Rapid Photoinduced Single Cell Detachment from Gold Nanoparticle-Embedded Collagen Gels with Low Denaturation Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of AuNP-Embedded Collagen Gels
2.2. Characterization
2.3. Laser-Induced Cell Detachment
3. Results
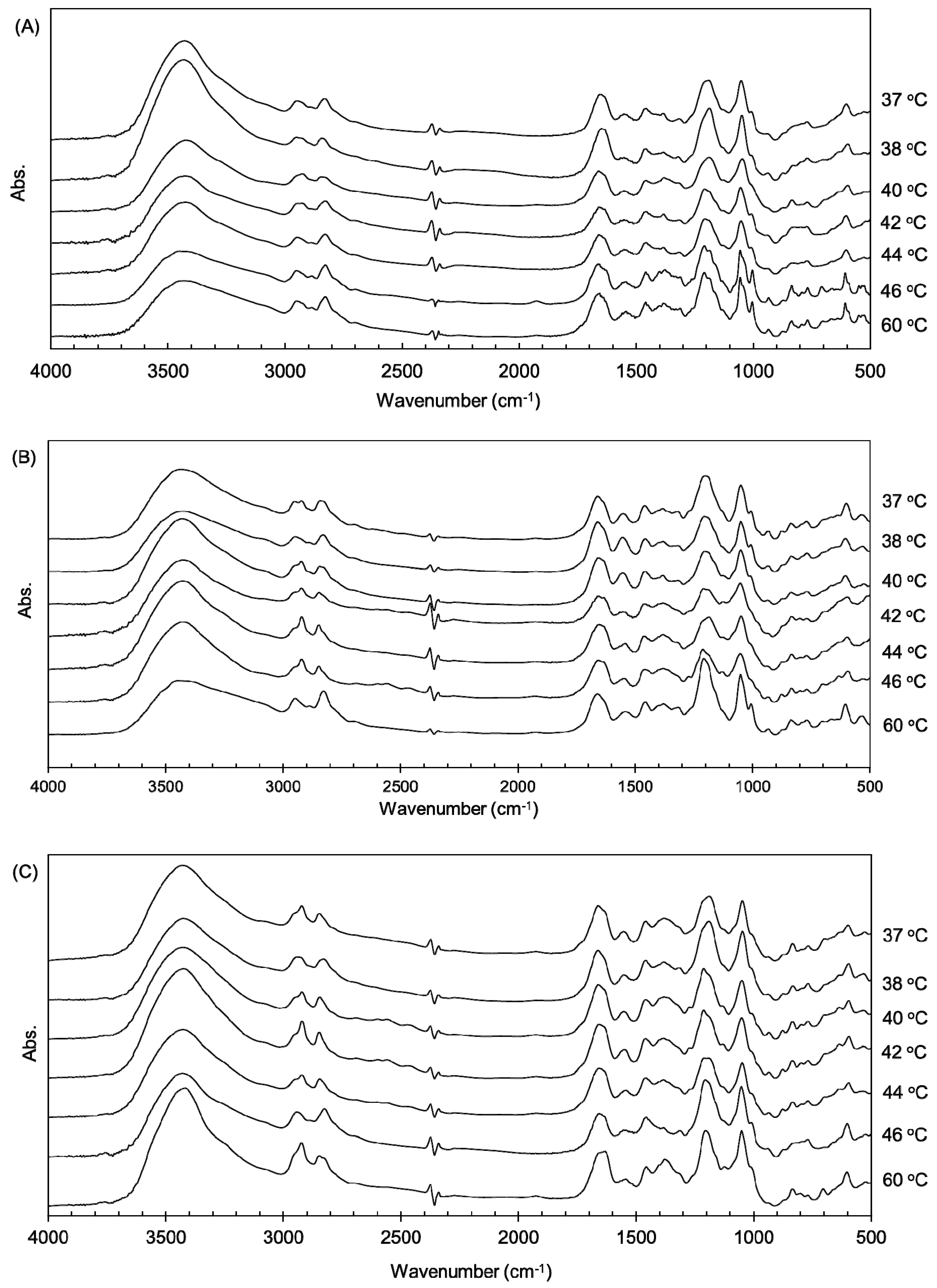
3.1. Thermal Denaturation of Different Collagen Gels
3.2. Thermal Changes of Collagen Fibers in Collagen Gels Containing AuNPs
3.3. Selective Cell Detachment from Various AuNP-Embedded Collagen Gels
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nagase, K.; Okano, T. Thermoresponsive-polymer-based materials for temperature-modulated bioanalysis and bioseparations. J. Mater. Chem. B 2016, 4, 6381–6397. [Google Scholar] [CrossRef] [Green Version]
- Bhagat, A.A.; Bow, H.; Hou, H.W.; Tan, S.J.; Han, J.; Lim, C.T. Microfluidics for cell separation. Med. Biol. Eng. Comput. 2010, 48, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Gossett, D.R.; Weaver, W.M.; MacH, A.J.; Hur, S.C.; Tse, H.T.; Lee, W.; Amini, H.; Di Carlo, D. Label-free cell separation and sorting in microfluidic systems. Anal. Bioanal. Chem. 2010, 397, 3249–3267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schütze, K.; Niyaz, Y.; Stich, M.; Buchstaller, A. Noncontact laser microdissection and catapulting for pure sample capture. Methods Cell Biol. 2007, 82, 649–673. [Google Scholar] [PubMed]
- Koller, M.R.; Hanania, E.G.; Stevens, J.; Eisfeld, T.M.; Sasaki, G.C.; Fieck, A.; Palsson, B.Ø. High-throughput laser-mediated in situ cell purification with high purity and yield. Cytometry A 2004, 61, 153–161. [Google Scholar] [CrossRef]
- Kojima, C.; Nakajima, Y.; Oeda, N.; Kawano, T.; Taki, Y. Visible laser-induced in situ cell detachment from gold nanoparticle-embedded collagen gel. Macromol. Biosci. 2017, 17, 1600341. [Google Scholar] [CrossRef]
- Nakajima, Y.; Kawano, T.; Taki, Y.; Kojima, C. Visible light-responsive cell scaffolds with bilayer structures for single cell separation. Res. Chem. Intermed. 2018, 44, 4745–4754. [Google Scholar] [CrossRef]
- Falahati, M.; Attar, F.; Sharifi, M.; Saboury, A.A.; Salihi, A.; Aziz, F.M.; Kostova, I.; Burda, C.; Priecel, P.; Lopez-Sanchez, J.A.; et al. Gold nanomaterials as key suppliers in biological and chemical sensing, catalysis, and medicine. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129435. [Google Scholar] [CrossRef]
- Govorov, A.O.; Richardson, H.H. Generating heat with metal nanoparticles. Nano Today 2007, 2, 30–38. [Google Scholar] [CrossRef]
- Djabourov, M.; Lechaire, J.-P.; Gaill, F. Structure and rheology of gelatin and collagen gels. Biorheology 1993, 30, 191–205. [Google Scholar] [CrossRef] [Green Version]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Ann. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, R.A.; Prockop, D.J. The thermal transition of a non-hydroxylated form of collagen. Evidence for a role for hydroxyproline in stabilizing the triple-helix of collagen. Biochem. Biophys. Res. Commun. 1973, 52, 115–120. [Google Scholar] [CrossRef]
- Holmes, R.; Kirk, S.; Tronci, G.; Yang, X.; Wood, D. Influence of telopeptides on the structural and physical properties of polymeric and monomeric acid-soluble type I collagen. Mater. Sci. Eng. C 2017, 77, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Lynn, A.K.; Yannas, I.V.; Bonfield, W. Antigenicity and immunogenicity of collagen. J. Biomed. Mater. Res. B 2004, 71B, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Yagi, S.; Oeda, N.; Kojima, C. Electroless growth of size-controlled gold nanoparticles using hydroquinone. J. Electrochem. Soc. 2012, 159, H668–H673. [Google Scholar] [CrossRef]
- Jackson, M.; Choo, L.P.; Watson, P.H.; Halliday, W.C.; Mantsch, H.H. Beware of connective tissue proteins: Assignment and implications of collagen absorptions in infrared spectra of human tissues. Biochim. Biophys. Acta 1995, 1270, 1–6. [Google Scholar] [CrossRef]
- Tian, Z.; Wu, K.; Liu, W.; Shen, L.; Li, G. Two-dimensional infrared spectroscopic study on the thermally induced structural changes of glutaraldehyde-crosslinked collagen. Spectrochim. Acta A 2015, 140, 356–363. [Google Scholar] [CrossRef]
- Pielesz, A. Temperature-dependent FTIR spectra of collagen and protective effect of partially hydrolysed fucoidan. Spectrochim. Acta A 2014, 118, 287–293. [Google Scholar] [CrossRef]
- Suwa, Y.; Nam, K.; Ozeki, K.; Kimura, T.; Kishida, A.; Masuzawa, T. Thermal denaturation behavior of collagen fibrils in wet and dry environment. J. Biomed. Mater. Res. B 2016, 104B, 538–545. [Google Scholar] [CrossRef]
- Minai, L.; Yeheskely-Hayon, D.; Golan, L.; Bisker, G.; Dann, E.J.; Yelin, D. Optical nanomanipulations of malignant cells: Controlled cell damage and fusion. Small 2012, 8, 1732–1739. [Google Scholar] [CrossRef]
- Misawa, M.; Takahashi, J. Generation of reactive oxygen species induced by gold nanoparticles under X-ray and UV Irradiations. Nanomedicine 2011, 7, 604–614. [Google Scholar] [CrossRef] [PubMed]









| Collagen | Origin | Preparation |
|---|---|---|
| Cellmatrix Type I-A | porcine tendons | acid |
| Atelocell IAC-50 | bovine dermis | acid |
| Cellmatrix Type I-C | porcine skin | pepsin |
| Collagen | Telopeptide | Au | FT-IR (°C) | Rheology (°C) | Sol–gel (°C) |
|---|---|---|---|---|---|
| Cellmatrix Type I-A | + | - | 38 | 51 1 | 50 ± 2 |
| + | 41 | 55 1 | 50 ± 2 | ||
| Attelocell IAC | + | - | 42 | 48 2 | 52 ± 0 |
| + | 45 | 54 2 | 52 ± 0 | ||
| Cellmatrix Type I-C | + | - | 40 | 43 2 | 46 ± 0 |
| + | 39 | 45 2 | 46 ± 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kojima, C.; Nishio, M.; Nakajima, Y.; Kawano, T.; Takatsuka, K.; Matsumoto, A. Rapid Photoinduced Single Cell Detachment from Gold Nanoparticle-Embedded Collagen Gels with Low Denaturation Temperature. Polymers 2020, 12, 213. https://doi.org/10.3390/polym12010213
Kojima C, Nishio M, Nakajima Y, Kawano T, Takatsuka K, Matsumoto A. Rapid Photoinduced Single Cell Detachment from Gold Nanoparticle-Embedded Collagen Gels with Low Denaturation Temperature. Polymers. 2020; 12(1):213. https://doi.org/10.3390/polym12010213
Chicago/Turabian StyleKojima, Chie, Misaki Nishio, Yusuke Nakajima, Takeshi Kawano, Kenji Takatsuka, and Akikazu Matsumoto. 2020. "Rapid Photoinduced Single Cell Detachment from Gold Nanoparticle-Embedded Collagen Gels with Low Denaturation Temperature" Polymers 12, no. 1: 213. https://doi.org/10.3390/polym12010213







