Review on Blueprint of Designing Anti-Wetting Polymeric Membrane Surfaces for Enhanced Membrane Distillation Performance
Abstract
1. Introduction
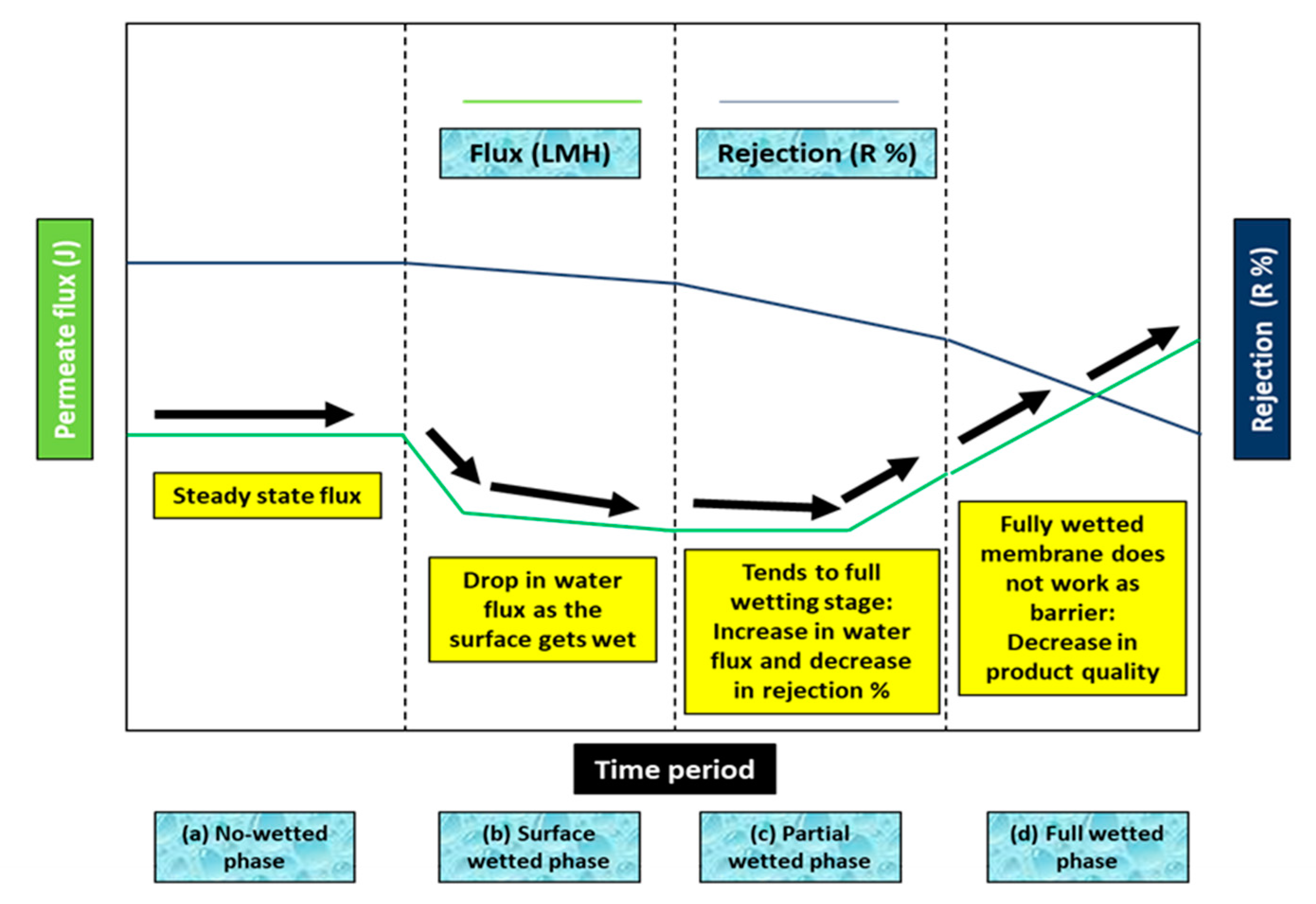
2. Wetting of MD Membrane and Prevention of Wetting
3. Geometrical Models for Predicting Wetting Behavior
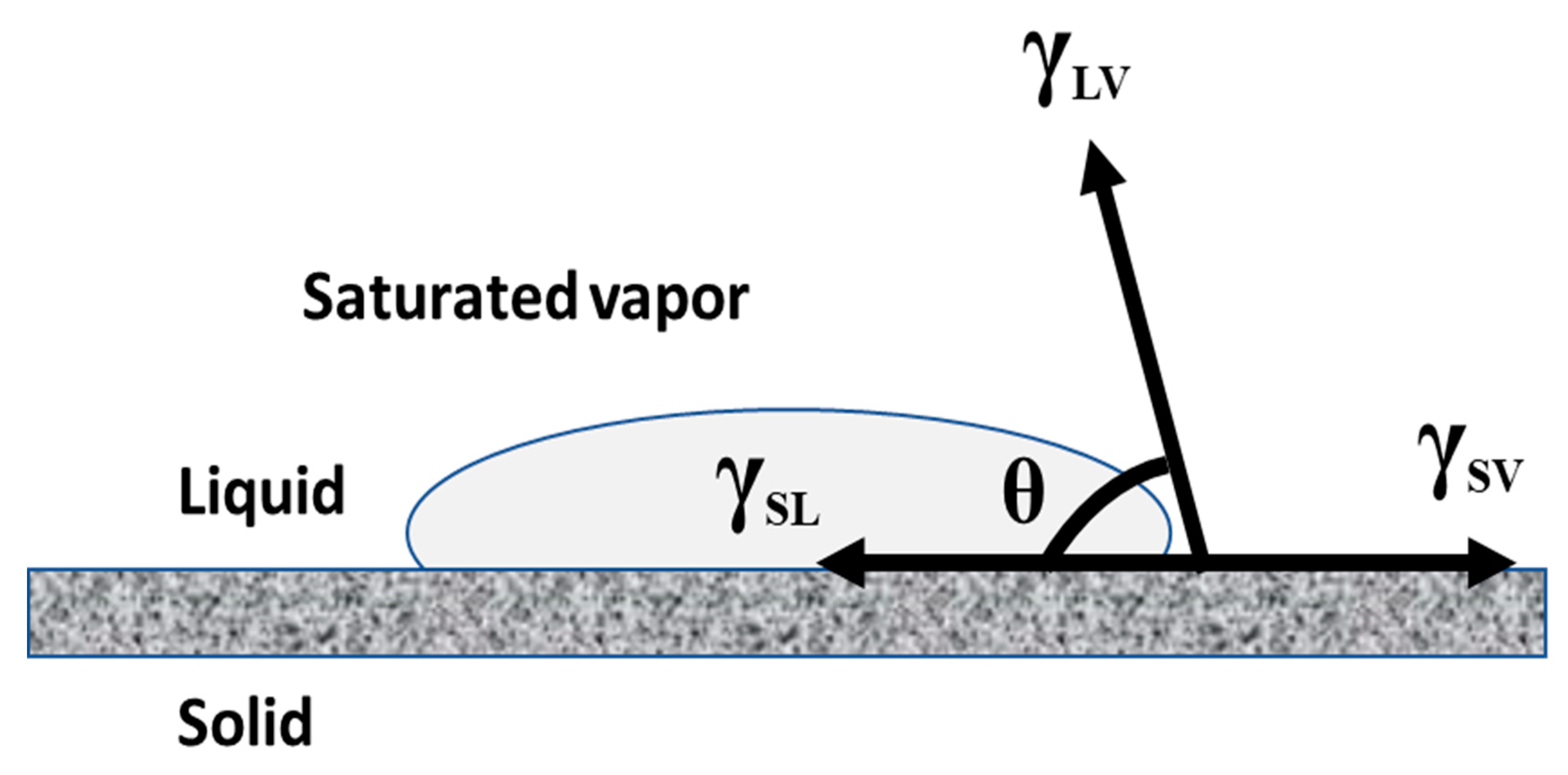
3.1. Correlation of Contact Angle and Wetting of an Ideal Solid Surface
3.2. Wetting State of a Real Solid Surface
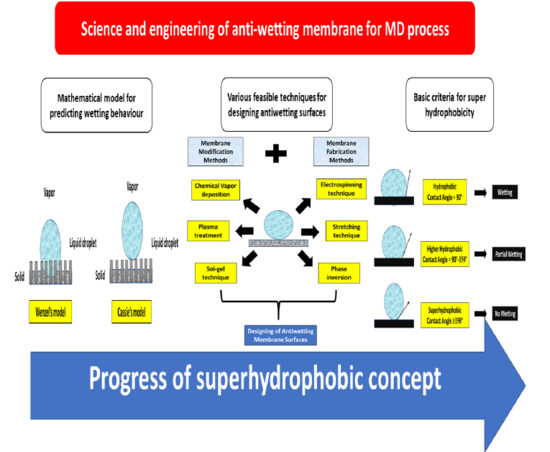
4. Science and Engineering of Antiwetting Interfaces
4.1. Basics of Wetting Issue in MD
4.2. Factors Affecting Membrane Wetting
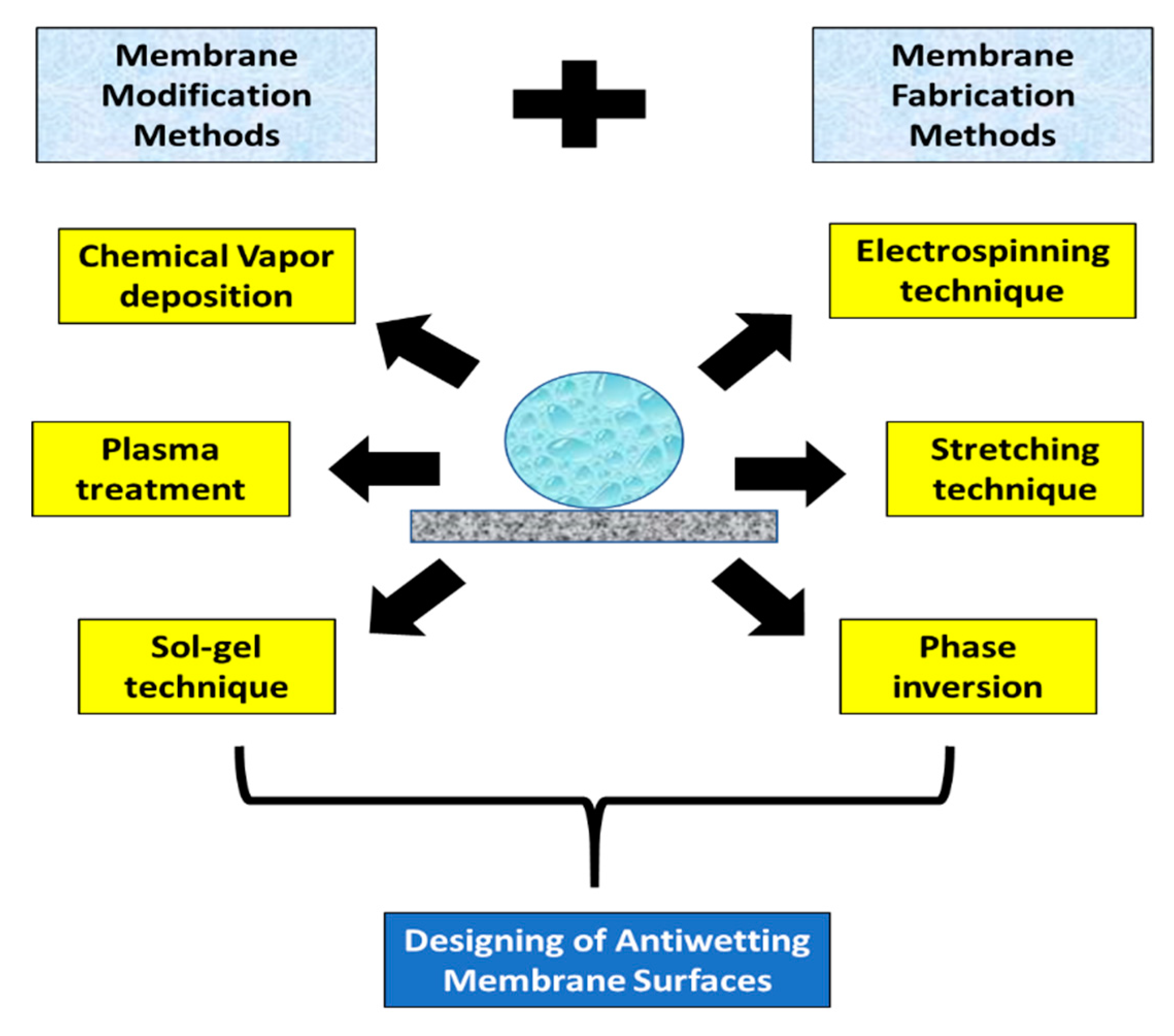
4.3. Feasible Techniques to Fabricate Antiwetting Surfaces
4.3.1. Feasible Membrane Fabrication Techniques for MD Membranes
Electrospinning Technique for Membrane Fabrication
Phase Inversion Membrane Casting Technique
- First, polymer pellets are dissolved in a particular organic solvent to form a polymeric casting solution, which is then cast on a flat plate up to the desired thickness with a knife casting device.
- Next, the semi-liquid film is cast onto the plate and is allowed to be immersed in a nonsolvent bath for precipitation.
- Finally, a polymeric film is rapidly formed with an asymmetric structure because of the exchange of solvent and nonsolvent across the interface, which can be explained based on the absorption of water molecules by the polymeric substrate and simultaneous loss of solvent. Figure 9 indicates the phase inversion concept utilized for fabricating self-cleaning membrane surfaces for MD application.
Mechanical Stretching for Hollow Fiber Membrane Fabrication
4.3.2. Membrane Surface Modification Methodologies
Blending or Coating for Antiwetting Membrane Surfaces
Plasma Treatment for Membrane Surface Modification
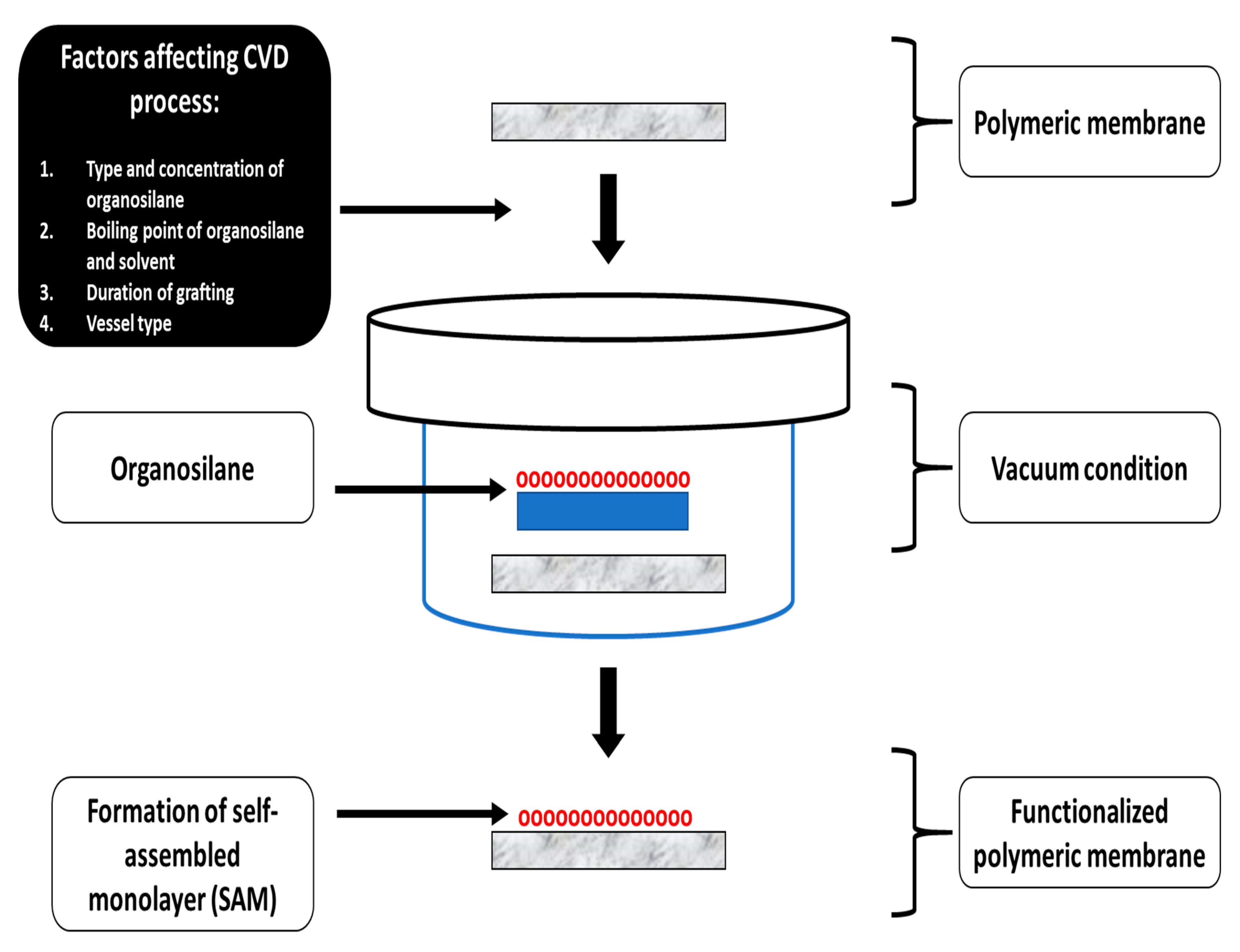
Chemical Vapor Deposition for Membrane Modification
4.3.3. Overall Perspective
5. Research Challenges and Future Perspectives
- (1)
- The durability of superhydrophobic coating must be higher. A facile pathway to design mechanically robust interconnected nanostructures/microstructures with overhanging geometries must be invented.
- (2)
- An easy approach to characterize antiwetting surfaces should be utilized. Even though CA analysis is easy and straight forward, it does not describe the feed-membrane interface in MD application. In addition, CA must be measured in a high temperature environment as the liquid surface tension is closely related to temperature.
- (3)
- The coatings must resist the acidic and basic nature of the feed stream. The properties of coating depend on the chemical nature of the material utilized.
- (4)
- Typically, during long-term operation, surface structures with overhanging features show weaker mechanical resistance because they may influence the surface chemistry, as well as geometry, of the pore structures.
- (5)
- Precise control of surface protrusions, roughness, and geometry is crucial to achieve Cassie stable state for high liquid repellency.
- (6)
- Finally, the surface tension of the feed stream is another major issue, while applying antiwetting surfaces in MD. Furthermore, the operating parameters must be optimized for better performance because a higher water flux (i.e., higher evaporation rate) leads to more severe concentration polarization, and thus, maximizes the chances of wetting.
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lawson, K.W.; Lloyd, D.R. Membrane distillation. J. Membr. Sci. 1997, 124, 1–25. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Kaplan, R.; Mamrosh, D.; Salih, H.H.; Dastgheib, S.A. Assessment of desalination technologies for treatment of a highly saline brine from a potential CO2 storage site. Desalination 2017, 404, 87–101. [Google Scholar] [CrossRef]
- El-Bourawi, M.; Ding, Z.; Ma, R.; Khayet, M. A framework for better understanding membrane distillation separation process. J. Membr. Sci. 2006, 285, 4–29. [Google Scholar] [CrossRef]
- Pangarkar, B.L.; Sane, M.G.; Guddad, M. Reverse osmosis and membrane distillation for desalination of groundwater: A review. ISRN Mater. Sci. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Kiss, A.A.; Kattan Readi, O.M. An industrial perspective on membrane distillation processes. J. Chem. Technol. Biotechnol. 2018, 93, 2047–2055. [Google Scholar] [CrossRef]
- Nawi, N.I.M.; Bilad, M.R.; Zolkhiflee, N.; Nordin, N.A.H.; Lau, W.J.; Narkkun, T.; Faungnawakij, K.; Arahman, N.; Mahlia, T.M.I. Development of A Novel Corrugated Polyvinylidene difluoride Membrane via Improved Imprinting Technique for Membrane Distillation. Polymers 2019, 11, 865. [Google Scholar] [CrossRef]
- Ray, S.S.; Chen, S.S.; Sangeetha, D.; Chang, H.M.; Thanh, C.N.D.; Le, Q.H.; Ku, H.M. Developments in forward osmosis and membrane distillation for desalination of waters. Environ. Chem. Lett. 2018, 16, 1247–1265. [Google Scholar] [CrossRef]
- Lu, K.J.; Chen, Y.; Chung, T.-S. Design of omniphobic interfaces for membrane distillation—A review. Water Res. 2019. [Google Scholar] [CrossRef]
- Guillen-Burrieza, E.; Mavukkandy, M.; Bilad, M.; Arafat, H. Understanding wetting phenomena in membrane distillation and how operational parameters can affect it. J. Membr. Sci. 2016, 515, 163–174. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Esmaeilbeig, M.A. Membrane Wetting in Membrane Distillation. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2019; pp. 143–174. [Google Scholar]
- Guillen-Burrieza, E.; Thomas, R.; Mansoor, B.; Johnson, D.; Hilal, N.; Arafat, H. Effect of dry-out on the fouling of PVDF and PTFE membranes under conditions simulating intermittent seawater membrane distillation (SWMD). J. Membr. Sci. 2013, 438, 126–139. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Servi, A.; Connors, G.B.; Mavukkandy, M.O.; Arafat, H.A.; Gleason, K.K. Reversing membrane wetting in membrane distillation: Comparing dryout to backwashing with pressurized air. Environ. Sci. Water Res. Technol. 2017, 3, 930–939. [Google Scholar] [CrossRef]
- Tijing, L.D.; Woo, Y.C.; Choi, J.-S.; Lee, S.; Kim, S.-H.; Shon, H.K. Fouling and its control in membrane distillation—A review. J. Membr. Sci. 2015, 475, 215–244. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Fei, X.; Sun, M.; Zhang, C.; Li, Y.; Yang, Q.; Hong, X. Preparation of a durable superhydrophobic membrane by electrospinning poly (vinylidene fluoride) (PVDF) mixed with epoxy–siloxane modified SiO2 nanoparticles: A possible route to superhydrophobic surfaces with low water sliding angle and high water contact angle. J. Colloid Interface Sci. 2011, 359, 380–388. [Google Scholar]
- Cao, L.; Jones, A.K.; Sikka, V.K.; Wu, J.; Gao, D. Anti-icing superhydrophobic coatings. Langmuir 2009, 25, 12444–12448. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Wang, R.; Fane, A.G. Engineering superhydrophobic surface on poly (vinylidene fluoride) nanofiber membranes for direct contact membrane distillation. J. Membr. Sci. 2013, 440, 77–87. [Google Scholar] [CrossRef]
- Mosadegh-Sedghi, S.; Rodrigue, D.; Brisson, J.; Iliuta, M.C. Wetting phenomenon in membrane contactors–causes and prevention. J. Membr. Sci. 2014, 452, 332–353. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Y.; Shi, L.; Li, J.; Guo, Z. Advances in the theory of superhydrophobic surfaces. J. Mater. Chem. 2012, 22, 20112–20127. [Google Scholar] [CrossRef]
- Gao, N.; Yan, Y. Modeling superhydrophobic contact angles and wetting transition. J. Bionic Eng. 2009, 6, 335–340. [Google Scholar] [CrossRef]
- Whyman, G.; Bormashenko, E.; Stein, T. The rigorous derivation of Young, Cassie–Baxter and Wenzel equations and the analysis of the contact angle hysteresis phenomenon. Chem. Phys. Lett. 2008, 450, 355–359. [Google Scholar] [CrossRef]
- Good, R.J. Contact angle, wetting, and adhesion: A critical review. J. Adhes. Sci. Technol. 1992, 6, 1269–1302. [Google Scholar] [CrossRef]
- Eick, J.; Good, R.; Neumann, A. Thermodynamics of contact angles. II. Rough solid surfaces. J. Colloid Interface Sci. 1975, 53, 235–248. [Google Scholar] [CrossRef]
- Li, D.; Neumann, A. Contact angles on hydrophobic solid surfaces and their interpretation. J. Colloid Interface Sci. 1992, 148, 190–200. [Google Scholar] [CrossRef]
- Li, W.; Amirfazli, A. A thermodynamic approach for determining the contact angle hysteresis for superhydrophobic surfaces. J. Colloid Interface Sci. 2005, 292, 195–201. [Google Scholar] [CrossRef]
- Zuo, Y.Y.; Do, C.; Neumann, A.W. Automatic measurement of surface tension from noisy images using a component labeling method. Colloids Surf. A Physicochem. Eng. Asp. 2007, 299, 109–116. [Google Scholar] [CrossRef]
- Dorrer, C.; Rühe, J. Some thoughts on superhydrophobic wetting. Soft Matter 2009, 5, 51–61. [Google Scholar] [CrossRef]
- Butt, H.-J.; Roisman, I.V.; Brinkmann, M.; Papadopoulos, P.; Vollmer, D.; Semprebon, C. Characterization of super liquid-repellent surfaces. Curr. Opin. Colloid Interface Sci. 2014, 19, 343–354. [Google Scholar] [CrossRef]
- Zhu, J.; Dai, X. A new model for contact angle hysteresis of superhydrophobic surface. AIP Adv. 2019, 9, 065309. [Google Scholar] [CrossRef]
- Chen, W.; Fadeev, A.Y.; Hsieh, M.C.; Öner, D.; Youngblood, J.; McCarthy, T.J. Ultrahydrophobic and ultralyophobic surfaces: Some comments and examples. Langmuir 1999, 15, 3395–3399. [Google Scholar] [CrossRef]
- Murase, H.; Nanishi, K.; Kogure, H.; Fujibayashi, T.; Tamura, K.; Haruta, N. Interactions between heterogeneous surfaces of polymers and water. J. Appl. Polym. Sci. 1994, 54, 2051–2062. [Google Scholar] [CrossRef]
- Franken, A.; Nolten, J.; Mulder, M.; Bargeman, D.; Smolders, C. Wetting criteria for the applicability of membrane distillation. J. Membr. Sci. 1987, 33, 315–328. [Google Scholar] [CrossRef]
- Jung, Y.C.; Bhushan, B. Wetting behavior of water and oil droplets in three-phase interfaces for hydrophobicity/philicity and oleophobicity/philicity. Langmuir 2009, 25, 14165–14173. [Google Scholar] [CrossRef] [PubMed]
- Nosonovsky, M. On the range of applicability of the Wenzel and Cassie equations. Langmuir 2007, 23, 9919–9920. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; McCarthy, T.J. How Wenzel and Cassie were wrong. Langmuir 2007, 23, 3762–3765. [Google Scholar] [CrossRef] [PubMed]
- Murakami, D.; Jinnai, H.; Takahara, A. Wetting transition from the Cassie–Baxter state to the Wenzel state on textured polymer surfaces. Langmuir 2014, 30, 2061–2067. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.C.; Bhushan, B. Wetting transition of water droplets on superhydrophobic patterned surfaces. Scr. Mater. 2007, 57, 1057–1060. [Google Scholar] [CrossRef]
- Vrancken, R.J.; Kusumaatmaja, H.; Hermans, K.; Prenen, A.M.; Pierre-Louis, O.; Bastiaansen, C.W.; Broer, D.J. Fully reversible transition from Wenzel to Cassie−Baxter states on corrugated superhydrophobic surfaces. Langmuir 2009, 26, 3335–3341. [Google Scholar] [CrossRef]
- Pan, Z.; Cheng, F.; Zhao, B. Bio-inspired polymeric structures with special wettability and their applications: An overview. Polymers 2017, 9, 725. [Google Scholar] [CrossRef]
- Iqbal, M.; Dinh, D.K.; Abbas, Q.; Imran, M.; Sattar, H.; Ul Ahmad, A. Controlled Surface Wettability by Plasma Polymer Surface Modification. Surfaces 2019, 2, 349–371. [Google Scholar] [CrossRef]
- Mohamed, A.M.; Abdullah, A.M.; Younan, N.A. Corrosion behavior of superhydrophobic surfaces: A review. Arab. J. Chem. 2015, 8, 749–765. [Google Scholar] [CrossRef]
- Milne, A.; Amirfazli, A. The Cassie equation: How it is meant to be used. Adv. Colloid Interface Sci. 2012, 170, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Peng, Y.; Qiu, H.; Liu, X.; Ge, L. Anti-fouling membranes by manipulating surface wettability and their anti-fouling mechanism. Desalination 2017, 413, 127–135. [Google Scholar] [CrossRef]
- Ray, S.S.; Gandhi, M.; Chen, S.-S.; Chang, H.-M.; Dan, C.T.N.; Le, H.Q. Anti-wetting behaviour of a superhydrophobic octadecyltrimethoxysilane blended PVDF/recycled carbon black composite membrane for enhanced desalination. Environ. Sci. Water Res. Technol. 2018, 4, 1612–1623. [Google Scholar] [CrossRef]
- Fürstner, R.; Barthlott, W.; Neinhuis, C.; Walzel, P. Wetting and self-cleaning properties of artificial superhydrophobic surfaces. Langmuir 2005, 21, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Koishi, T.; Yasuoka, K.; Fujikawa, S.; Zeng, X.C. Measurement of contact-angle hysteresis for droplets on nanopillared surface and in the Cassie and Wenzel states: A molecular dynamics simulation study. ACS Nano 2011, 5, 6834–6842. [Google Scholar] [CrossRef] [PubMed]
- Marmur, A. Wetting on hydrophobic rough surfaces: To be heterogeneous or not to be? Langmuir 2003, 19, 8343–8348. [Google Scholar] [CrossRef]
- Khayet, M.; Matsuura, T. Membrane Distillation: Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane distillation: Recent developments and perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- Dommati, H.; Ray, S.S.; Wang, J.C.; Chen, S.S.A. A comprehensive review of recent developments in 3D printing technique for ceramic membrane fabrication for water purification. RSC Adv. 2019, 9, 16869–16883. [Google Scholar] [CrossRef]
- Subramanian, N.; Qamar, A.; Alsaadi, A.; Gallo, A., Jr.; Ridwan, M.G.; Lee, J.G.; Pillai, S.; Arunachalam, S.; Anjum, D.; Sharipov, F.; et al. Evaluating the potential of superhydrophobic nanoporous alumina membranes for direct contact membrane distillation. J. Colloid Interface Sci. 2019, 533, 723–732. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Swaminathan, J.; Guillen-Burrieza, E.; Arafat, H.A.; Lienhard V, J.H. Scaling and fouling in membrane distillation for desalination applications: A review. Desalination 2015, 356, 294–313. [Google Scholar] [CrossRef]
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van der Bruggen, B. How to optimize the membrane properties for membrane distillation: A review. Ind. Eng. Chem. Res. 2016, 55, 9333–9343. [Google Scholar] [CrossRef]
- Luo, A.; Lior, N. Critical review of membrane distillation performance criteria. Desalin. Water Treat. 2016, 57, 20093–20140. [Google Scholar] [CrossRef]
- Goh, S.; Zhang, J.; Liu, Y.; Fane, A.G. Fouling and wetting in membrane distillation (MD) and MD-bioreactor (MDBR) for wastewater reclamation. Desalination 2013, 323, 39–47. [Google Scholar] [CrossRef]
- Nguyen, Q.-M.; Lee, S. Fouling analysis and control in a DCMD process for SWRO brine. Desalination 2015, 367, 21–27. [Google Scholar] [CrossRef]
- Naidu, G.; Jeong, S.; Vigneswaran, S.; Hwang, T.-M.; Choi, Y.-J.; Kim, S.-H. A review on fouling of membrane distillation. Desalin. Water Treat. 2016, 57, 10052–10076. [Google Scholar] [CrossRef]
- Naidu, G.; Jeong, S.; Kim, S.-J.; Kim, I.S.; Vigneswaran, S. Organic fouling behavior in direct contact membrane distillation. Desalination 2014, 347, 230–239. [Google Scholar] [CrossRef]
- Rezaei, M.; Warsinger, D.M.; Duke, M.C.; Matsuura, T.; Samhaber, W.M. Wetting phenomena in membrane distillation: Mechanisms, reversal, and prevention. Water Res. 2018, 139, 329–352. [Google Scholar] [CrossRef]
- Gryta, M. Fouling in direct contact membrane distillation process. J. Membr. Sci. 2008, 325, 383–394. [Google Scholar] [CrossRef]
- Tow, E.W.; Warsinger, D.M.; Trueworthy, A.M.; Swaminathan, J.; Thiel, G.P.; Zubair, S.M.; Myerson, A.S. Comparison of fouling propensity between reverse osmosis, forward osmosis, and membrane distillation. J. Membr. Sci. 2018, 556, 352–364. [Google Scholar] [CrossRef]
- Gryta, M.; Grzechulska-Damszel, J.; Markowska, A.; Karakulski, K. The influence of polypropylene degradation on the membrane wettability during membrane distillation. J. Membr. Sci. 2009, 326, 493–502. [Google Scholar] [CrossRef]
- Tijing, L.D.; Woo, Y.C.; Shim, W.-G.; He, T.; Choi, J.-S.; Kim, S.-H.; Shon, H.K. Superhydrophobic nanofiber membrane containing carbon nanotubes for high-performance direct contact membrane distillation. J. Membr. Sci. 2016, 502, 158–170. [Google Scholar] [CrossRef]
- Ray, S.S.; Chen, S.-S.; Chang, H.-M.; Thanh, C.N.D.; Le, H.Q.; Nguyen, N.C. Enhanced desalination using a three-layer OTMS based superhydrophobic membrane for a membrane distillation process. RSC Adv. 2018, 8, 9640–9650. [Google Scholar] [CrossRef]
- Guillén-Burrieza, E.; Blanco, J.; Zaragoza, G.; Alarcón, D.-C.; Palenzuela, P.; Ibarra, M.; Gernjak, W. Experimental analysis of an air gap membrane distillation solar desalination pilot system. J. Membr. Sci. 2011, 379, 386–396. [Google Scholar] [CrossRef]
- Guillen-Burrieza, E.; Servi, A.; Lalia, B.S.; Arafat, H.A. Membrane structure and surface morphology impact on the wetting of MD membranes. J. Membr. Sci. 2015, 483, 94–103. [Google Scholar] [CrossRef]
- Gilron, J.; Ladizansky, Y.; Korin, E. Silica fouling in direct contact membrane distillation. Ind. Eng. Chem. Res. 2013, 52, 10521–10529. [Google Scholar] [CrossRef]
- Curcio, E.; Drioli, E. Membrane distillation and related operations—A review. Sep. Purif. Rev. 2005, 34, 35–86. [Google Scholar] [CrossRef]
- Lawson, K.W.; Lloyd, D.R. Membrane distillation. II. Direct contact MD. J. Membr. Sci. 1996, 120, 123–133. [Google Scholar] [CrossRef]
- Schofield, R.; Fane, A.; Fell, C.; Macoun, R. Factors affecting flux in membrane distillation. Desalination 1990, 77, 279–294. [Google Scholar] [CrossRef]
- Ray, S.S.; Deb, C.K.; Chang, H.M.; Chen, S.S.; Ganesapillai, M. Crosslinked PVDF-HFP-based hydrophobic membranes incorporated with CNF for enhanced stability and permeability in membrane distillation. J. Appl. Polym. Sci. 2019, 48021. [Google Scholar] [CrossRef]
- Gryta, M. Long-term performance of membrane distillation process. J. Membr. Sci. 2005, 265, 153–159. [Google Scholar] [CrossRef]
- Gryta, M. Influence of polypropylene membrane surface porosity on the performance of membrane distillation process. J. Membr. Sci. 2007, 287, 67–78. [Google Scholar] [CrossRef]
- Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C.Q. Enhanced performance of PVDF nanocomposite membrane by nanofiber coating: A membrane for sustainable desalination through MD. Water Res. 2016, 89, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Eykens, L.; De Sitter, K.; Dotremont, C.; De Schepper, W.; Pinoy, L.; Van Der Bruggen, B. Wetting resistance of commercial membrane distillation membranes in waste streams containing surfactants and oil. Appl. Sci. 2017, 7, 118. [Google Scholar] [CrossRef]
- Shirazi, M.M.A.; Kargari, A.; Tabatabaei, M. Evaluation of commercial PTFE membranes in desalination by direct contact membrane distillation. Chem. Eng. Process. Process. Intensif. 2014, 76, 16–25. [Google Scholar] [CrossRef]
- Tijing, L.D.; Choi, J.-S.; Lee, S.; Kim, S.-H.; Shon, H.K. Recent progress of membrane distillation using electrospun nanofibrous membrane. J. Membr. Sci. 2014, 453, 435–462. [Google Scholar] [CrossRef]
- De Zarate, J.O.; Pen, L.; Mengual, J.I. Characterization of membrane distillation membranes prepared by phase inversion. Desalination 1995, 100, 139–148. [Google Scholar] [CrossRef]
- Ma, M.; Mao, Y.; Gupta, M.; Gleason, K.K.; Rutledge, G.C. Superhydrophobic fabrics produced by electrospinning and chemical vapor deposition. Macromolecules 2005, 38, 9742–9748. [Google Scholar] [CrossRef]
- Xu, W.; Ning, T.; Yang, X.; Lu, S. Fabrication of superhydrophobic surfaces on zinc substrates. Appl. Surf. Sci. 2011, 257, 4801–4806. [Google Scholar] [CrossRef]
- Blanco, M.; Monteserín, C.; Angulo, A.; Pérez-Márquez, A.; Maudes, J.; Murillo, N.; Aranzabe, E.; Ruiz-Rubio, L.; Vilas, J.L. TiO2-Doped Electrospun Nanofibrous Membrane for Photocatalytic Water Treatment. Polymers 2019, 11, 747. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, D.; Zhao, J.; Feng, Q.; Li, Z.; Bai, H.; Sun, D.D. Efficient Oil/Water Separation Membrane Derived from Super-Flexible and Superhydrophilic Core–Shell Organic/Inorganic Nanofibrous Architectures. Polymers 2019, 11, 974. [Google Scholar] [CrossRef]
- Ray, S.S.; Chen, S.-S.; Li, C.-W.; Nguyen, N.C.; Nguyen, H.T. A comprehensive review: Electrospinning technique for fabrication and surface modification of membranes for water treatment application. RSC Adv. 2016, 6, 85495–85514. [Google Scholar] [CrossRef]
- Ray, S.S.; Chen, S.-S.; Hsu, H.-T.; Cao, D.-T.; Nguyen, H.-T.; Nguyen, N.C. Uniform hydrophobic electrospun nanofibrous layer composed of polysulfone and sodium dodecyl sulfate for improved desalination performance. Sep. Purif. Technol. 2017, 186, 352–365. [Google Scholar] [CrossRef]
- Khayet, M.; García-Payo, C.; Matsuura, T. Superhydrophobic nanofibers electrospun by surface segregating fluorinated amphiphilic additive for membrane distillation. J. Membr. Sci. 2019, 117215. [Google Scholar] [CrossRef]
- Dong, Z.-Q.; Ma, X.-H.; Xu, Z.-L.; You, W.-T.; Li, F.-B. Superhydrophobic PVDF–PTFE electrospun nanofibrous membranes for desalination by vacuum membrane distillation. Desalination 2014, 347, 175–183. [Google Scholar] [CrossRef]
- Su, C.; Li, Y.; Dai, Y.; Gao, F.; Tang, K.; Cao, H. Fabrication of three-dimensional superhydrophobic membranes with high porosity via simultaneous electrospraying and electrospinning. Mater. Lett. 2016, 170, 67–71. [Google Scholar] [CrossRef]
- An, X.; Liu, Z.; Hu, Y. Amphiphobic surface modification of electrospun nanofibrous membranes for anti-wetting performance in membrane distillation. Desalination 2018, 432, 23–31. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Yang, Y.; Wang, X.; Zhu, M.; Hsiao, B.S. Dual-biomimetic superhydrophobic electrospun polystyrene nanofibrous membranes for membrane distillation. ACS Appl. Mater. Interfaces 2014, 6, 2423–2430. [Google Scholar] [CrossRef]
- Liao, Y.; Loh, C.-H.; Wang, R.; Fane, A.G. Electrospun superhydrophobic membranes with unique structures for membrane distillation. ACS Appl. Mater. Interfaces 2014, 6, 16035–16048. [Google Scholar] [CrossRef]
- Nuraje, N.; Khan, W.S.; Lei, Y.; Ceylan, M.; Asmatulu, R. Superhydrophobic electrospun nanofibers. J. Mater. Chem. A 2013, 1, 1929–1946. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, Z.; Zhang, F.; Liu, X.; Jin, J.; Jiang, L. Superhydrophobic and superoleophilic PVDF membranes for effective separation of water-in-oil emulsions with high flux. Adv. Mater. 2013, 25, 2071–2076. [Google Scholar] [CrossRef]
- Yao, M.; Woo, Y.C.; Tijing, L.D.; Shim, W.G.; Choi, J.S.; Kim, S.H.; Shon, H.K. Effect of heat-press conditions on electrospun membranes for desalination by direct contact membrane distillation. Desalination 2016, 378, 80–91. [Google Scholar] [CrossRef]
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N. A review on membrane fabrication: Structure, properties and performance relationship. Desalination 2013, 326, 77–95. [Google Scholar] [CrossRef]
- Liu, S.; Li, K.; Hughes, R. Preparation of porous aluminium oxide (Al2O3) hollow fibre membranes by a combined phase-inversion and sintering method. Ceram. Int. 2003, 29, 875–881. [Google Scholar] [CrossRef]
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van der Bruggen, B. Membrane synthesis for membrane distillation: A review. Sep. Purif. Technol. 2017, 182, 36–51. [Google Scholar] [CrossRef]
- Peng, M.; Li, H.; Wu, L.; Zheng, Q.; Chen, Y.; Gu, W. Porous poly (vinylidene fluoride) membrane with highly hydrophobic surface. J. Appl. Polym. Sci. 2005, 98, 1358–1363. [Google Scholar] [CrossRef]
- Munirasu, S.; Banat, F.; Durrani, A.A.; Haija, M.A. Intrinsically superhydrophobic PVDF membrane by phase inversion for membrane distillation. Desalination 2017, 417, 77–86. [Google Scholar] [CrossRef]
- Wu, C.; Tang, W.; Zhang, J.; Liu, S.; Wang, Z.; Wang, X.; Lu, X. Preparation of super-hydrophobic PVDF membrane for MD purpose via hydroxyl induced crystallization-phase inversion. J. Membr. Sci. 2017, 543, 288–300. [Google Scholar] [CrossRef]
- Xiao, Z.; Zheng, R.; Liu, Y.; He, H.; Yuan, X.; Ji, Y.; Li, D.; Yin, H.; Zhang, Y.; Li, X.-M. Slippery for scaling resistance in membrane distillation: A novel porous micropillared superhydrophobic surface. Water Res. 2019, 155, 152–161. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R. A review on electrospinning for membrane fabrication: Challenges and applications. Desalination 2015, 356, 15–30. [Google Scholar] [CrossRef]
- Yuan, Z.; Chen, H.; Zhang, J.; Zhao, D.; Liu, Y.; Zhou, X.; Li, S.; Shi, P.; Tang, J.; Chen, X. Preparation and characterization of self-cleaning stable superhydrophobic linear low-density polyethylene. Sci. Technol. Adv. Mater. 2008, 9, 045007. [Google Scholar] [CrossRef]
- Wang, K.Y.; Chung, T.S.; Gryta, M. Hydrophobic PVDF hollow fiber membranes with narrow pore size distribution and ultra-thin skin for the fresh water production through membrane distillation. Chem. Eng. Sci. 2008, 63, 2587–2594. [Google Scholar] [CrossRef]
- Kurumada, K.-I.; Kitamura, T.; Fukumoto, N.; Oshima, M.; Tanigaki, M.; Kanazawa, S.-I. Structure generation in PTFE porous membranes induced by the uniaxial and biaxial stretching operations. J. Membr. Sci. 1998, 149, 51–57. [Google Scholar] [CrossRef]
- Huang, L.-T.; Hsu, P.-S.; Kuo, C.-Y.; Chen, S.-C.; Lai, J.-Y. Pore size control of PTFE membranes by stretch operation with asymmetric heating system. Desalination 2008, 233, 64–72. [Google Scholar] [CrossRef]
- Huang, Q.-L.; Xiao, C.F.; Feng, X.S.; Hu, X.Y. Design of super-hydrophobic microporous polytetrafluoroethylene membranes. New J. Chem. 2013, 37, 373–379. [Google Scholar] [CrossRef]
- Fang, H.; Gao, J.F.; Wang, H.T.; Chen, C.S. Hydrophobic porous alumina hollow fiber for water desalination via membrane distillation process. J. Membr. Sci. 2012, 403, 41–46. [Google Scholar] [CrossRef]
- Zhang, J.; Song, Z.; Li, B.; Wang, Q.; Wang, S. Fabrication and characterization of superhydrophobic poly (vinylidene fluoride) membrane for direct contact membrane distillation. Desalination 2013, 324, 1–9. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, Z.; Song, P.; Xiao, C. Fabrication of super-hydrophobic polypropylene hollow fiber membrane and its application in membrane distillation. Desalination 2017, 414, 10–17. [Google Scholar] [CrossRef]
- Razmjou, A.; Arifin, E.; Dong, G.; Mansouri, J.; Chen, V. Superhydrophobic modification of TiO2 nanocomposite PVDF membranes for applications in membrane distillation. J. Membr. Sci. 2012, 415, 850–863. [Google Scholar] [CrossRef]
- Ma, M.; Hill, R.M.; Lowery, J.L.; Fridrikh, S.V.; Rutledge, G.C. Electrospun poly (styrene-block-dimethylsiloxane) block copolymer fibers exhibiting superhydrophobicity. Langmuir 2005, 21, 5549–5554. [Google Scholar] [CrossRef]
- Wang, Y.; He, G.; Shao, Y.; Zhang, D.; Ruan, X.; Xiao, W.; Li, X.; Wu, X.; Jiang, X. Enhanced performance of superhydrophobic polypropylene membrane with modified antifouling surface for high salinity water treatment. Sep. Purif. Technol. 2019, 214, 11–20. [Google Scholar] [CrossRef]
- Liao, Y.; Loh, C.-H.; Tian, M.; Wang, R.; Fane, A.G. Progress in electrospun polymeric nanofibrous membranes for water treatment: Fabrication, modification and applications. Prog. Polym. Sci. 2018, 77, 69–94. [Google Scholar] [CrossRef]
- Lee, E.-J.; An, A.K.; He, T.; Woo, Y.C.; Shon, H.K. Electrospun nanofiber membranes incorporating fluorosilane-coated TiO2 nanocomposite for direct contact membrane distillation. J. Membr. Sci. 2016, 520, 145–154. [Google Scholar] [CrossRef]
- Gethard, K.; Sae-Khow, O.; Mitra, S. Water desalination using carbon-nanotube-enhanced membrane distillation. ACS Appl. Mater. Interfaces 2010, 3, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.C.; Tijing, L.D.; Shim, W.-G.; Choi, J.-S.; Kim, S.-H.; He, T.; Drioli, E.; Shon, H.K. Water desalination using graphene-enhanced electrospun nanofiber membrane via air gap membrane distillation. J. Membr. Sci. 2016, 520, 99–110. [Google Scholar] [CrossRef]
- Prince, J.; Singh, G.; Rana, D.; Matsuura, T.; Anbharasi, V.; Shanmugasundaram, T. Preparation and characterization of highly hydrophobic poly (vinylidene fluoride)–Clay nanocomposite nanofiber membranes (PVDF–clay NNMs) for desalination using direct contact membrane distillation. J. Membr. Sci. 2012, 397, 80–86. [Google Scholar] [CrossRef]
- Kim, Y.J.; Ahn, C.H.; Choi, M.O. Effect of thermal treatment on the characteristics of electrospun PVDF− silica composite nanofibrous membrane. Eur. Polym. J. 2010, 46, 1957–1965. [Google Scholar] [CrossRef]
- Gontarek, E.; Macedonio, F.; Militano, F.; Giorno, L.; Lieder, M.; Politano, A.; Drioli, E.; Gugliuzza, A. Adsorption-assisted transport of water vapour in super-hydrophobic membranes filled with multilayer graphene platelets. Nanoscale 2019. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Gutierrez, L.; Khumalo, N.; Derese, S.; Mamba, B.B.; Verliefde, A.R.; Mhlanga, S.D. Superhydrophobic PVDF nanofibre membranes coated with an organic fouling resistant hydrophilic active layer for direct-contact membrane distillation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 575, 363–372. [Google Scholar] [CrossRef]
- An, A.K.; Lee, E.J.; Guo, J.; Jeong, S.; Lee, J.G.; Ghaffour, N. Enhanced vapor transport in membrane distillation via functionalized carbon nanotubes anchored into electrospun nanofibres. Sci. Rep. 2017, 7, 41562. [Google Scholar]
- Yang, F.; Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C. Metal–organic frameworks supported on nanofiber for desalination by direct contact membrane distillation. ACS Appl. Mater. Interfaces 2018, 10, 11251–11260. [Google Scholar] [CrossRef]
- Laganà, F.; Barbieri, G.; Drioli, E. Direct contact membrane distillation: Modelling and concentration experiments. J. Membr. Sci. 2000, 166, 1–11. [Google Scholar] [CrossRef]
- Gnus, M.; Dudek, G.; Turczyn, R. The influence of filler type on the separation properties of mixed-matrix membranes. Chem. Pap. 2018, 72, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.L.; Morales-Torres, S.; Figueiredo, J.L.; Silva, A.M. Multi-walled carbon nanotube/PVDF blended membranes with sponge-and finger-like pores for direct contact membrane distillation. Desalination 2015, 357, 233–245. [Google Scholar] [CrossRef]
- Qtaishat, M.; Khayet, M.; Matsuura, T. Guidelines for preparation of higher flux hydrophobic/hydrophilic composite membranes for membrane distillation. J. Membr. Sci. 2009, 329, 193–200. [Google Scholar] [CrossRef]
- Wang, K.Y.; Foo, S.W.; Chung, T.S. Mixed matrix PVDF hollow fiber membranes with nanoscale pores for desalination through direct contact membrane distillation. Ind. Eng. Chem. Res. 2009, 48, 4474–4483. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Iglesia, O.D.L.; Fíla, V.; Téllez, C.; Coronas, J. Pervaporation-assisted esterification reactions by means of mixed matrix membranes. Ind. Eng. Chem. Res. 2018, 57, 15998–16011. [Google Scholar]
- Weigelt, F.; Georgopanos, P.; Shishatskiy, S.; Filiz, V.; Brinkmann, T.; Abetz, V. Development and characterization of defect-free matrimid® mixed-matrix membranes containing activated carbon particles for gas separation. Polymers 2018, 10, 51. [Google Scholar] [CrossRef]
- Zuo, J.; Chung, T.S. Metal–organic framework-functionalized alumina membranes for vacuum membrane distillation. Water 2016, 8, 586. [Google Scholar] [CrossRef]
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van der Bruggen, B. Coating techniques for membrane distillation: An experimental assessment. Sep. Purif. Technol. 2018, 193, 38–48. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.a.; Hu, X.; Jin, P.; Song, X. Surface modification to produce superhydrophobic hollow fiber membrane contactor to avoid membrane wetting for biogas purification under pressurized conditions. Sep. Purif. Technol. 2018, 194, 222–230. [Google Scholar] [CrossRef]
- Kim, H.I.; Kim, S.S. Plasma treatment of polypropylene and polysulfone supports for thin film composite reverse osmosis membrane. J. Membr. Sci. 2006, 286, 193–201. [Google Scholar] [CrossRef]
- Kawakami, M.; Yamashita, Y.; Iwamoto, M.; Kagawa, S. Modification of gas permeabilities of polymer membranes by plasma coating. J. Membr. Sci. 1984, 19, 249–258. [Google Scholar] [CrossRef]
- Woo, Y.C.; Chen, Y.; Tijing, L.D.; Phuntsho, S.; He, T.; Choi, J.-S.; Kim, S.-H.; Shon, H.K. CF4 plasma-modified omniphobic electrospun nanofiber membrane for produced water brine treatment by membrane distillation. J. Membr. Sci. 2017, 529, 234–242. [Google Scholar]
- Lee, H.K.; Kim, W.; Kim, Y.M.; Kwon, Y.-N. Surface modification of polyvinylidene fluoride membrane for enhanced wetting resistance. Appl. Surf. Sci. 2019. [Google Scholar] [CrossRef]
- Wei, X.; Zhao, B.; Li, X.-M.; Wang, Z.; He, B.-Q.; He, T.; Jiang, B. CF4 plasma surface modification of asymmetric hydrophilic polyethersulfone membranes for direct contact membrane distillation. J. Membr. Sci. 2012, 407, 164–175. [Google Scholar] [CrossRef]
- Tian, M.; Yin, Y.; Yang, C.; Zhao, B.; Song, J.; Liu, J.; Li, X.-M.; He, T. CF4 plasma modified highly interconnective porous polysulfone membranes for direct contact membrane distillation (DCMD). Desalination 2015, 369, 105–114. [Google Scholar] [CrossRef]
- Zuo, G.; Wang, R. Novel membrane surface modification to enhance anti-oil fouling property for membrane distillation application. J. Membr. Sci. 2013, 447, 26–35. [Google Scholar] [CrossRef]
- Choi, J.; Lee, K.H.; Yang, S. Fabrication of PDMS through-holes using the MIMIC method and the surface treatment by atmospheric-pressure CH4/He RF plasma. J. Micromech. Microeng. 2011, 21, 097001. [Google Scholar] [CrossRef]
- Kim, H.I.; Kim, S.S. Fabrication of reverse osmosis membrane via low temperature plasma polymerization. J. Membr. Sci. 2001, 190, 21–33. [Google Scholar] [CrossRef]
- Choi, J.H.; Jegal, J.; Kim, W.N. Fabrication and characterization of multi-walled carbon nanotubes/polymer blend membranes. J. Membr. Sci. 2006, 284, 406–415. [Google Scholar] [CrossRef]
- Kaur, S.; Ma, Z.; Gopal, R.; Singh, G.; Ramakrishna, S.; Matsuura, T. Plasma-induced graft copolymerization of poly (methacrylic acid) on electrospun poly (vinylidene fluoride) nanofiber membrane. Langmuir 2007, 23, 13085–13092. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Gu, Z.; Huo, R.; Ye, Y. Superhydrophobicity of polyvinylidene fluoride membrane fabricated by chemical vapor deposition from solution. Appl. Surf. Sci. 2009, 255, 7263–7267. [Google Scholar] [CrossRef]
- Guo, F.; Servi, A.; Liu, A.; Gleason, K.K.; Rutledge, G.C. Desalination by membrane distillation using electrospun polyamide fiber membranes with surface fluorination by chemical vapor deposition. ACS Appl. Mater. Interfaces 2015, 7, 8225–8232. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Vargas, C.S.; Zhuang, H.L.; Huang, P.Y.; Van Der Zande, A.M.; Garg, S.; McEuen, P.L.; Muller, D.A.; Hennig, R.G.; Park, J. Softened elastic response and unzipping in chemical vapor deposition graphene membranes. Nano Lett. 2011, 11, 2259–2263. [Google Scholar] [CrossRef] [PubMed]
- Warsinger, D.M.; Servi, A.; Van Belleghem, S.; Gonzalez, J.; Swaminathan, J.; Kharraz, J.; Chung, H.W.; Arafat, H.A.; Gleason, K.K. Combining air recharging and membrane superhydrophobicity for fouling prevention in membrane distillation. J. Membr. Sci. 2016, 505, 241–252. [Google Scholar] [CrossRef]
- Huang, Y.-X.; Wang, Z.; Jin, J.; Lin, S. Novel Janus membrane for membrane distillation with simultaneous fouling and wetting resistance. Environ. Sci. Technol. 2017, 51, 13304–13310. [Google Scholar] [CrossRef]
- Servi, A.T.; Guillen-Burrieza, E.; Warsinger, D.M.; Livernois, W.; Notarangelo, K.; Kharraz, J.; Arafat, H.A.; Gleason, K.K. The effects of iCVD film thickness and conformality on the permeability and wetting of MD membranes. J. Membr. Sci. 2017, 523, 470–479. [Google Scholar] [CrossRef]
- Ray, S.S.; Chen, S.-S.; Nguyen, N.C.; Nguyen, H.T. Electrospinning: A Versatile Fabrication Technique for Nanofibrous Membranes for Use in Desalination. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019; pp. 247–273. [Google Scholar]
- Bonyadi, S.; Chung, T.S. Flux enhancement in membrane distillation by fabrication of dual layer hydrophilic–hydrophobic hollow fiber membranes. J. Membr. Sci. 2007, 306, 134–146. [Google Scholar]
- Haponska, M.; Trojanowska, A.; Nogalska, A.; Jastrzab, R.; Gumi, T.; Tylkowski, B. PVDF membrane morphology—Influence of polymer molecular weight and preparation temperature. Polymers 2017, 9, 718. [Google Scholar] [CrossRef]
- Rao, A.V.; Latthe, S.S.; Nadargi, D.Y.; Hirashima, H.; Ganesan, V. Preparation of MTMS based transparent superhydrophobic silica films by sol–gel method. J. Colloid Interface Sci. 2009, 332, 484–490. [Google Scholar]
- Yang, C.; Li, X.-M.; Gilron, J.; Kong, D.-F.; Yin, Y.; Oren, Y.; Linder, C.; He, T. CF4 plasma-modified superhydrophobic PVDF membranes for direct contact membrane distillation. J. Membr. Sci. 2014, 456, 155–161. [Google Scholar] [CrossRef]
- Ganesh, V.A.; Raut, H.K.; Nair, A.S.; Ramakrishna, S.A. A review on self-cleaning coatings. J. Mater. Chem. 2011, 21, 16304–16322. [Google Scholar] [CrossRef]
- An, A.K.; Guo, J.; Lee, E.-J.; Jeong, S.; Zhao, Y.; Wang, Z.; Leiknes, T. PDMS/PVDF hybrid electrospun membrane with superhydrophobic property and drop impact dynamics for dyeing wastewater treatment using membrane distillation. J. Membr. Sci. 2017, 525, 57–67. [Google Scholar] [CrossRef]
- Li, X.; García-Payo, M.; Khayet, M.; Wang, M.; Wang, X. Superhydrophobic polysulfone/polydimethylsiloxane electrospun nanofibrous membranes for water desalination by direct contact membrane distillation. J. Membr. Sci. 2017, 542, 308–319. [Google Scholar] [CrossRef]
- Deng, L.; Li, P.; Liu, K.; Wang, X.; Hsiao, B.S. Robust superhydrophobic dual layer nanofibrous composite membranes with a hierarchically structured amorphous polypropylene skin for membrane distillation. J. Mater. Chem. A 2019, 7, 11282–11297. [Google Scholar] [CrossRef]
- Efome, J.E.; Baghbanzadeh, M.; Rana, D.; Matsuura, T.; Lan, C.Q. Effects of superhydrophobic SiO2 nanoparticles on the performance of PVDF flat sheet membranes for vacuum membrane distillation. Desalination 2015, 373, 47–57. [Google Scholar] [CrossRef]
- Li, K.; Zhang, Y.; Xu, L.; Zeng, F.; Hou, D.; Wang, J. Optimizing stretching conditions in fabrication of PTFE hollow fiber membrane for performance improvement in membrane distillation. J. Membr. Sci. 2018, 550, 126–135. [Google Scholar] [CrossRef]
- Nair, R.; Wu, H.A.; Jayaram, P.N.; Grigorieva, I.V.; Geim, A.K. Unimpeded permeation of water through helium-leak–tight graphene-based membranes. Science 2012, 335, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Jeevahan, J.; Chandrasekaran, M.; Joseph, G.B.; Durairaj, R.; Mageshwaran, G. Superhydrophobic surfaces: A review on fundamentals, applications, and challenges. J. Coat. Technol. Res. 2018, 15, 231–250. [Google Scholar] [CrossRef]
- Deshmukh, A.; Boo, C.; Karanikola, V.; Lin, S.; Straub, A.P.; Tong, T.; Warsinger, D.M.; Elimelech, M. Membrane distillation at the water-energy nexus: Limits, opportunities, and challenges. Energy Environ. Sci. 2018, 11, 1177–1196. [Google Scholar] [CrossRef]




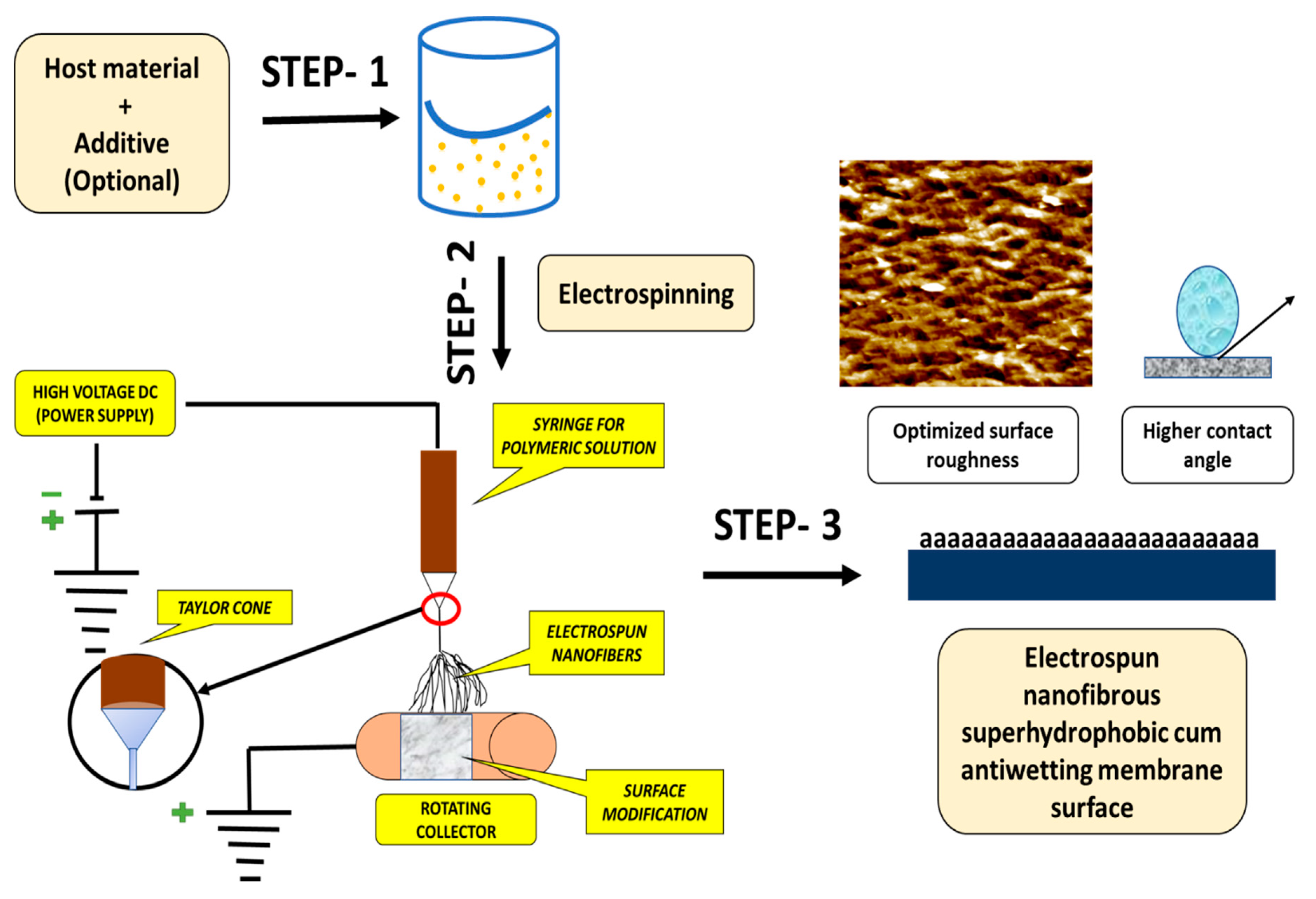
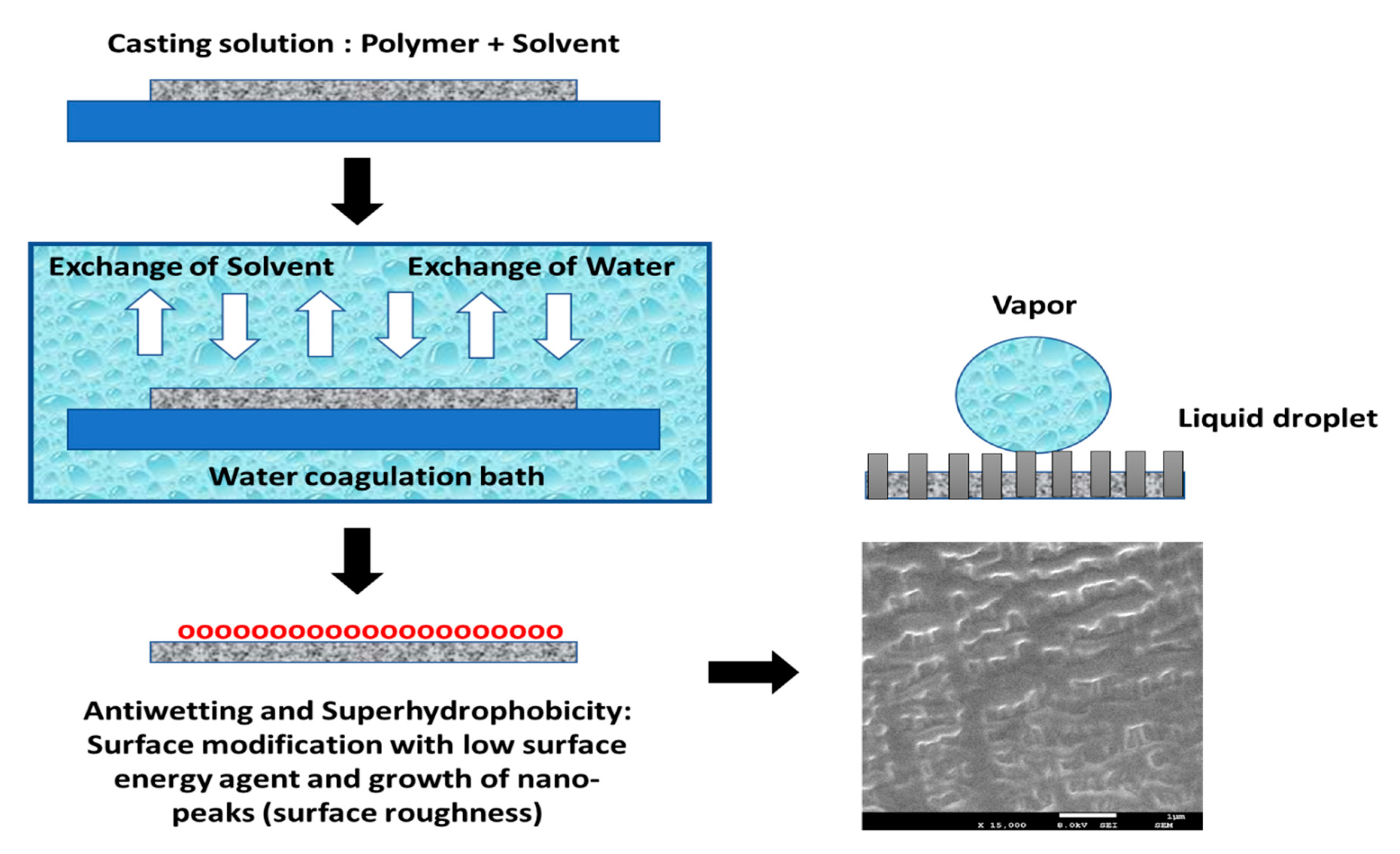
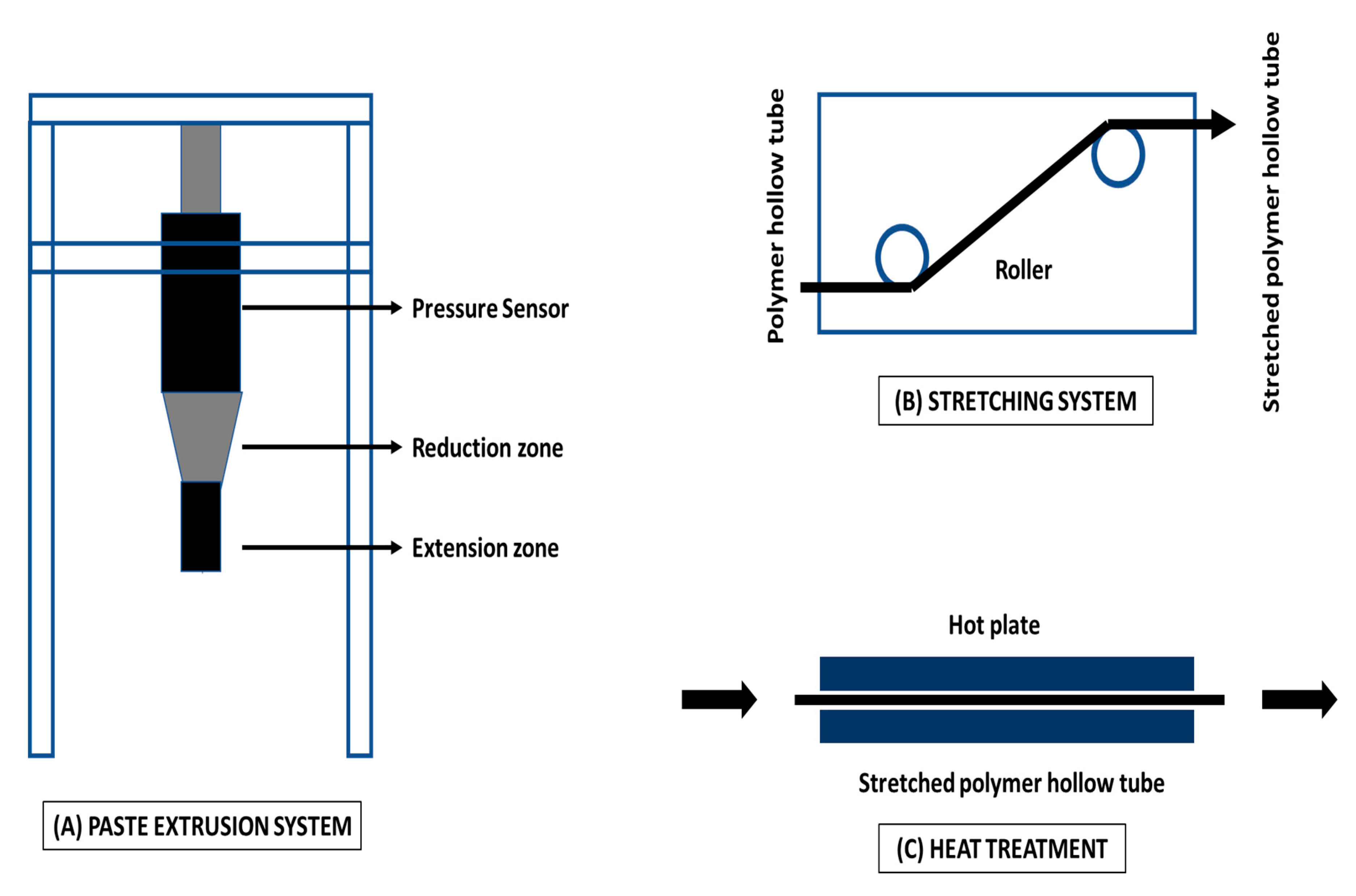
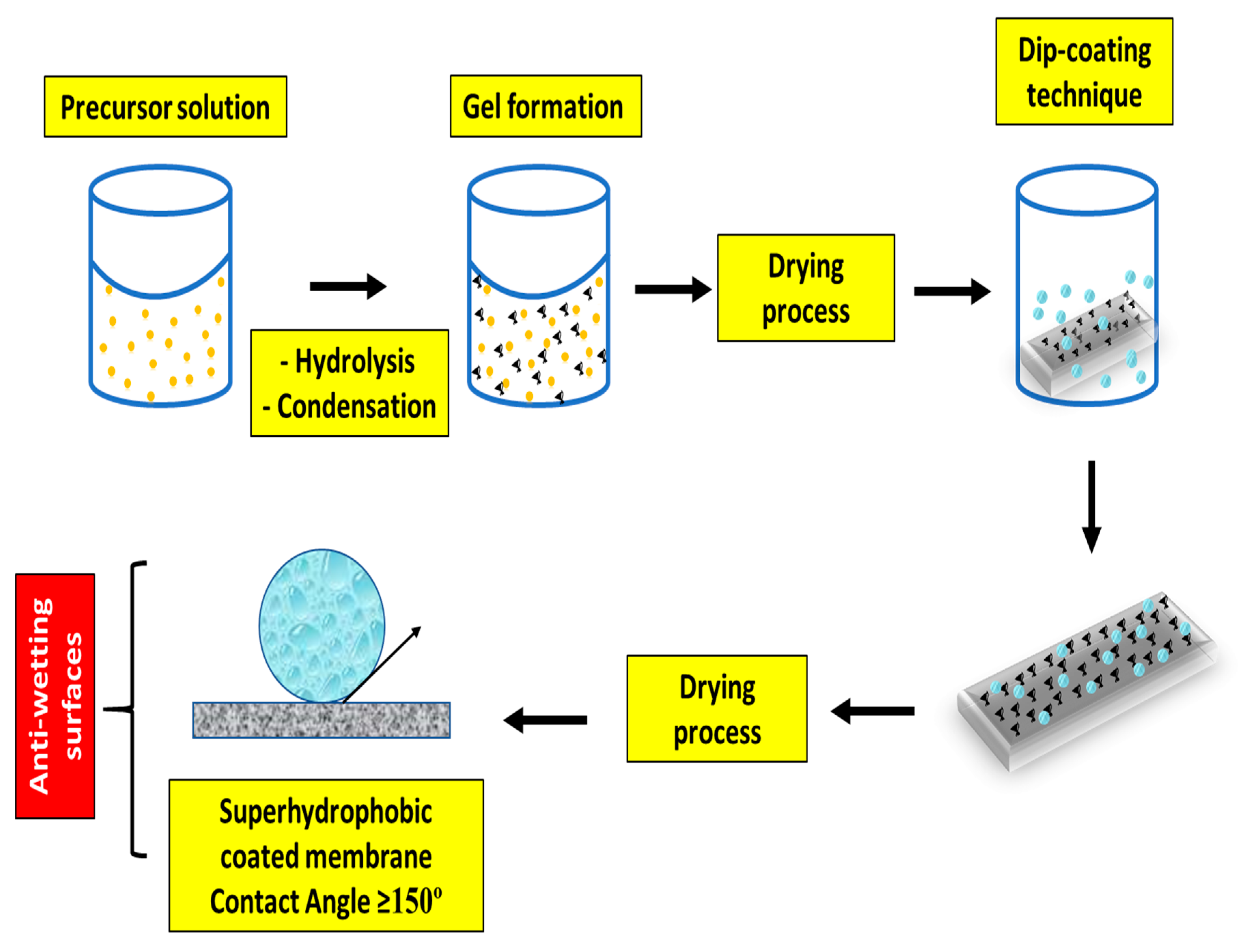



| Technique | Working Condition | Overall Outcomes | References |
|---|---|---|---|
| Drying up | Simple air jets are used onto the membrane surface | Minimized surface wetting Salt remains in membrane pores | [12] |
| Air backwashing | Pressurized air forces water and salt out of pores | Minimized pore and surface wetting | [13] |
| Chemical treatment | Acid or other chemicals may dissolve the deposits (May involve a drying up technique) | Minimized pore and surface wetting | [14] |
| Quantitative Equation | Contact Angle Relationship | Overall Prediction (State) |
|---|---|---|
| γSV − γSL > 0 | 0° ≤ θ ≤ 90° | High wettability |
| γSV − γSL > γLV | θ = 0° | Complete wetting (spreading) |
| γSV − γSL < 0 | 90° ≤ θ ≤ 180° | Low wetting |
| γSL − γSV > γLV | θ = 180° | No wetting |
| Factors | Conditions | Reference |
|---|---|---|
| Layers | At least the top layer must be hydrophobic or superhydrophobic in nature to avoid wetting in the MD process | [48] |
| Pore size distribution | Selection of a narrow pore size range with a high liquid entry pressure (LEP) value | [32,49,50] |
| Tortuosity factor | Must be as lower as possible: This is a measure of the deviation in pore structure from normal straight cylindrical pores. Typically, the tortuosity factor is inversely proportional to MD permeability | [4] |
| Thermal conductivity of the membrane surface | The thermal conductivity of the membrane surface must be as low as possible | [51] |
| Fouling resistance | The polymeric membrane can be coated with fouling resistant materials to ensure high permeate flux | [52] |
| Thermal stability | The MD polymeric membrane must show high thermal stability up to 80 °C. | [53] |
| Chemical resistance | The MD membranes must exhibit good chemical resistance because they may come in contact with acids and bases | [54] |
| Effects | Reasons | Comments | Reference |
|---|---|---|---|
| Scaling of membrane | Scaling occurs because of the deposition of inorganic ions and components that reduce the hydrophobicity of the MD membrane | Deposition of crystalline inorganic corrosive foulants | [57,60] |
| Organic and biofouling | Growth of biofoulants reduces the hydrophobicity and surface tension | Interaction between the aqueous medium and hydrophobic surface | [58,61] |
| Degradation of membrane | Formation of hydrophilic chemical groups, due to long term use of the membrane | Loss of chemical and mechanical stability makes MD process difficult | [62] |
| Feed stream-based wetting | Surfactant-based feed stream reduces the liquid entry pressure (LEP) value | The liquid entry pressure (LEP) is directly proportional to the surface tension | [62] |
| Parameters | Factors | Comments | References |
|---|---|---|---|
| Operational conditions | Effect of temperature | Salts, such as CaSO4 and CaCO3, which have a negative correlation of solubility with respect to temperature also tend to become saturated in the feed stream of desalination. Typically, for commonly utilized feed solutions, increased temperature leads to increased risk of scaling and fouling | [69,70] |
| Effect of dissolved gases | The presence of dissolved gases in the feed stream leads to chemical processes, such as the breakdown of bicarbonates which penetrate the membrane pores along with water vapor, exerting an additional diffusive resistance for the water vapor. | [52] | |
| Membrane properties | Thickness of the membrane | Membrane thickness seems to be inversely proportional to the mass and heat transfer rate across the MD membrane. Thus, an optimized membrane thickness must be utilized. | [71] |
| Pore size distribution | The pore size of the MD membrane ranges from 0.1 µm to 1 µm In general, pore size affects the mass transfer mechanism. For instance, one side of the membrane, the pore size should be small so that water or liquid cannot penetrate into the pores, while, the pore size must be large on another side, in order to achieve high permeate flux. | [72] | |
| Porosity | Higher porosity of the membrane offers more water flux, but rapidly wets the membrane | [73] | |
| Surface energy | A low surface energy offers high hydrophobicity | [74] | |
| Feed solution chemistry | Surface tension | If the feed solution is composed of surfactants higher than the critical value, membrane and pore wetting occurs. Thus, feed streams with a low surface tension must be avoided | [75] |
| Concentration of non-volatile solutes | A higher concentration of inorganic salts may result in the formation of salt crystals onto the membrane surface, leading to wetting of membrane portions occupied by salt crystals. Therefore, a higher concentration of inorganic salts may lead to the formation of a cake layer of inorganic foulants that leads to membrane and pore wetting | [76] |
| Criteria | Description | Reference |
|---|---|---|
| Hierarchical structure and lower surface energy | A hierarchical structure blended with lower surface energy materials are required to prevent membrane wetting which occurs because of the presence of a hot feed stream consisting of an NaCl solution. | [89] |
| Porous skin layer | The top active layer must be porous in nature with proper porosity, pore size range, and interconnected pore channels without blocking the support layer from achieving a high-water flux | [90] |
| Mechanical and chemical stability | The mechanical and chemical stability between the active layer and support layer must be high, so that it can perform efficiently during the hydraulic impact in MD application | [91] |
| Polymeric Solution | Additives or Nanoparticle Type | Overall Outcome | Reference |
|---|---|---|---|
| Polyvinylidene fluoride (PVDF) solution | TiO2 nanoparticles | High and stable flux | [114] |
| PVDF solution | Carbon nanotubes | Flux improvement | [115] |
| Poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP) solution | Fluorosilane coated TiO2 nanoparticles | Wetting resistance | [114] |
| PVDF-HFP solution | Graphene | Stable flux | [116] |
| PVDF solution | Clay particles | Wetting resistance | [117] |
| PVDF solution | SiO2 nanoparticles | Wetting and fouling resistance | [118] |
| PVDF solution | Multilayer graphene platelets | Improved productivity–efficiency trade-off and enhanced wetting resistance | [119] |
| PVDF solution | Silver nanoparticles/Multi-walled carbon nanotubes (AgNPs/f-MWCNTs) | High anti-biofouling and separation efficiency | [120] |
| PVDF-HFP solution | Carbon nanotubes (CNTs) | Superhydrophobicity and efficient desalination performance | [121] |
| PVDF solution | Metal organic framework: MOF (iron 1,3,5-benzenetricarboxylate) | Stable performance and stable liquid entry pressure | [122] |
| Advantages | Explanation | Reference |
|---|---|---|
| Facile approach | 1. Simple methodology 2. Formation of uniform, consistent, thin, and clear coating layer | [140] |
| Strong adhesion | 1. Chemically and mechanically stable coating 2. Dense layer formation onto the polymeric surface | [141] |
| Surface roughening | 1. Transformation of hydrophobic to superhydrophobic surface | [142] |
| Antifouling and antiwetting | 1. Membranes with improved fouling resistance can be fabricated 2. Superhydrophobicity can be achieved and results in antiwetting properties | [143] |
| Major Advantages | Limitations |
|---|---|
| 1. Avoids the line of sight | 1. Requirement of high temperature |
| 2. High deposition rate | 2. High possibility of toxic precursor |
| 3. Production of thick coating layers | 3. Mostly inorganic materials can be used |
| 4. Co-deposition of material at same time |
| Table 10 (a) | ||||
| Methodologies | Casting Parameters | Membrane Characteristics | Membrane Performance | Reference |
| Electrospinning technique | Ease: Moderate Replication: Moderate | Pore size distribution: Maximum Surface roughness: Maximum | Overall cost: Moderate Permeate flux: Maximum Wetting tendency: Minimum | [111,150] |
| Mechanical stretching technique | Ease: Moderate Replication: Moderate | Pore size distribution: Same range Surface roughness: Lower | Overall cost: Moderate Permeate flux: Moderate Wetting tendency: Minimum | [151] |
| Phase inversion technique | Ease: Maximum Replication: Maximum | Pore size distribution: Same range Surface roughness: Minimum | Overall cost: Minimum Permeate flux: Maximum Wetting tendency: Moderate | [152] |
| Table 10 (b) | ||||
| Membrane Modification Techniques | Modification Process | Processing Time | Overall Outcome | References |
| Blending or coating technique | Coated with nanoparticles or nanosols | 1. Bit slow | Chemically stable | [153] |
| Plasma treatment | Growth of nanoparticles by etching | 1. Moderate 2. Requires particular equipment | Self-cleaning ability | [138,154] |
| Chemical vapor deposition | Formation of nanostructures by polymerization | 1. Slow 2. Require heating treatment | Good separation efficiency | [79] |
| Technique Utilized | Polymer Type | Additive or Agent/Processing | Permeate Flux | Salt Rejection | Reference |
|---|---|---|---|---|---|
| Phase inversion Technique | Polyvinylidene fluoride (PVDF) | Mechanical scratching | 90 LMH | 99.99% | [98] |
| Phase inversion Technique | Polyvinylidene fluoride (PVDF) | SiO2 nanoparticles | 3 kg/m2 h | 99.98% | [159] |
| Electrospinning Technique | Polyvinylidene fluoride- Polytetrafluoroethylene (PVDF-PTFE) | PTFE micro powders | 18.5 kg/m2 h | 99.9% | [86] |
| Electrospinning Technique | Poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP) | Graphene | 22.9 LMH | 100% | [116] |
| Mechanical Stretching Method | Polytetrafluoroethylene (PTFE) | Stretching | 5 LMH | 99.99% | [160] |
| Mechanical Stretching Method | Polyvinylidene fluoride (PVDF) | Stretching | 41.5 kg/m2 h | 99.99% | [103] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinha Ray, S.; Lee, H.-K.; Kwon, Y.-N. Review on Blueprint of Designing Anti-Wetting Polymeric Membrane Surfaces for Enhanced Membrane Distillation Performance. Polymers 2020, 12, 23. https://doi.org/10.3390/polym12010023
Sinha Ray S, Lee H-K, Kwon Y-N. Review on Blueprint of Designing Anti-Wetting Polymeric Membrane Surfaces for Enhanced Membrane Distillation Performance. Polymers. 2020; 12(1):23. https://doi.org/10.3390/polym12010023
Chicago/Turabian StyleSinha Ray, Saikat, Hyung-Kae Lee, and Young-Nam Kwon. 2020. "Review on Blueprint of Designing Anti-Wetting Polymeric Membrane Surfaces for Enhanced Membrane Distillation Performance" Polymers 12, no. 1: 23. https://doi.org/10.3390/polym12010023
APA StyleSinha Ray, S., Lee, H.-K., & Kwon, Y.-N. (2020). Review on Blueprint of Designing Anti-Wetting Polymeric Membrane Surfaces for Enhanced Membrane Distillation Performance. Polymers, 12(1), 23. https://doi.org/10.3390/polym12010023