Characterization of Engineering Plastics Plasticized Using Supercritical CO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Materials
2.3. Procedure
2.4. Characterization
3. Results and Discussion
3.1. Supercritical Treatment of PLLA
3.2. Plasticization Behavior of Engineering Plastics
3.3. Physical Properties
3.4. Electrical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Migahed, M.D.; Zidan, H.M. Influence of UV-irradiation on the structure and optical properties of polycarbonate films. Curr. Appl. Phys. 2006, 6, 91–96. [Google Scholar] [CrossRef]
- Mares, J.; Thongboonkerd, V.; Tuma, Z.; Moravec, J.; Matejovic, M. Specific adsorption of some complement activation proteins to polysulfone dialysis membranes during hemodialysis. Kidney Int. 2009, 76, 404–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDonald, W.A. Engineered films for display technologies. J. Mater. Chem. 2004, 14, 4–10. [Google Scholar] [CrossRef]
- Wagner, A.H.; Yu, J.S.; Kalyon, D.M. Injection molding of engineering plastics. Adv. Polym. Technol. 1989, 9, 17–32. [Google Scholar] [CrossRef]
- Hu, B.; Duan, X.; Xing, Z.; Xu, Z.; Du, C.; Zhou, H.; Chen, R.; Shan, B. Improved design of fused deposition modeling equipment for 3D printing of high-performance PEEK parts. Mech. Mater. 2019, 137, 103139. [Google Scholar] [CrossRef]
- Hassanajili, S.; Pour, A.; Oryan, A.; Khozani, T.T. Preparation and characterization of PLA/PCL/HA composite scaffolds using indirect 3D printing for bone tissue engineering. Mater. Sci. Eng. C 2019, 104, 109960. [Google Scholar] [CrossRef]
- Teo, N.; Gu, Z.; Jana, S.C. Polyimide-based aerogel foams, via emulsion-templating. Polymer 2018, 157, 95–102. [Google Scholar] [CrossRef]
- Youn, S.W.; Noguchi, T.; Takahashi, M.; Maeda, R. Fabrication of micro mold for hot-embossing of polyimide microfluidic platform by using electron beam lithography combined with inductively coupled plasma. Microelectron. Eng. 2008, 85, 918–921. [Google Scholar] [CrossRef]
- Tom, J.W.; Debenedetti, P.G. Formation of bioerodible polymeric microspheres and microparticles by rapid expansion of supercritical solutions. Biotechnol. Prog. 1991, 7, 403–411. [Google Scholar] [CrossRef]
- Reverchon, E.; Porta, G.D.; Taddeo, R. Solubility and micronization of griseofulvin in supercritical CHF3. Ind. Eng. Chem. Res. 1995, 34, 4087–4091. [Google Scholar] [CrossRef]
- Kim, J.H.; Paxton, T.E.; Tomasko, D.L. Microencapsulation of naproxen using rapid expansion of supercritical solutions. Biotechnol. Prog. 1996, 12, 650–661. [Google Scholar] [CrossRef]
- Jung, J.; Perrut, M. Particle design using supercritical fluids: Literature and patent survey. J. Supercrit. Fluids 2001, 20, 179–219. [Google Scholar] [CrossRef]
- Turk, M.; Hils, P.; Helfgen, B.; Schaber, K.; Martin, H.J.; Wahl, M.A. Micronization of pharmaceutical substances by the Rapid Expansion of Supercritical Solution (RESS): A promising method to improve bioavailability of poorly soluble pharmaceutical agents. J. Supercrit. Fluids. 2002, 22, 75–84. [Google Scholar] [CrossRef]
- Kayrak, D.; Akman, U.; Hortacsu, O. Micronization of ibuprofen by RESS. J. Supercrit. Fluids 2003, 26, 17–31. [Google Scholar] [CrossRef]
- Alnaief, M.; Antonyuk, S.; Hentzschel, C.M.; Leopold, C.S.; Heinrich, S.; Smirnova, I. A novel process for coating silica aerosol microsphere for controlled drug release applications. Microporous Mesoporous Mater. 2012, 160, 167–173. [Google Scholar] [CrossRef]
- Tomasko, D.L.; Li, H.; Liu, D.; Han, X.; Wingert, M.J.; Lee, L.J.; Koelling, K.W. A review of CO2 applications in the processing of polymers. Ind. Eng. Chem. Res. 2003, 42, 6431–6456. [Google Scholar] [CrossRef]
- Kiran, E. Polymer miscibility, phase separation, morphological modifications and polymorphic transformations in dense fluids. J. Supercrit. Fluids 2009, 47, 466–483. [Google Scholar] [CrossRef]
- Nalawade, S.P.; Picchioni, F.; Janssen, L.P.B.M. Supercritical carbon dioxide as a green solvent for processing polymer melts: Processing aspects and applications. Prog. Poly. Sci. 2006, 31, 19–43. [Google Scholar] [CrossRef] [Green Version]
- Kiran, E. Supercritical fluids and polymers—The year in review—2014. J. Supercrit. Fluids 2016, 110, 126–153. [Google Scholar] [CrossRef]
- Matson, D.W.; Fulton, J.L.; Petersen, R.C.; Smith, R.D. Rapid expansion of supercritical fluid solutions: Solute formation of powders, thin films, and fibers. Ind. Eng. Chem. Res. 1987, 26, 2298–2306. [Google Scholar] [CrossRef]
- Petersen, R.C.; Matson, D.W.; Smith, R.D. Rapid precipitation of low vapor pressure solids from supercritical fluid solutions: The formation of thin films and powders. J. Am. Chem. Soc. 1986, 108, 2100–2102. [Google Scholar] [CrossRef]
- Kongsombut, B.; Chen, W.; Tsutsumi, A.; Tanthapanichakoon, W.; Charinpanitkul, T. Formation of deagglomerated PLGA particles and PLGA-coated ultra fine powders by rapid expansion of supercritical solution with ethanol cosolvent. Korean J. Chem. Eng. 2008, 25, 838–845. [Google Scholar] [CrossRef]
- Lee, J.W.S.; Wang, K.; Park, C.B. Challenge to extrusion of low-density microcellular polycarbonate foams using supercritical carbon dioxide. Ind. Eng. Chem. Res. 2005, 44, 92–99. [Google Scholar] [CrossRef]
- Kirby, C.F.; McHugh, M.A. Phase behavior of polymers in supercritical fluid solvents. Chem. Rev. 1999, 99, 565–602. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Yurugi, M.; Fujiwara, K.; Takishima, S.; Masuoka, H. Solubilities of carbon dioxide and nitrogen in polystyrene under high temperature and pressure. Fluid Phase Equilib. 1996, 125, 129–138. [Google Scholar] [CrossRef]
- Sato, Y.; Takikawa, T.; Takishima, S.; Masuoka, H. Solubilities and diffusion coefficients of carbon dioxide in poly(vinyl acetate) and polystyrene. J. Supercrit. Fluids 2001, 19, 187–198. [Google Scholar] [CrossRef]
- Nemoto, T.; Tanaka, C. Method for Producing Polymer and Device for Producing Polymer. U.S. Patent 9346915, 24 May 2016. [Google Scholar]
- Nishikawa, S. The modification and processing of polymers with supercritical carbon dioxide. Soc. Rubber Sci. Technol. Jpn. 2004, 77, 19–23. [Google Scholar]







| Polymer (Abbreviation) | Molecular Structure | Crystal Structure | Tg/°C | Tm/°C |
|---|---|---|---|---|
| Polycarbonate (PC) | 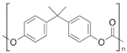 | Amorphous | 150 | - |
| Polysulfone (PSU) | 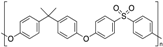 | Amorphous | 190 | - |
| Polyarylate (PAR) | 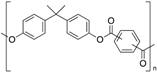 | Amorphous | 193 | - |
| Polyetherimide (PEI) |  | Amorphous | 217 | - |
| Polyphenylsulfone (PPSU) | 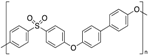 | Amorphous | 220 | - |
| Polylactic acid (PLLA) | 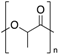 | Crystalline | 60 | 175 |
| Polyethylene terephthalate (PET) | 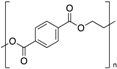 | Crystalline | 69 | 260 |
| Polybutylene terephthalate (PBT) | 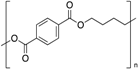 | Crystalline | 50 | 225 |
| Polyamide 6 (PA 6) | 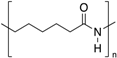 | Crystalline | 50 | 225 |
| Number | Polymer | Temperature/°C | Pressure/MPa | Time/h |
|---|---|---|---|---|
| 1 | PLLA | 150 | 5 | 1 |
| 2 | 15 | 0.5 | ||
| 3 | 15 | 1 | ||
| 4 | 15 | 2 | ||
| 5 | 25 | 1 | ||
| 6 | PC | 120 | 25 | 1 |
| 7 | PSU | 150 | 25 | 1 |
| 8 | PAR | 150 | 25 | 1 |
| 9 | PEI | 150 | 25 | 1 |
| 10 | PPSU | 150 | 25 | 1 |
| 11 | PET | 150 | 25 | 1 |
| 12 | PBT | 150 | 25 | 1 |
| 13 | PA6 | 150 | 25 | 1 |
| Weight-Averaged Molecular Weight (Mw) | Number-Averaged Molecular Weight (Mn) | |
|---|---|---|
| Before | 186,000 ± 1000 | 138,000 ± 1000 |
| After | 182,000 ± 1000 | 131,000 ± 1000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, M.; Hashimoto, Y.; Kimura, T.; Kishida, A. Characterization of Engineering Plastics Plasticized Using Supercritical CO2. Polymers 2020, 12, 134. https://doi.org/10.3390/polym12010134
Watanabe M, Hashimoto Y, Kimura T, Kishida A. Characterization of Engineering Plastics Plasticized Using Supercritical CO2. Polymers. 2020; 12(1):134. https://doi.org/10.3390/polym12010134
Chicago/Turabian StyleWatanabe, Masaki, Yoshihide Hashimoto, Tsuyoshi Kimura, and Akio Kishida. 2020. "Characterization of Engineering Plastics Plasticized Using Supercritical CO2" Polymers 12, no. 1: 134. https://doi.org/10.3390/polym12010134
APA StyleWatanabe, M., Hashimoto, Y., Kimura, T., & Kishida, A. (2020). Characterization of Engineering Plastics Plasticized Using Supercritical CO2. Polymers, 12(1), 134. https://doi.org/10.3390/polym12010134






