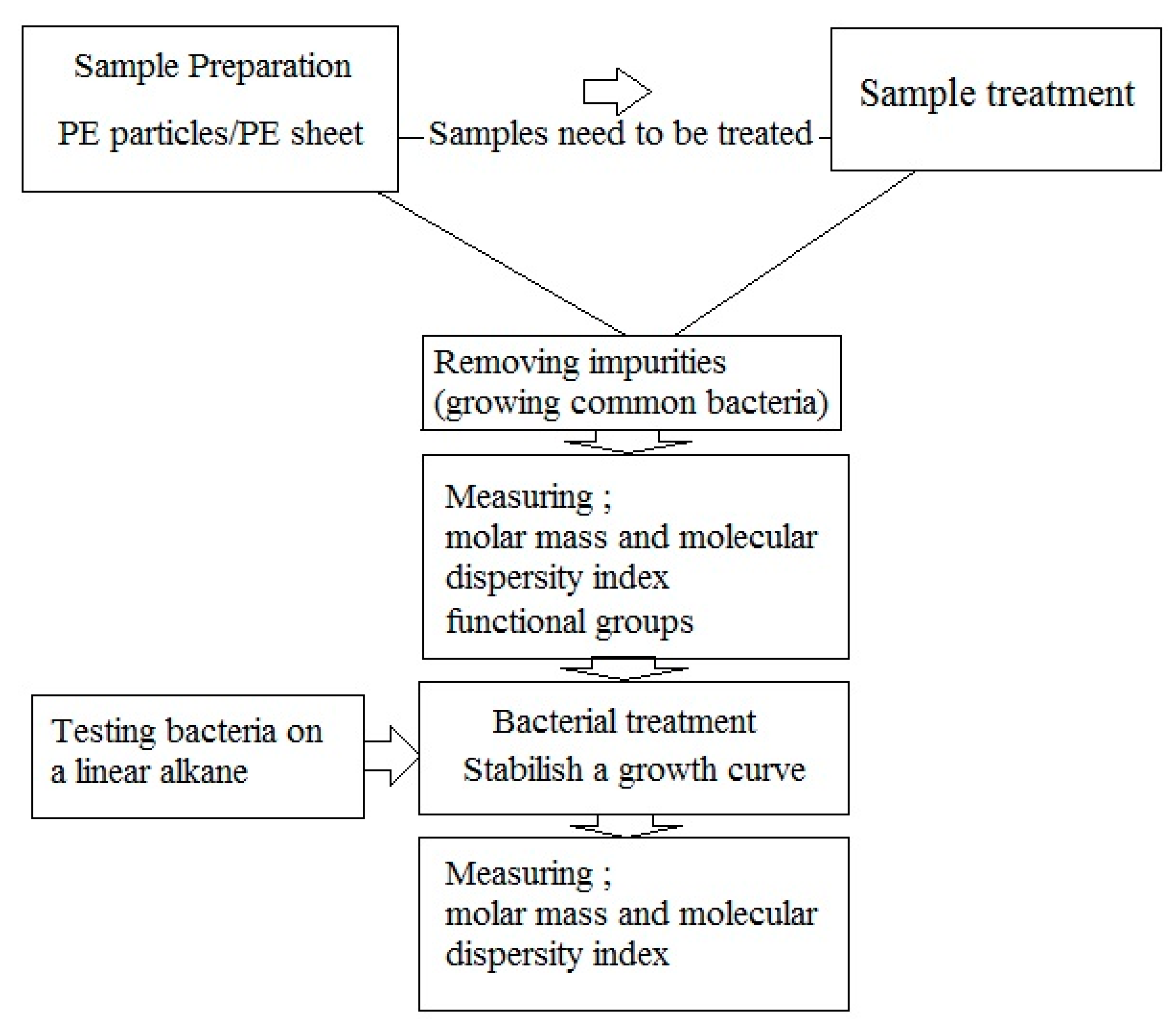
8. Guidelines for Studying Microbial Degradation of Polyethylene
- Sample preparation: PE samples can be in form of particles or films. In case of particles, the surface area for bacterial colonization is greater. Disadvantages of particle use include a) measurement of weight loss, which is prone to errors, and b) SEM analysis for assessment the penetration of microorganisms, which is impossible because of uneven surface. If any pretreatments are used, these should be done before bacterial treatment;
- Removing impurities: Impurities can be removed easily by washing the sample, and subsequently growing the common bacteria on culture media containing PE sample to remove the usual impurities added in previous steps through consumption;
- Measuring molar mass, molecular dispersity index and functional groups; Measurements of molar mass and molar mass number are still the best biodegradation assays. These must be used both immediately before and after of microbial degradation. An increase in molar mass after incubation with bacteria suggests that the bacteria consumed branches and other low molar mass portions of the polymers. An increase in the molecular dispersity index indicates that chain breakage occurred at the ends of the polymer chains or branches rather than at the center of the molecule. FTIR analysis can show changes in functional groups in the polymer structure.
- Bacterial treatment: In biodegradation experiments, it is assumed that bacteria able to degrade PE (by testing the growth on n-hexadecane or paraffin [67] and forming colony on polyethylene surface [31]). Bacterial treatment should be performed under optimum conditions for the microbe(s) used in the experiments. Bacteria are inoculated on culture media containing PE samples for further experiment.
- Establish microbial growth curves on polyethylene: It is essential to establish growth curves of the microorganisms used in biodegradation assays When comparing the biodegradation of PE between two bacteria, it is important to have accurate growth curves of the bacteria.
9. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef] [PubMed]
- Harshvardhan, K.; Jha, B. Biodegradation of low-density polyethylene by marine bacteria from pelagic waters Arabian Sea, India. Mar. Pollut. Bull. 2013, 77, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Hernández, C.; Cairós, C.; López-Darias, J.; Mazzetti, E.; Hernández-Sánchez, C.; González-Sálamo, J.; Hernández-Borges, J. Microplastic debris in beaches of Tenerife (Canary Islands, Spain). Mar. Pollut. Bull. 2019, 146, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Foley, C.J.; Feiner, Z.S.; Malinich, T.D.; Hook, T.O. A meta-analysis of the effects of exposure to microplastics on fish and aquatic invertebrates. Sci. Total Environ. 2018, 631, 550–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rochman, C.M.; Browne, M.A.; Underwood, A.J.; Van Franeker, J.A.; Thompson, R.C.; Amaral-Zettler, L.A. The ecological impacts of marine debris: Unraveling the demonstrated evidence from what is perceived. Ecology 2016, 97, 302–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, M.; Zhang, Y.; Zhu, Y.; Song, B.; Zeng, G.; Hu, D.; Wen, X.; Ren, X. Recent advances in toxicological research of nanoplastics in the environment: A review. Environ. Pollut. 2019, 252, 511–521. [Google Scholar] [CrossRef]
- Bergmann, M.; Mützel, S.; Primpke, S.; Tekman, M.B.; Trachsel, J.; Gerdts, G. White and wonderful? Microplastics prevail in snow from the Alps to the Arctic. Sci. Adv. 2019, 5, eaax1157. [Google Scholar] [CrossRef] [Green Version]
- Peixoto, J.; Silva, P.L.; Krüger, R.H. Brazilian Cerrado soil reveals an untapped microbial potential forunpretreated polyethylene biodegradation. J. Hazard. Mater. 2017, 324, 634–644. [Google Scholar] [CrossRef]
- Shimao, M. Biodegradation of plastics. Curr. Opin. Biotechnol. 2001, 12, 242–247. [Google Scholar] [CrossRef]
- Koutny, M.; Lemaire, J.; Delort, A.M. Biodegradation of polyethylene films with prooxidant additives. Chemosphere 2006, 64, 1243–1252. [Google Scholar] [CrossRef] [Green Version]
- Arutchelvi, J.; Sudhakar, M.; Arkatkar, A.; Doble, M.; Bhaduri, S.; Uppara, P.V. Biodegradation of polyethylene and polypropylene. Indian J. Biotechnol. 2008, 7, 9–22. [Google Scholar]
- Shah, A.A.; Hasan, F.; Hameed, A.; Ahmed, S. Biological degradation of plastics: A comprehensive review. Biotechnol. Adv. 2008, 26, 246–265. [Google Scholar] [CrossRef]
- Lucas, N.; Bienaime, C.; Belloy, C.; Queneudec, M.; Silvestre, F.; Nava-Saucedo, J.E. Polymer biodegradation: Mechanisms and estimation techniques. Chemosphere 2008, 73, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef] [PubMed]
- Sivan, A. New perspectives in plastic biodegradation. Curr. Opin. Biotechnol. 2011, 22, 422–426. [Google Scholar] [CrossRef]
- Ammala, A.; Bateman, S.; Deana, K.; Petinakis, E.; Sangwan, P.; Wong, S.; Yuan, Q.; Yu, L.; Patrick, C.; Leong, K.H. An overview of degradable and biodegradable polyolefins. Prog. Polym. Sci. 2011, 36, 1015–1049. [Google Scholar] [CrossRef]
- Restrepo-Flórez, J.M.; Bassi, A.; Thompson, M.R. Microbial degradation and deterioration of polyethylene: A review. Int. Biodeterior. Biodegrad. 2014, 88, 83–90. [Google Scholar] [CrossRef]
- Sen, S.; Raut, S. Microbial degradation of low density polyethylene (LDPE): A review. J. Environ. Chem. Eng. 2015, 3, 462–473. [Google Scholar]
- Raziyafathima, M.; Praseetha, P.K.; Rimal Isaac, R.S. Microbial degradation of plastic waste: A review. J. Pharm. Chem. Biol. Sci. 2016, 4, 231–242. [Google Scholar]
- Emadian, S.M.; Onat, T.T.; Demirel, B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017, 59, 526–536. [Google Scholar] [CrossRef]
- Harrison, J.P.; Boardman, C.; O’Callaghan, K.; Delort, A.M.; Song, J. Biodegradability standards for carrier bags and plastic films in aquatic environments: A critical review. R. Soc. Open Sci. 2018, 5, 171792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albertsson, A.C.; Karlsson, S. The Influence of biotic and abiotic environments on the degradation of polyethylene. Prog. Polym. Sci. 1990, 15, 177–192. [Google Scholar] [CrossRef]
- Pirt, S.J. Microbial degradation of synthetic polymers. J. Chem. Technol. Biotechnol. 1980, 30, 176–179. [Google Scholar] [CrossRef]
- Otake, Y.; Kobayashi, T.; Asabe, H.; Murakami, N. Biodegradation of low density polyethylene, polystyrene, polyvinyl chloride, and urea formaldehyde resin buried under soil for over 32 years. Appl. Polym. Sci. 1995, 56, 1789–1796. [Google Scholar] [CrossRef]
- Chiellini, E.; Cortia, A.; Swift, G. Biodegradation of thermally-oxidized, fragmented low-density polyethylenes. Polym. Degrad. Stab. 2003, 81, 341–351. [Google Scholar] [CrossRef]
- Yashchuk, O.; Portillo, F.S.; Hermida, E.B. Degradation of polyethylene film samples containing oxodegradable additives. Procedia Mater. Sci. 2012, 1, 439–445. [Google Scholar] [CrossRef] [Green Version]
- Chiellini, E.; Corti, A.; D’Antone, S. Oxo-biodegradable full carbon backbone polymers biodegradation behaviour of thermally oxidized polyethylene in an aqueous medium. Polym. Degrad. Stab. 2007, 92, 1378–1383. [Google Scholar] [CrossRef]
- Veethahavya, K.S.; Rajath, B.S.; Noobia, S.; Kumar, M.B. Biodegradation of low density polyethylene in aqueous media. Procedia Environ. Sci. 2016, 35, 709–713. [Google Scholar] [CrossRef]
- Nowak, B.; Pajak, J.; Drozd-Bratkowicz, M.; Rymarz, G. Microorganisms participating in the biodegradation of modified polyethylene films in different soils under laboratory conditions. Int. Biodeterior. Biodegrad. 2011, 65, 757–767. [Google Scholar] [CrossRef]
- Kathiresan, K. Polythene and plastics-degrading microbes from the mangrove soil. Rev. Biol. Trop. 2003, 51, 629–633. [Google Scholar]
- Abrusci, C.; Pablos, J.L.; Corrales, T.; López-Marín, J.; Marín, I.; Catalina, F. Biodegradation of photo-degraded mulching films based on polyethylenes and stearates of calcium and iron as pro-oxidant additives. Int. Biodeterior. Biodegrad. 2011, 65, 451–459. [Google Scholar] [CrossRef]
- Divyalakshmi, S.; Subhashini, A. Screening and isolation of polyethylene degrading bacteria from various soil environments. IOSR J. Environ. Sci. Toxicol. Food Technol. 2016, 10, 1–7. [Google Scholar]
- Hassan, F.; Shah, A.A.; Hameed, A.; Ahmed, S. Synergistic effect of photo and chemical treatment on the rate of biodegradation of low density polyethylene by Fusarium sp. AF4. J. Appl. Polym. Sci. 2007, 105, 1466–1470. [Google Scholar] [CrossRef]
- Usha, R.; Sangeetha, T.; Palaniswamy, M. Screening of polyethylene degrading microorganisms from garbage soil. Libyan Agric. Res. Cent. J. Intern. 2011, 2, 200–204. [Google Scholar]
- Koutny, M.; Amato, P.; Muchova, M.; Ruzicka, J.; Delort, A.M. Soil bacterial strains able to grow on the surface of oxidized polyethylene film containing prooxidant additives. Int. Biodeterior. Biodegrad. 2009, 63, 354–357. [Google Scholar] [CrossRef] [Green Version]
- Gilan, I.; Hadar, Y.; Sivan, A. Colonization, biofilm formation and biodegradation of polyethylene by a strain of Rhodococcus ruber. Appl. Microbiol. Biotechnol. 2004, 65, 97–104. [Google Scholar]
- Montazer, Z.; Habibi-Najafi, M.B.; Mohebbi, M.; Oromiehei, A. Microbial degradation of UV-pretreated low-density polyethylene films by novel polyethylene-degrading bacteria isolated from plastic-dump soil. J. Polym. Environ. 2018, 26, 3613–3625. [Google Scholar] [CrossRef]
- Bhatia, M.; Girdhar, A.; Tiwari, A.; Nayarisseri, A. Implications of a novel Pseudomonas species on low density polyethylene biodegradation: An in vitro to in silico approach. SpringerPlus 2014, 3, 497. [Google Scholar] [CrossRef] [Green Version]
- Das, M.P.; Kumar, S. An approach to low-density polyethylene biodegradation by Bacillus amyloliquefaciens. 3 Biotech 2015, 5, 81–86. [Google Scholar] [CrossRef] [Green Version]
- Mehmood, C.T.; Qazi, I.A.; Hashmi, I.; Bhargava, S.; Deepa, S. Biodegradation of low density polyethylene (LDPE) modified with dye sensitized titania and starch blend using Stenotrophomonas pavanii. Int. Biodeterior. Biodegrad. 2016, 113, 276–286. [Google Scholar] [CrossRef]
- Nanda, S.; Sahu, S.S. Biodegradability of polyethylene by Brevibacillus, Pseudomonas, and Rhodococcus spp. N. Y. Sci. J. 2010, 3, 95–98. [Google Scholar]
- Pramila, R.; Ramesh, K.V. Potential biodegradation of low-density polyethylene (LDPE) by Acinetobacter bumannii. Afr. J. Bacteriol. Res. 2015, 7, 24–28. [Google Scholar]
- Sudhakar, M.; Doble, M.; Sriyutha Murthy, P.; Venkatesan, R. Marine microbe-mediated biodegradation of low- and high-density polyethylenes. Int. Biodeterior. Biodegrad. 2008, 61, 203–213. [Google Scholar] [CrossRef]
- Jeon, H.J.; Kim, M.N. Degradation of linear low density polyethylene (LLDPE) exposed to UV-irradiation. Eur. Polym. J. 2014, 52, 146–153. [Google Scholar] [CrossRef]
- Jeon, H.J.; Kim, M.N. Functional analysis of alkane hydroxylase system derived from Pseudomonas aeruginosa E7 for low molecular weight polyethylene biodegradation. Int. Biodeterior. Biodegrad. 2015, 103, 141–146. [Google Scholar] [CrossRef]
- Yoon, M.G.; Jeon, J.H.; Kim, M.N. Biodegradation of polyethylene by a soil bacterium and AlkB cloned recombinant cell. J. Bioremed. Biodegrad. 2012, 3, 145. [Google Scholar]
- Yang, J.; Yang, Y.; Wu, W.M.; Zhao, J.; Jiang, L. Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating waxworms. Environ. Sci. Technol. 2014, 48, 13776–13784. [Google Scholar] [CrossRef]
- Thakur, P. Screening of Plastic Degrading Bacteria from Dumped Soil Area. Ph.D. Thesis, National Institue of Technology of Rourkela, Odisha, India, 2012. [Google Scholar]
- Das, M.P.; Kumar, S. Influence of cell surface hydrophobicity in colonization and biofilm formation on LDPE biodegradation. Int. J. Pharm. Pharm. Sci. 2013, 4, 690–694. [Google Scholar]
- Vimala, P.P.; Mathew, L. Biodegradation of polyethylene using Bacillus subtilis. Procedia Technol. 2016, 24, 232–239. [Google Scholar] [CrossRef] [Green Version]
- Hadad, D.; Geresh, S.; Sivan, A. Biodegradation of polyethylene by the thermophilic bacterium Brevibacillus borstelensis. J. Appl. Microbiol. 2005, 98, 1093–1100. [Google Scholar] [CrossRef]
- Rajandas, H.; Parimannan, S.; Sathasivam, K.; Ravichandran, M.; Yin, L.S. A novel FTIR-ATR spectroscopy based technique for the estimation of low-density polyethylene biodegradation. Polym. Test. 2012, 3, 1094–1099. [Google Scholar] [CrossRef]
- Kyaw, B.M.; Champakalakshmi, R.; Sakharkar, M.K.; Lim, C.S.; Sakharkar, K.R. Biodegradation of low-density polythene (LDPE) by Pseudomonas species. Indian J. Microbiol. 2012, 52, 411–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivan, A.; Santo, M.; Pavlov, V. Biofilm development of the polyethylene-degrading bacterium Rhodococcus ruber. Appl. Microbiol. Biotechnol. 2006, 72, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Santo, M.; Weitsman, R.; Sivan, A. The role of the copper-binding enzyme, laccase, in the biodegradation of polyethylene by the actinomycete Rhodococcus ruber. Int. Biodeterior. Biodegrad. 2013, 84, 204–210. [Google Scholar] [CrossRef]
- Bonhomme, S.; Cuer, A.; Delort, A.M.; Lemaire, J.; Sancelme, M.; Scott, C. Environmental biodegradation of polyethylene. Polym. Degrad. Stab. 2003, 81, 441–452. [Google Scholar] [CrossRef]
- Fontanella, S.; Bonhomme, S.; Koutny, M.; Husarova, L.; Brusso, J.M.; Courdavault, J.P.; Pitteri, S.; Pichon, S.G.; Emaire, G.J.; Delort, A.M. Comparison of the biodegradability of various polyethylene films containing pro-oxidant additives. Polym. Degrad. Stab. 2010, 95, 1011–1021. [Google Scholar] [CrossRef]
- El-Shafei, H.; EI-Nasser, N.H.A.; Kansoh, A.L.; Ali, A.M. Biodegradation of disposable polyethylene by fungi Streptomyces species. Polym. Degrad. Stab. 1998, 62, 361–365. [Google Scholar] [CrossRef]
- Ranjan, V.P.; Goel, S. Degradation of Low-density polyethylene film exposed to UV radiation in four environments. J. Hazard. Toxic Radioact. Waste 2019, 23, 04019015. [Google Scholar] [CrossRef]
- Celina, M.; Linde, E.; Brunson, D.; Quintana, A.; Giron, N. Overview of accelerated aging and polymer degradation kinetics for combined radiation-thermal environments. Polym. Degrad. Stab. 2019, 166, 353–378. [Google Scholar] [CrossRef]
- Kelkar, V.P.; Rolsky, C.B.; Pant, A.; Green, M.D.; Tongay, S.; Halden, R.U. Chemical and physical changes of microplastics during sterilization by chlorination. Water Res. 2019, 163, 114871. [Google Scholar] [CrossRef]
- Albertsson, A.C.; Anderson, S.O.; Karlsson, S. Mechanism of biodegradation of polyethylene. Polym. Degrad. Stab. 1987, 18, 73–87. [Google Scholar] [CrossRef]
- Abrusci, C.; Pablos, J.L.; Marín, I.; Espí, E.; Corrales, T.; Catalina, F. Comparative effect of metal stearates as pro-oxidant additives on bacterial biodegradation of thermal- and photo-degraded low density polyethylene mulching films. Int. Biodeterior. Biodegrad. 2013, 83, 25–32. [Google Scholar] [CrossRef]
- Reddy, M.M.; Deighton, M.; Gupta, R.K.; Bhattacharya, S.N.; Parthasarathy, R. Biodegradation of montmorillonite filled oxo-biodegradable polyethylene. J. Appl. Polym. Sci. 2009, 113, 826–832. [Google Scholar] [CrossRef]
- Yamada-Onodera, K.; Mukumoto, H.; Katsuyama, Y.; Saiganji, A.; Tani, Y. Degradation of polyethylene by a fungus, Penicillium simplicissimum YK. Polym. Degrad. Stab. 2001, 72, 323–327. [Google Scholar] [CrossRef]
- Montazer, Z.; Habibi-Najafi, M.B.; Levin, D.B. Microbial degradation of low-density polyethylene and synthesis of polyhydroxyalkanoate polymers. Can. J. Microbiol. 2019, 65, 1–11. [Google Scholar] [CrossRef]
- Haines, J.R. Microbial degradation of high-molecular-weight alkanes. Appl. Microbiol. 1975, 28, 1084–1085. [Google Scholar] [CrossRef] [Green Version]
- Álvarez, H.M. Relationship between β-oxidation pathway and the hydrocarbon-degrading profile in actinomycetes bacteria. Int. Biodeterior. Biodegrad. 2003, 52, 35–42. [Google Scholar] [CrossRef]
- Eubeler, J.P.; Bernhard, M.; Knepper, T.P. Environmental biodegradation of synthetic polymers: Biodegradation of different polymer groups. Trends Analyt. Chem. 2010, 29, 84–100. [Google Scholar] [CrossRef]
- Gewert, B.; Plassmann, M.M.; MacLeod, M. Pathways for degradation of plastic polymers floating in the marine environment. Environ. Sci. Process. Impacts 2015, 17, 1513–1521. [Google Scholar] [CrossRef] [Green Version]
- Jeon, H.J.; Kim, M.N. Comparison of the functional characterization between alkane monooxygenases for low-molecular-weight polyethylene biodegradation. Int. Biodeterior. Biodegrad. 2016, 114, 202–208. [Google Scholar] [CrossRef]
- Takei, D.; Washio, K.; Morikawa, M. Identification of alkane hydroxylase genes in Rhodococcus sp. strain TMP2 that degrades a branched alkane. Biotechnol. Lett. 2008, 30, 1447–1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojo, F. Degradation of alkanes by bacteria. Environ. Microbiol. 2009, 11, 2477–2490. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.S.; Kannan, V.R.; Nivas, D.; Kannan, K.; Chandru, S.; Antony, A.R. Biodegradation of HDPE by Aspergillus spp. from marine ecosystem of Gulf of Mannar, India. Mar. Pollut. Bull. 2015, 96, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Stempfle, L.; Ortmann, P.; Mecking, S. Long-Chain aliphatic polymers to bridge the gap between semicrystalline polyolefins and traditional polycondensates. Chem. Rev. 2016, 116, 4597–4641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimi, M.; Biria, D. The promiscuous activity of alpha-amylase in biodegradation of low density polyethylene in a polymer-starch blend. Nat. Sci. Rep. 2018, 9, 2612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawai, K.; Watanabe, M.; Shibata, M.; Yokoyama, S.; Sudate, Y.; Hayashi, S. Comparative study on biodegradability of polyethylene wax by bacteria and fungi. Polym. Degrad. Stab. 2004, 86, 105–114. [Google Scholar] [CrossRef]
- Albertsson, A.C.; Erlandsson, B.; Hakkarainen, M.; Karlsson, S. Molecular weight changes and polymeric matrix changes correlated with the formation of degradation products in biodegraded polyethylene. Environ. Polym. Degrad. 1998, 6, 187–195. [Google Scholar] [CrossRef]
- Eyheraguibel, B.; Traikia, M.; Fontanella, S.; Sancelme, M.; Bonhomme, S.; Fromageot, D.; Lemaire, L.; Lauranson, G.; Lacoste, J.; Delort, A.M. Characterization of oxidized oligomers from polyethylene films by mass spectrometry and NMR spectroscopy before and after biodegradation by a Rhodococcus rhodochrous strain. Chemosphere 2017, 184, 366–374. [Google Scholar] [CrossRef]
- Ojeda, T.; Freitas, A.; Dalmolin, E.; Dal Pizzol, M.; Vignol, L.; Melnik, J.; Jacques, R.; Bento, F.; Camargo, F. Abiotic and biotic degradation of oxo-biodegradable foamed polystyrene. Polym. Degrad. Stab. 2009, 94, 2128–2133. [Google Scholar] [CrossRef]
- Hua, Z.; Song, R.; Du, G.; Li, H.; Chen, J. Effects of EDTA and Tween60 on biodegradation of n-hexadecane with two strains of Pseudomonas aeruginosa. Biochem. Eng. J. 2007, 36, 66–71. [Google Scholar] [CrossRef]
- Gravouil, K.; Ferru-Clément, R.; Colas, S.; Helye, R.; Kadri, L.; Bourdeau, L.; Moumen, B.; Mercier, A.; Ferreira, T. Transcriptomics and lipidomics of the environmental strain Rhodococcus ruber point out consumption pathways and potential metabolic bottlenecks for polyethylene degradation. Environ. Sci. Technol. 2017, 51, 5172–5181. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha Devi, R.; Ramya, R.; Kannan, K.; Antony, A.R.; Kannan, V.R. Investigation of biodegradation potentials of high density polyethylene degrading marine bacteria isolated from the coastal regions of Tamil Nadu, India. Mar. Pollut. Bull. 2019, 138, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Chaudhary, D.R.; Jha, B. Destabilization of polyethylene and polyvinylchloride structure by marine bacterial strain. Environ. Sci. Pollut. Res. 2019, 26, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Delacuvellerie, A.; Cyriaque, V.; Gobert, S.; Benali, S.; Wattiez, R. The plastisphere in marine ecosystem hosts potential specific microbial degraders including Alcanivorax borkumensis as a key player for the low density polyethylene degradation. J. Hazard. Mater. 2019, 380, 120899. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, D.; Chirsteen, J.; Doble, M. Synergistic effects of pretreatment and blending on fungi mediated biodegradation of polypropylenes. Bioresour. Technol. 2013, 148, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Ahebnazar, Z.; Shojaosadati, S.A.; Mohammad-Taheri, M.; Nosrati, M. Biodegradation of low-density polyethylene (LDPE) by isolated fungi in solid waste medium. Waste Manag. 2010, 30, 396–401. [Google Scholar]
- Sheik, S.; Chandrashekar, K.R.; Swaroop, K.; Somashekarappa, H.M. Biodegradation of gamma irradiated low density polyethylene and polypropylene by endophytic fungi. Int. Biodeterior. Biodegrad. 2015, 105, 21–29. [Google Scholar] [CrossRef]
- Paco, A.; Duarte, K.; da Costa, J.P.; Santos, P.S.M.; Pereira, R.; Pereira, M.E.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A.P. Biodegradation of polyethylene microplastics by the marine fungus Zalerion maritimum. Sci. Total Environ. 2017, 586, 10–15. [Google Scholar] [CrossRef]
- Gilan, I.; Sivan, A. Effect of proteases on biofilm formation of the plastic-degrading actinomycete Rhodococcus ruber C208. FEMS Microbiol. Lett. 2013, 342, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Muenmee, S.; Chiemchaisri, W.; Chiemchaisri, C. Enhancement of biodegradation of plastic wastes via methane oxidation in semi-aerobic landfill. Int. Biodeterior. Biodegrad. 2016, 113, 244–255. [Google Scholar] [CrossRef]




| Authors | Year of Publication | Topic | References |
|---|---|---|---|
| Shimao | 2001 | Biodegradation of plastics | [9] |
| Koutny et al. | 2006 | Biodegradation of polyethylene films with prooxidant additives | [10] |
| Arutchelvi et al. | 2008 | Biodegradation of polyethylene and polypropylene | [11] |
| Shah et al. | 2008 | Biological degradation of plastics | [12] |
| Lucas et al. | 2008 | Polymer biodegradation: Mechanisms and estimation techniques | [13] |
| Tokiwa et al. | 2009 | Biodegradability of Plastics | [14] |
| Sivan | 2011 | New perspectives in plastic biodegradation | [15] |
| Ammala et al. | 2011 | An overview of degradable and biodegradable polyolefin | [16] |
| Restrepo-Flórez et al. | 2014 | Microbial degradation and deterioration of polyethylene | [17] |
| Sen and Raut | 2015 | Microbial degradation of low density polyethylene | [18] |
| Raziyafathima et al. | 2016 | Microbial Degradation of Plastic Waste: A Review | [19] |
| Emadian et al. | 2017 | Biodegradation of bioplastics in natural environments | [20] |
| Harrison et al. | 2018 | Biodegradability standards for carrier bags and plastic films in aquatic environments: A critical review | [21] |
| Genus (and Species) | Source | Experiment Duration | Experiment Condition | Biodegradation Result | Reference |
|---|---|---|---|---|---|
| Acinetobacter bumannii | Municipal landfill | 30 days | 37 °C Non-pretreated PE | Biomass production | [42] |
| Arthobacter defluvii | Dumped soil area | 1 month | PE bags | 20%–30% W.L. * | [48] |
| Bacillus amyloliquefaciens Bacillussubtilis | |||||
| Bacillus pumilus Bacillus subtillis | Pelagic waters | 30 days | PE bags | 1.5%–1.75% W.L. | [2] |
| Bacillus ssp. | Waste coal, a forest and an extinct volcano crater | 225 days | Modified PE | Reduction of mechanical properties by 98% No W.L. detected | [29] |
| Bacillus sphericus | Shallow waters of ocean | 1 year | HDPE and LDPE; Untreated and Heat treated | 3.5% and 10% 9% and 19% | [43] |
| Bacillus megaterium Bacillus subtilis Bacillus cereus (MIX together) | Soil | 90 days | 45 °C photo-degraded oxobiodegradable PE | 7%–10% mineralization | [31] |
| Bacillus amyloliquefaciens | Solid waste dumped | 60 days | LDPE | 11%–16% | [49] |
| Bacillus subtilis | MCC No. 2183 | 30 days | Adding Biosurfactant Unpretreated 18 μm thickness PE | 9.26% W.L. | [50] |
| Bacillus pumilus M27 Bacillus subtilis H1584 | Pelagic waters | 30 days | PE bags | 1.5–1.75 W.L. % | [2] |
| Brevibacillus borstelensis | DSMZ | 90 days | 50 °C Irradiated LDPE | 17% W.L. | [51] |
| Brevibacillus | Waste disposal site | 3 weeks | Pretreated PE | 37.5% W.L. | [41] |
| Chryseobacterium gleum | Waste water activated sludge soil | 1 month | UV-radiated LLDPE | - | [44] |
| Comamonas sp. | Plastic debris in soil | 90 days | Non-treated LDPE | Changing in chemical properties | [8] |
| Delftia sp. | Plastic debris in soil | 90 days | Non-treated LDPE | Changing in chemical properties | [8] |
| Kocuria palustris M16, | Pelagic waters | 30 days | PE bags | 1% | [2] |
| Microbacterium paraoxydans | Having Gene bank ID | 2 months | Pretreated LDPE | 61% W.L. | [52] |
| Pseudomonas sp. | Mangrove soil | 1 month | PE | 20.54% W.L. | [30] |
| Pseudomonas aeroginosa | Petroleum contaminated beach soil | 80 days | LMWPE | 40.8% W.L. | [45] |
| Pseudomonas sp. | Beach soil contaminated with crude oil | 80 days | 37 °C LMWPE | 4.9%–28.6% CO2 production | [46] |
| Pseudomonas sp. | Garbage soil | 6 months | PE bags | 37.09% W.L. | [34] |
| Pseudomonas citronellolis | Municipal Landfill | 4 days | LDPE | 17.8% W.L. | [38] |
| Pseudomonas sp. | Having Gene bank ID | 2 months | Pretreated LDPE | 50.5% W.L. | [52] |
| Pseudomonas aeroginosa Pseudomonas putida Pseudomonas siringae | ATCC | 120 days | Untreated PE | 9%–20% | [53] |
| Pseudomonas sp. | Waste disposal site | 3 weeks | Pretreated PE | 40.5% W.L. | [41] |
| Rhodococcus ruber | PE agricultural waste in soil | 4 weeks | Treated LDPE | Up to 8% W.L. | [36] |
| Rhodococcus ruber | PE agricultural waste in soil | 60 days | LDPE | 0.86% W.L./week | [54] |
| Rhodococcus ruber | PE agricultural waste in soil | 30 days | LDPE | 1.5%–2.5% W.L. Reduction of 20%.in Mw and 15%.in Mn | [55] |
| Rhodococcus rhorocuros | ATCC | 6 months | 27 °C Degradable PE | 60% mineralization | [56] |
| Rhodococcus rhorocuros | ATCC 29672 | 6 month | PE containing prooxidant additives | Different amount of mineralization | [57] |
| Rhodococcus sp. | Waste disposal site | 3 weeks | Pretreated PE | 33% W.L. | [41] |
| Rhodococcus sp. | Three forest soil | 30 days | LDPE containing prooxidant additives | Confirmation of Adhering | [35] |
| Staphylococcus arlettae | Various soil environments | 30 days | PE | 13.6% W.L. | [32] |
| Stentrophomonas sp. | Plastic debris in soil | 90 days | Non-treated LDPE | Changing in chemical properties | [8] |
| Stentrophomonas pavanii | Solid waste dump site | 56 days | Modified LDPE | Confirmed by FTIR | [40] |
| Streptomyces spp. | Nile River Delta | 1 month | 30 °C Heat treated degradable PE bags | 3 species showed slight W.L. | [58] |
| SI No. | Wave Number (cm−1) | Bond | Functional Group |
|---|---|---|---|
| 1 | 3000–2850 | –C–H stretch | Alkanes |
| 2 | 2830–2695 | H–C = O: C–H stretch | Aldehyde |
| 3 | 1710–1665 | –C = O stretch | Ketones, Aldehyde |
| 4 | 1470–1450 | –C–H Bend | Alkanes |
| 5 | 1320–1000 | –C–O stretch | Alcohol, Carboxylic acid, esters, ethers |
| 6 | 1000–650 | =C–H Bond | Alkenes |
| Species | Reference |
|---|---|
| Mucor rouxii; Aspergillus flavus | [58] |
| Penicillium simplicissimum | [65] |
| Cladosporium cladosporoides; Nocardia asteroides | [56] |
| Fusarium sp. | [33] |
| Aspergilus sp.; Fusarium sp | [87] |
| Gliocladium viride, Aspergillus awamori, and Mortierella subtilissima | [29] |
| Aspergilus sp. | [88] |
| Zalerion maritimum | [89] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montazer, Z.; Habibi Najafi, M.B.; Levin, D.B. Challenges with Verifying Microbial Degradation of Polyethylene. Polymers 2020, 12, 123. https://doi.org/10.3390/polym12010123
Montazer Z, Habibi Najafi MB, Levin DB. Challenges with Verifying Microbial Degradation of Polyethylene. Polymers. 2020; 12(1):123. https://doi.org/10.3390/polym12010123
Chicago/Turabian StyleMontazer, Zahra, Mohammad B. Habibi Najafi, and David B. Levin. 2020. "Challenges with Verifying Microbial Degradation of Polyethylene" Polymers 12, no. 1: 123. https://doi.org/10.3390/polym12010123






