Core-Shell Graphitic Carbon Nitride/Zinc Phytate as a Novel Efficient Flame Retardant for Fire Safety and Smoke Suppression in Epoxy Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of g-C3N4/PAZn
2.3. Preparation of EP Composites
2.4. Characterization
3. Results and Discussion
3.1. Characterization of g-C3N4/PAZn
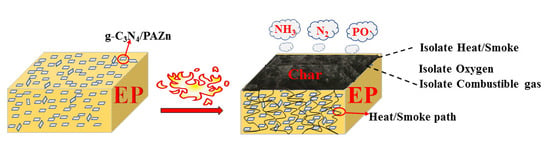
3.2. Fire and Smoke Hazards of EP Composites
3.3. Research on Flame-Retardant Mechanism
3.3.1. Thermal Stability Analysis of EP Composites
3.3.2. Char Residue Analysis
3.4. Mechanical Property
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wu, H.Y.; Zeng, B.R.; Chen, J.M.; Wu, T.; Li, Y.T.; Liu, Y.Z.; Dai, L.Z. An intramolecular hybrid of metal polyhedral oligomeric silsesquioxanes with special titanium-embedded cage structure and flame retardant functionality. Chem. Eng. J. 2019, 374, 1304–1316. [Google Scholar] [CrossRef]
- Chen, L.M.; Song, T.Y.; Wang, J.Y.; Shi, H.; Hao, J.W. Intrinsic flame-retardant epoxy resin composites with benzoxazine: Effect of a catalyst and a low curing temperature. J. Appl. Polym. Sci. 2019, 136. [Google Scholar] [CrossRef]
- Shahan, A.; Al-Shukriu, S.M.; Kahlaf, H.I.; Al Hanbali, O.A. Synthesis, characterization, thermal and fire retardant properties of new homo-and block copolymers of polyacrylate and epoxy resin with cyclotriphosphazene core. Polimery 2019, 64, 578–591. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.N.; Pan, H.F.; Ma, W.B.; Lian, J.L.; Shen, Q.; Zhu, Q.; Yang, X.F. Synthesis of CeO2-loaded titania nanotubes and its effect on the flame retardant property of epoxy resin. Polym. Adv. Technol. 2019, 30, 2136–2142. [Google Scholar] [CrossRef]
- Huang, S.C.; Deng, C.; Wang, S.X.; Wei, W.C.; Chen, H.; Wang, Y.Z. Electrostatic action induced interfacial accumulation of layered double hydroxides towards highly efficient flame retardance and mechanical enhancement of thermoplastic polyurethane/ammonium polyphosphate. Polym. Degrad. Stab. 2019, 165, 126–136. [Google Scholar] [CrossRef]
- Tawiah, B.; Yu, B.; Yuen, R.K.K.; Hu, Y.; Wei, R.C.; Xin, J.H.; Fei, B. Highly efficient flame retardant and smoke suppression mechanism of boron modified graphene Oxide/Poly(Lactic acid) nanocomposites. Carbon 2019, 150, 8–20. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Fu, L.B.; Chen, X.L.; Guo, J.; Yang, F.Q.; Wang, J.G.; Zheng, Y.Y.; Hu, Y. Hypophosphite/graphitic carbon nitride hybrids: Preparation and flame-retardant application in thermoplastic polyurethane. Nanomaterials 2017, 7, 259. [Google Scholar] [CrossRef] [Green Version]
- Yue, X.P.; Li, C.F.; Ni, Y.H.; Xu, Y.J.; Wang, J. Flame retardant nanocomposites based on 2D layered nanomaterials: A review. J. Mater. Sci. 2019, 54, 13070–13105. [Google Scholar] [CrossRef]
- Xu, L.F.; Tan, X.W.; Xu, R.J.; Xie, J.Y.; Lei, C.H. Influence of functionalized molybdenum disulfide (MoS2) with triazine derivatives on the thermal stability and flame retardancy of intumescent Poly(lactic acid) system. Polym. Compos. 2019, 40, 2244–2257. [Google Scholar] [CrossRef]
- Guo, F.; Li, M.Y.; Ren, H.J.; Huang, X.L.; Shu, K.K.; Shi, W.L.; Lu, C.Y. Facile bottom-up preparation of Cl-doped porous g-C3N4 nanosheets for enhanced photocatalytic degradation of tetracycline under visible light. Sep. Purif. Technol. 2019, 228. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Yu, B.; Duan, L.J.; Gui, Z.; Wang, B.B.; Hu, Y.; Yuen, R.K.K. Graphitic carbon nitride/phosphorus-rich aluminum phosphinates hybrids as smoke suppressants and flame retardants for polystyrene. J. Hazard. Mater. 2017, 332, 87–96. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Jiang, S.H.; Zhou, K.Q.; Bao, C.L.; Yu, B.; Qian, X.D.; Wang, B.B.; Hong, N.N.; Wen, P.Y.; Gui, Z.; et al. Influence of g-C3N4 nanosheets on thermal stability and mechanical properties of biopolymer electrolyte nanocomposite films: A novel investigation. ACS Appl. Mater. Interfaces 2014, 6, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Im, S.; Kim, J.C.; Hong, W.G.; Shin, K.; Jeong, H.Y.; Hong, Y.J. Phytic acid doped polyaniline nanofibers for enhanced aqueous copper(II) adsorption capability. ACS Sustain. Chem. Eng. 2017, 5, 6654–6664. [Google Scholar] [CrossRef]
- Shang, S.; Yuan, B.H.; Sun, Y.R.; Chen, G.Q.; Huang, C.Y.; Yu, B.; He, S.; Dai, H.M.; Chen, X.F. Facile preparation of layered melamine-phytate flame retardant via supramolecular self-assembly technology. J. Colloid Interface Sci. 2019, 553, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.Y.; Wang, D.; Li, M.; Zhang, L.P.; Liu, M.M.; Fu, S.H. N-P-Zn-containing 2D supermolecular networks grown on MoS2 nanosheets for mechanical and flame-retardant reinforcements of polyacrylonitrile fiber. Chem. Eng. J. 2019, 372, 873–885. [Google Scholar] [CrossRef]
- Liu, L.B.; Xu, Y.; Xu, M.J.; Li, Z.Q.; Hu, Y.M.; Li, B. Economical and facile synthesis of a highly efficient flame retardant for simultaneous improvement of fire retardancy, smoke suppression and moisture resistance of epoxy resins. Compos. Part B Eng. 2019, 167, 422–433. [Google Scholar] [CrossRef]
- Li, Z.W.; Jiang, G.D.; Zhang, Z.H.; Wu, Y.; Han, Y.H. Phosphorus-doped g-C3N4 nanosheets coated with square flake-like TiO2: Synthesis, characterization and photocatalytic performance in visible light. J. Mol. Catal. A Chem. 2016, 425, 340–348. [Google Scholar] [CrossRef]
- Cheng, L.Y.; Wu, W.H.; Meng, W.H.; Xu, S.; Han, H.D.; Yu, Y.F.; Qu, H.Q.; Xu, J.Z. Application of metallic phytates to poly(vinyl chloride) as efficient biobased phosphorous flame retardants. J. Appl. Polym. Sci. 2018, 135. [Google Scholar] [CrossRef]
- Shi, Y.H.; Huang, J.H.; Zeng, G.M.; Cheng, W.J.; Hu, J.L.; Shi, L.X.; Yi, K.X. Evaluation of self-cleaning performance of the modified g-C3N4 and GO based PVDF membrane toward oil-in-water separation under visible-light. Chemosphere 2019, 230, 40–50. [Google Scholar] [CrossRef]
- Cai, K.; Shen, W.; Ren, B.Y.; He, J.; Wu, S.Z.; Wang, W. A phytic acid modified CoFe2O4 magnetic adsorbent with controllable morphology, excellent selective adsorption for dyes and ultra-strong adsorption ability for metal ions. Chem. Eng. J. 2017, 330, 936–946. [Google Scholar] [CrossRef]
- Zeeshan, M.; Nozari, V.; Keskin, S.; Uzun, A. Structural factors determining thermal stability limits of ionic liquid/MOF composites: Lmidazolium ionic liquids combined with CuBTC and ZIF-8. Ind. Eng. Chem. Res. 2019, 58, 14124–14138. [Google Scholar] [CrossRef]
- Feng, Y.Z.; He, C.G.; Wen, Y.F.; Ye, Y.S.; Zhou, X.P.; Xie, X.L.; Mai, Y.W. Superior flame retardancy and smoke suppression of epoxy-based composites with phosphorus/nitrogen co-doped graphene. J. Hazard. Mater. 2018, 346, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.J.; Liao, D.J.; Hu, X.P.; Pan, N.; Li, W.X.; Wang, D.Y.; Yao, Y. Facile fabrication of biobased PeNeC-containing nano-layered hybrid: Preparation, growth mechanism and its efficient fire retardancy in epoxy. Polym. Degrad. Stab. 2019, 159, 153–162. [Google Scholar] [CrossRef]
- Geng, Y.J.; Liu, Z.H. Preparation and thermodynamic characterization of 2CaO·B2O3·H2O nanomaterials with enhanced flame retardant properties. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 563–568. [Google Scholar] [CrossRef]
- Zhang, Q.R.; Li, Z.W.; Li, X.H.; Yu, L.G.; Zhang, Z.J.; Wu, Z.S. Preparation of cobalt ferrite nanoparticle-decorated boron nitride nanosheet flame retardant and its flame retardancy in epoxy resin. Nano 2019, 14. [Google Scholar] [CrossRef]
- Wang, D.; Mu, X.W.; Cai, W.; Song, L.; Ma, C.; Hu, Y. Constructing phosphorus, nitrogen, silicon-co-contained boron nitride nanosheets to reinforce flame retardant properties of unsaturated polyester resin. Compos. Part A Appl. Sci. 2018, 109, 546–554. [Google Scholar] [CrossRef]
- Qiu, S.L.; Hou, Y.B.; Xing, W.Y.; Ma, C.; Zhou, X.; Liu, L.X.; Kan, Y.C.; Yuen, R.K.K.; Hu, Y. Self-assembled supermolecular aggregate supported on boron nitride nanoplatelets for flame retardant and friction application. Chem. Eng. J. 2018, 349, 223–234. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Yu, B.; Zheng, Y.Y.; Yang, J.; Duan, Z.P.; Hu, Y. Design of reduced graphene oxide decorated with DOPO-phosphanomidate for enhanced fire safety of epoxy resin. J. Colloid Interf. Sci. 2018, 521, 160–171. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Long, Z.; Yu, B.; Zhou, K.Q.; Gui, Z.; Yuen, R.K.K.; Hu, Y. Tunable thermal, flame retardant and toxic effluent suppression properties of polystyrene based on alternating graphitic carbon nitride and multi-walled carbon nanotubes. J. Mater. Chem. A 2015, 3, 17064–17073. [Google Scholar] [CrossRef]
- Zhang, W.W.; Wu, H.J.; Meng, W.H.; Zhang, M.J.; Xie, W.Y.; Bian, G.; Qu, H.Q. Synthesis of activated carbon and different types phosphomolybdate ionic liquid composites for flame retardancy of poly(vinyl chloride). Mater. Res. Express 2019, 6. [Google Scholar] [CrossRef]
- Xiao, F.; Wu, K.; Luo, F.B.; Yao, S.; Lv, M.P.; Zou, H.M.; Lu, M.G. Influence of ionic liquid-based metal-organic hybrid on thermal degradation, flame retardancy, and smoke suppression properties of epoxy resin composites. J. Mater. Sci. 2018, 53, 10135–10146. [Google Scholar] [CrossRef]
- Rong, M.C.; Cai, Z.X.; Xie, L.; Lin, C.S.; Song, X.H.; Luo, F.; Wang, Y.R.; Chen, X. Study on the Ultrahigh Quantum Yield of Fluorescent P,O-g-C3N4 Nanodots and its Application in Cell Imaging. Chem. Eur. J. 2016, 22, 9387–9395. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Cao, Y.F.; Qian, L.J.; Chen, Y.J.; Qiu, Y.; Xu, B.; Xin, F. Synergistic Charring Flame-Retardant Behavior of Polyimide and Melamine Polyphosphate in Glass Fiber-Reinforced Polyamide 66. Polymers 2019, 11, 1851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, J.W.; Liang, C.B.; Zhao, X.M.; Gan, B.; Qiu, H.; Guo, Y.Q.; Yang, X.T.; Zhang, Q.Y.; Wang, D.Y. Highly thermally conductive flame-retardant epoxy nanocomposites with reduced ignitability and excellent electrical conductivities. Compos. Sci. Technol. 2017, 139, 83–89. [Google Scholar] [CrossRef]
- Yao, Z.Y.; Liu, X.X.; Qian, L.J.; Chen, Y.J.; Xu, B.; Qiu, Y. Synthesis and Characterization of Aluminum 2-Carboxyethyl-Phenyl-Phosphinate and Its Flame-Retardant Application in Polyester. Polymers 2019, 12, 1969. [Google Scholar] [CrossRef] [Green Version]
- Walle, M.D.; Zhang, Z.F.; Zhang, M.Y.; You, X.L.; Li, Y.J.; Liu, Y.N. Hierarchical 3D nitrogen and phosphorous codoped graphene/carbon nanotubes-sulfur composite with synergistic effect for high performance of lithium-sulfur batteries. J. Mater. Sci. 2018, 53, 2685–2696. [Google Scholar] [CrossRef]
- Suresha, B.; Saini, M.S. Influence of organo-modified montmorillonite nanolayers on static mechanical and dynamic mechanical behavior of carbon/epoxy composites. J. Compos. Mater. 2016, 50, 3589–3601. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.C.; Liu, Q.; Cai, X.F. Synergistic effects between silicon-containing flame retardant and DOPO on flame retardancy of epoxy resins. J. Therm. Anal. Calorim. 2016, 123, 1343–1350. [Google Scholar] [CrossRef]
- Yang, G.; Wu, W.H.; Wang, Y.H.; Jiao, Y.H.; Lu, L.Y.; Qu, H.Q.; Qin, X.Y. Synthesis of a novel phosphazene-based flame retardant with active amine groups and its application in reducing the fire hazard of Epoxy Resin. J. Hazard. Mater. 2019, 366, 78–87. [Google Scholar] [CrossRef]









| Sample | EP (phr) | m-Phenylenediamine (phr) | g-C3N4 (phr) | g-C3N4/PAZn (phr) |
|---|---|---|---|---|
| Pure EP | 100 | 11 | 0 | 0 |
| g-C3N4-EP | 100 | 11 | 5 | 0 |
| g-C3N4/PAZn-EP | 100 | 11 | 0 | 5 |
| Sample | TTI (s) | PHRR (kW/m2) | THR (MJ/m2) | FGI (kW/m2 s) | PSPR (m2/s) | TSP (m2) | EHC (MJ/kg) | R-Mass (%) |
|---|---|---|---|---|---|---|---|---|
| Pure EP | 60 | 1458.14 | 109.79 | 11.22 | 0.48 | 35.19 | 24.88 | 4.58 |
| g-C3N4-EP | 61 | 906.28 | 101.25 | 6.71 | 0.34 | 37.85 | 20.91 | 7.60 |
| g-C3N4/PAZn-EP | 63 | 417.26 | 46.15 | 3.48 | 0.36 | 35.09 | 11.39 | 12.90 |
| Samples | T5% (°C) | Tmax (°C) | Vmax (%/min) | R-Mass (%) |
|---|---|---|---|---|
| Pure EP | 356.3 | 371.7 | 15.87 | 17.99 |
| g-C3N4-EP | 356.3 | 371.7 | 13.85 | 18.83 |
| g-C3N4/PAZn-EP | 346.7 | 361.2 | 11.99 | 23.21 |
| Samples | Tensile Strength (MPa) | Elongation at Break (%) | Impact Strength (kJ/m2) |
|---|---|---|---|
| Pure EP | 57.70 ± 0.84 | 14.67 ± 0.57 | 50.83 ± 1.21 |
| g-C3N4-EP | 51.59 ± 0.67 | 13.17 ± 0.51 | 35.619 ± 0.87 |
| g-C3N4/PAZn-EP | 35.11 ± 0.54 | 6.49 ± 0.25 | 26.04 ± 0.46 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Wu, W.; Meng, W.; Xie, W.; Cui, Y.; Xu, J.; Qu, H. Core-Shell Graphitic Carbon Nitride/Zinc Phytate as a Novel Efficient Flame Retardant for Fire Safety and Smoke Suppression in Epoxy Resin. Polymers 2020, 12, 212. https://doi.org/10.3390/polym12010212
Zhang W, Wu W, Meng W, Xie W, Cui Y, Xu J, Qu H. Core-Shell Graphitic Carbon Nitride/Zinc Phytate as a Novel Efficient Flame Retardant for Fire Safety and Smoke Suppression in Epoxy Resin. Polymers. 2020; 12(1):212. https://doi.org/10.3390/polym12010212
Chicago/Turabian StyleZhang, Weiwei, Weihong Wu, Weihua Meng, Weiya Xie, Yumeng Cui, Jianzhong Xu, and Hongqiang Qu. 2020. "Core-Shell Graphitic Carbon Nitride/Zinc Phytate as a Novel Efficient Flame Retardant for Fire Safety and Smoke Suppression in Epoxy Resin" Polymers 12, no. 1: 212. https://doi.org/10.3390/polym12010212
APA StyleZhang, W., Wu, W., Meng, W., Xie, W., Cui, Y., Xu, J., & Qu, H. (2020). Core-Shell Graphitic Carbon Nitride/Zinc Phytate as a Novel Efficient Flame Retardant for Fire Safety and Smoke Suppression in Epoxy Resin. Polymers, 12(1), 212. https://doi.org/10.3390/polym12010212