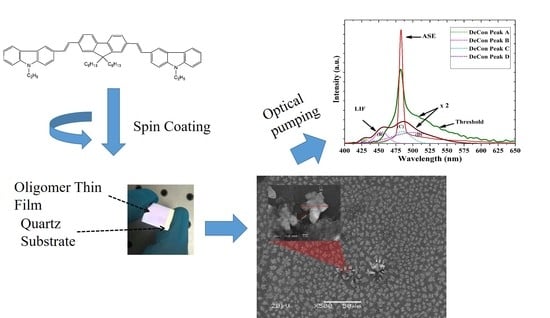
Narrowband Spontaneous Emission Amplification from a Conjugated Oligomer Thin Film
Abstract
:1. Introduction
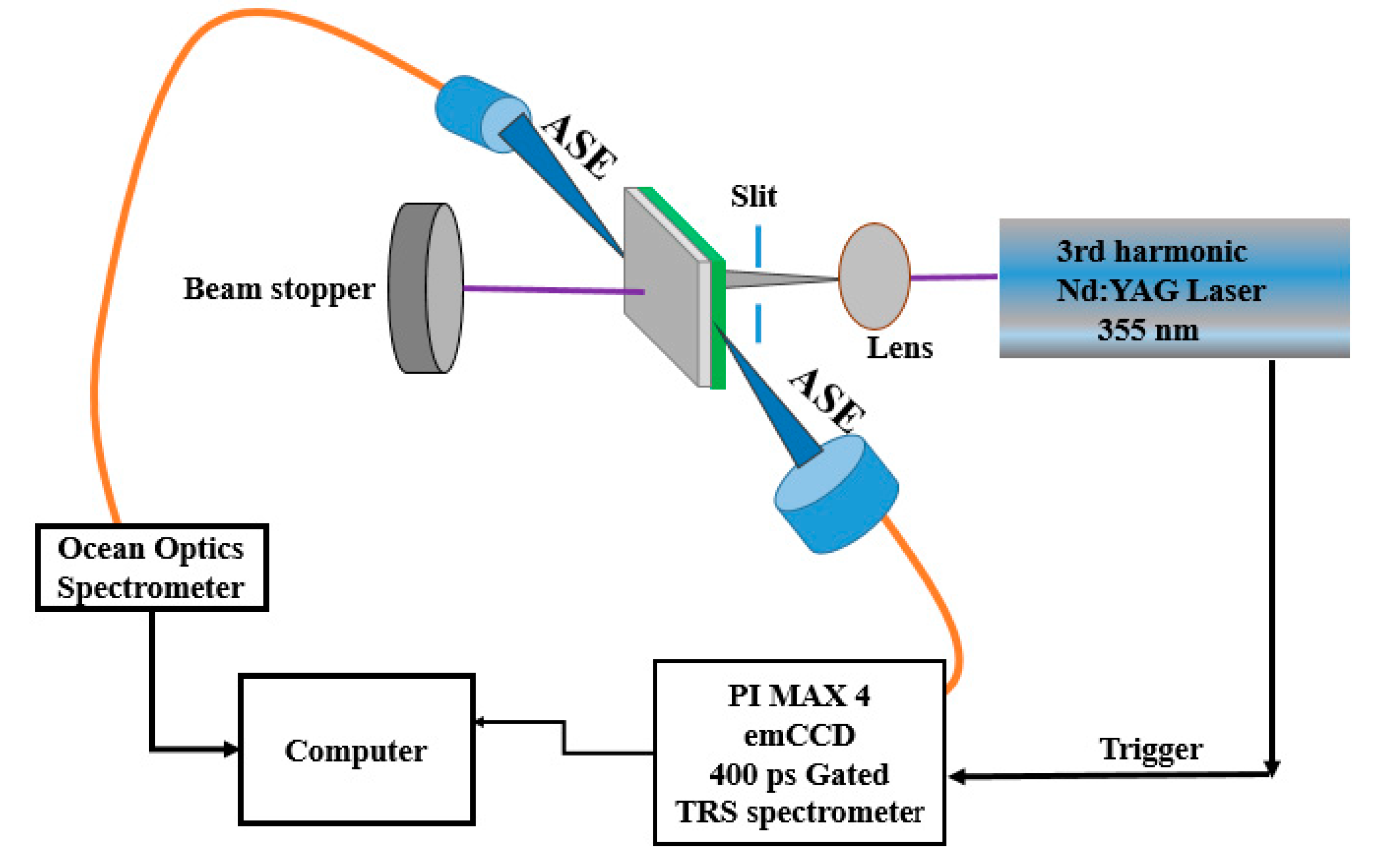
2. Materials and Methods
3. Results and Discussion
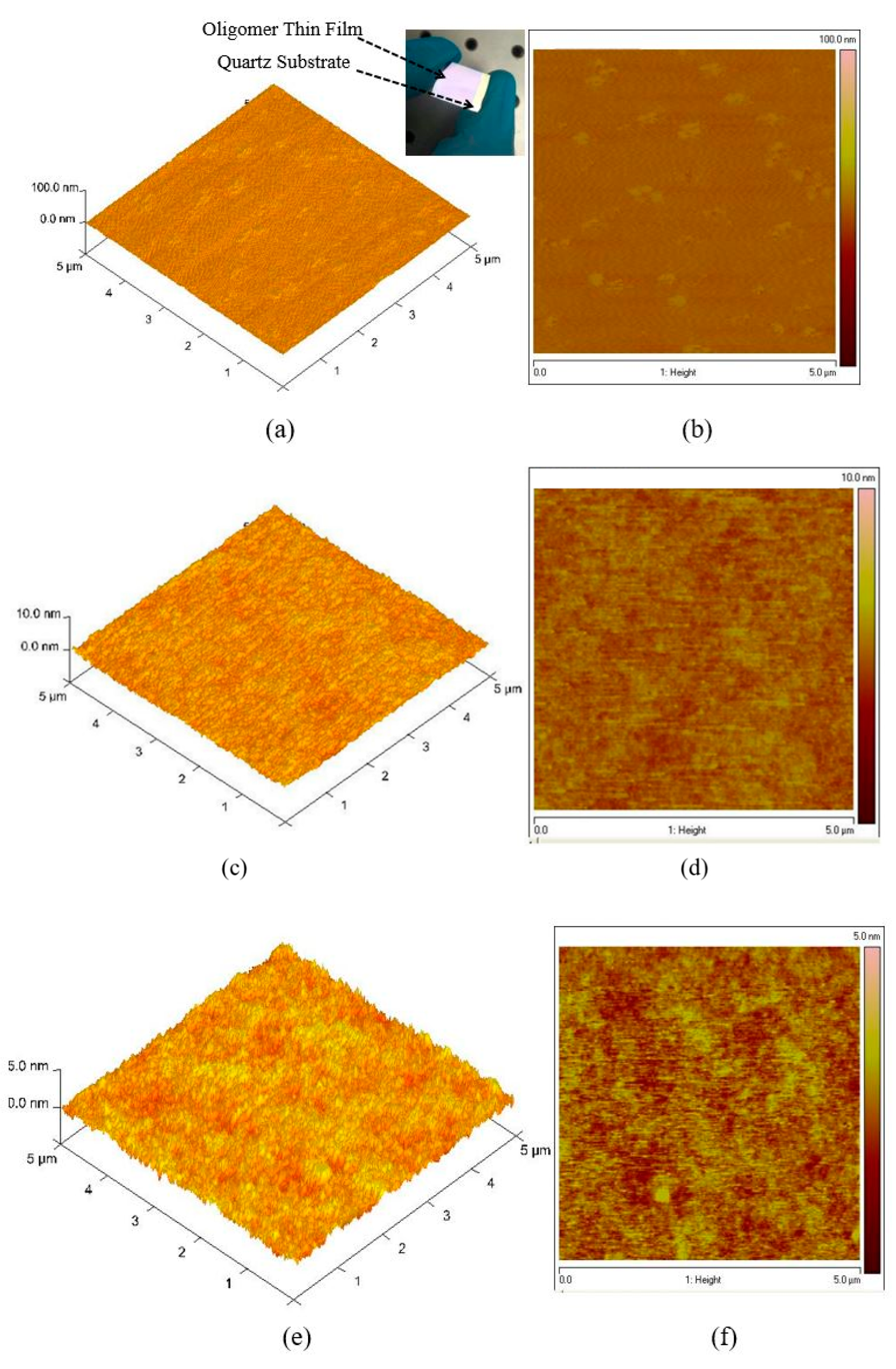
3.1. Atomic Force Microscopy (AFM)
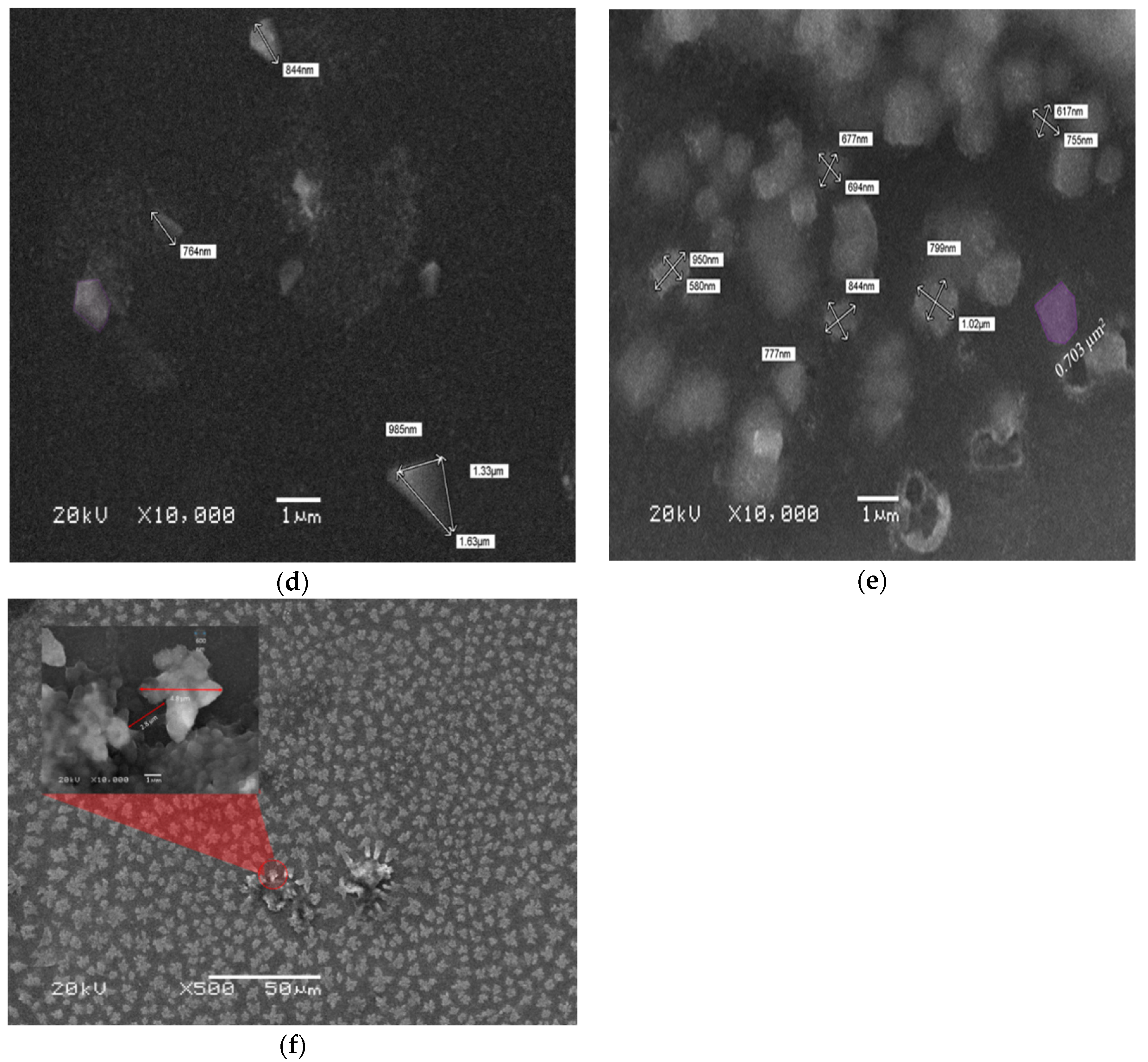
3.2. Scanning Electron Microscope Analysis
3.3. Absorption and Photoluminescence Properties of the Oligomer Film
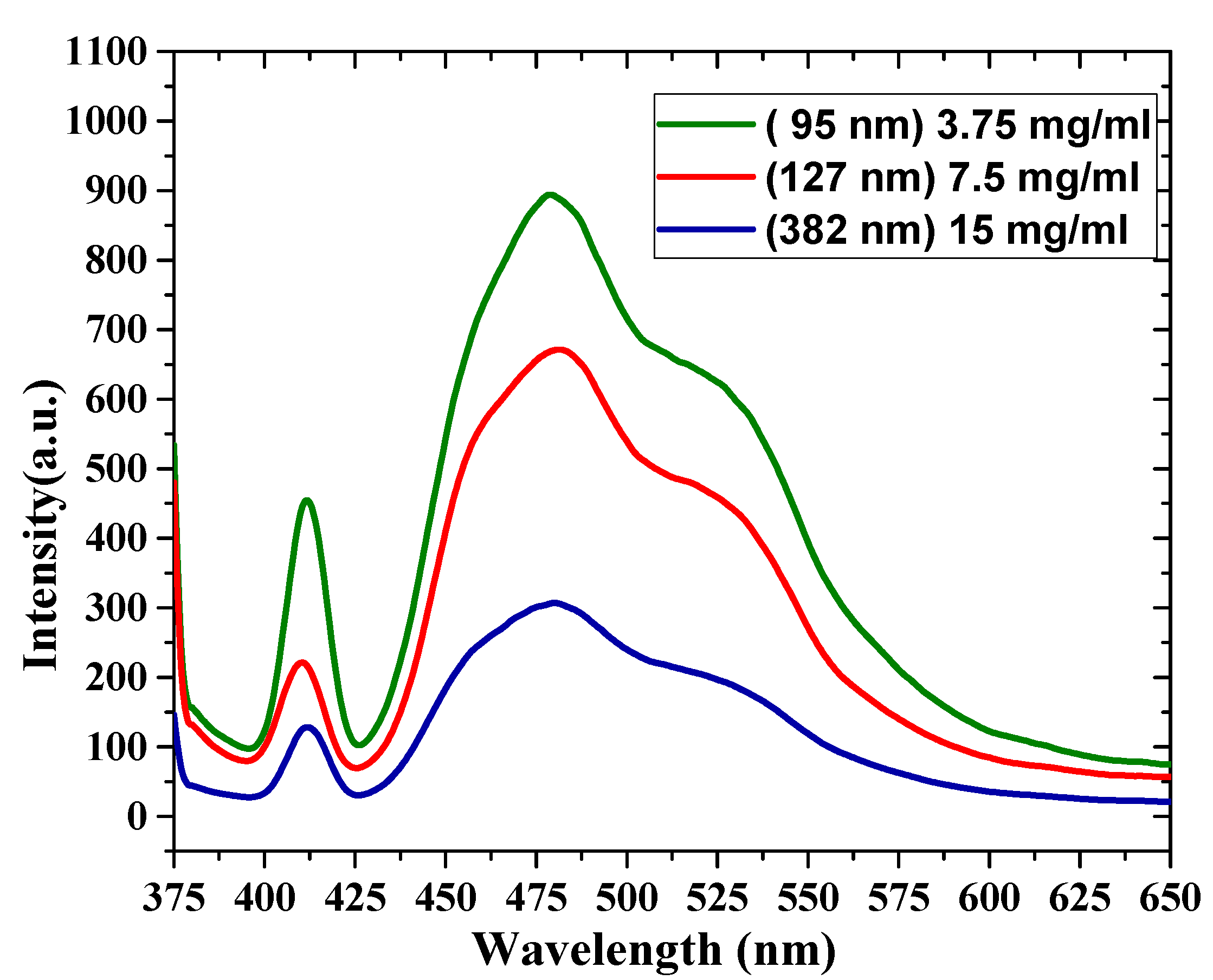
3.4. Photoluminescence (PL) Spectra of the Oligomer Thin Films
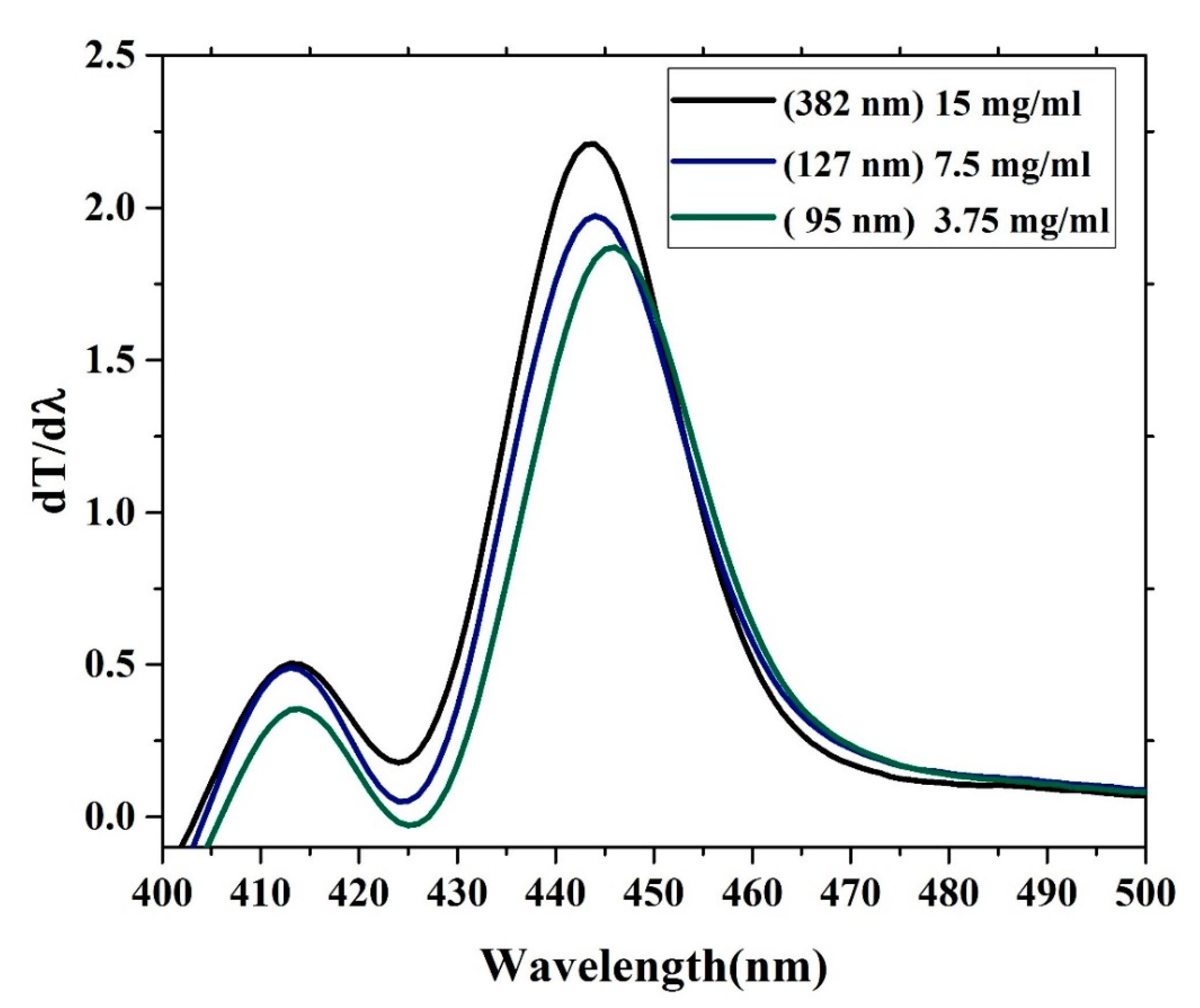
3.5. Band Gap Analysis
3.6. ASE (Mirrorless Laser) from CO BECV-DHF Films
3.7. TRS of the CO
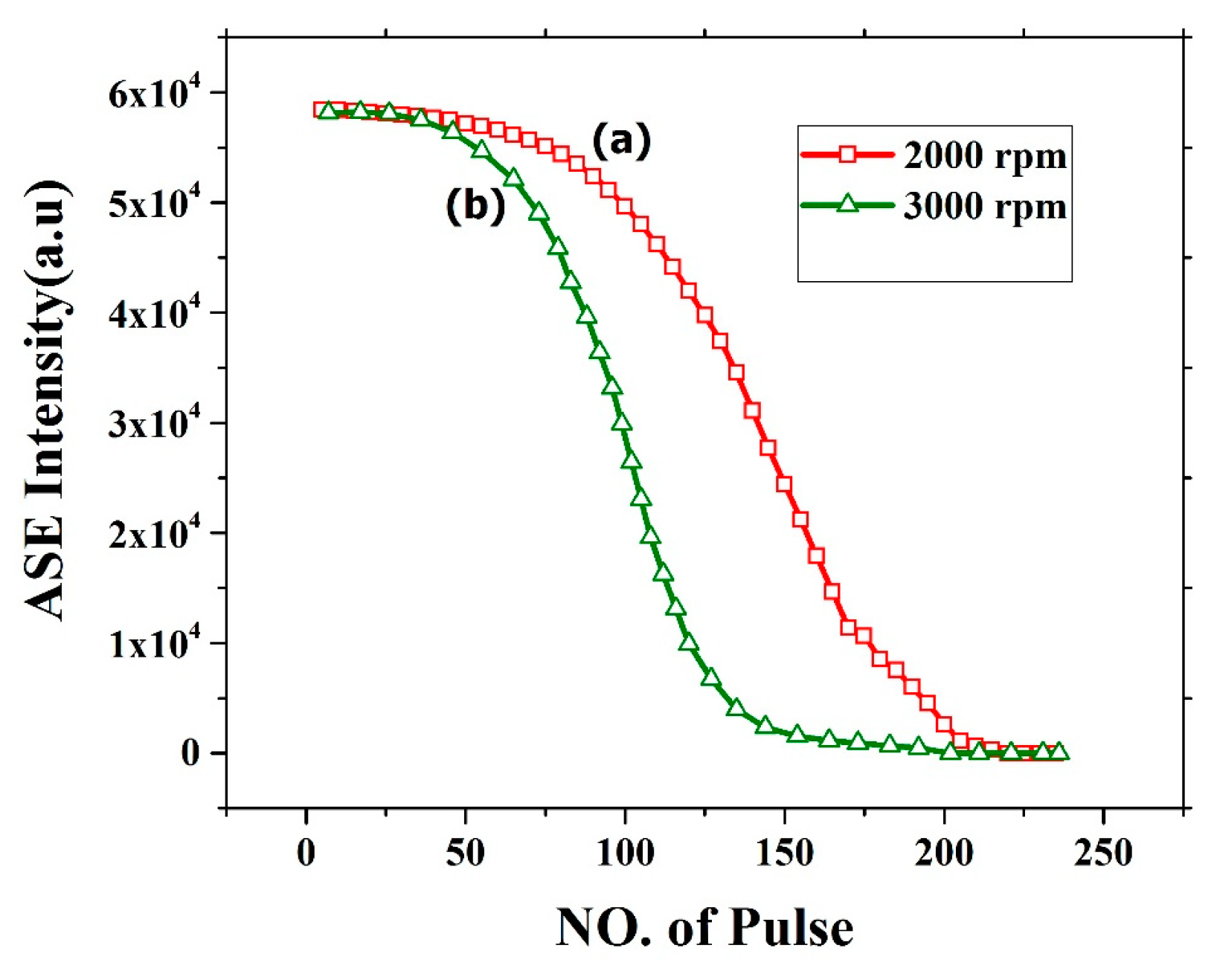
3.8. Stability of ASE from BECV-DHF films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Murphy, E. The semiconductor laser: Enabling optical communication. Nat. Photonics 2010, 4, 287. [Google Scholar] [CrossRef]
- Gupta, R.; Stevenson, M.; Dogariu, A.; McGehee, M.D.; Park, J.Y.; Srdanov, V.; Heeger, A.J.; Wang, H. Low-threshold amplified spontaneous emission in blends of conjugated polymers. Appl. Phys. Lett. 1998, 73, 3492–3494. [Google Scholar] [CrossRef]
- Hide, F.; Schwartz, B.J.; Díaz-García, M.A.; Heeger, A.J. Conjugated polymers as solid-state laser materials. Synth. Met. 1997, 91, 35–40. [Google Scholar] [CrossRef]
- Son, D.I.; Kim, H.H.; Cho, S.; Hwang, D.K.; Seo, J.W.; Choi, W.K. Carrier transport of inverted quantum dot LED with PEIE polymer. Org. Electron. physics, Mater. Appl. 2014, 15, 886–892. [Google Scholar] [CrossRef]
- Brabec, C.J.; Dyakonov, V.; Parisi, J.; Sariciftci, N.S. Organic Photovoltaics: Concepts and Realization; Springer: New York, NY, USA, 2003; pp. 57–84. [Google Scholar]
- Scherf, U.; Riechel, S.; Lemmer, U.; Mahrt, R.F. Conjugated polymers: Lasing and stimulated emission. Curr. Opin. Solid State Mater. Sci. 2001, 5, 143–154. [Google Scholar] [CrossRef]
- AlSalhi, M.S.; Alam, J.; Dass, L.A.; Raja, M. Recent advances in conjugated polymers for light emitting devices. Int. J. Mol. Sci. 2011, 12, 2036–2054. [Google Scholar] [CrossRef]
- Aljaafreh, M.J.; Prasad, S.; AlSalhi, M.S.; Alahmed, Z.A.; Al-Mogren, M.M. Optically pumped intensive light amplification from a blue oligomer. Polymers 2019, 11, 1534. [Google Scholar] [CrossRef] [Green Version]
- Anni, M. Polymer-II-VI Nanocrystals Blends: Basic Physics and Device Applications to Lasers and LEDs. Nanomaterials 2019, 9, 1036. [Google Scholar] [CrossRef] [Green Version]
- Rose, A.; Zhu, Z.; Madigan, C.F.; Swager, T.M.; Bulović, V. Sensitivity gains in chemosensing by lasing action in organic polymers. Nature 2005, 434, 876–879. [Google Scholar] [CrossRef]
- Alstrup, J.; Norrman, K.; Jørgensen, M.; Krebs, F.C. Lifetimes of organic photovoltaics: Design and synthesis of single oligomer molecules in order to study chemical degradation mechanisms. Sol. Energy Mater. Sol. Cells 2006, 90, 2777–2792. [Google Scholar] [CrossRef]
- McNeill, C.R.; Greenham, N.C. Conjugated-polymer blends for optoelectronics. Adv. Mater. 2009, 21, 3840–3850. [Google Scholar] [CrossRef]
- Prasad, S.; Ibnaouf, K.H.; Alsalhi, M.S.; Masilamani, V. Laser from the dimer state of a conjugated polymer (PFO) in solution. Polymer 2014, 55, 49. [Google Scholar] [CrossRef]
- Ibnaouf, K.H.; Prasad, S.; Masilamani, V.; Alsalhi, M.S.; Alaamer, A.S. Evidence for the double excimer state of conjugated polymer in a liquid solution. J. Eur. Opt. Soc. 2013, 8, 13001. [Google Scholar] [CrossRef]
- Ginzburg, N.S.; Baryshev, V.R.; Sergeev, A.S.; Malkin, A.M. Dynamics of semiconductor lasers with two-dimensional distributed feedback. Phys. Rev. A 2015, 91, 3806. [Google Scholar] [CrossRef]
- AlSalhi, M.S.; Almotiri, A.R.; Prasad, S.; Aljaafreh, M.J.; Othman, A.H.S.; Masilamai, V. A temperature-tunable thiophene polymer laser. Polymers 2018, 10, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chénais, S.; Forget, S. Recent advances in solid-state organic lasers. Polym. Int. 2012, 61, 390–406. [Google Scholar] [CrossRef] [Green Version]
- Kuehne, A.J.C.; Gather, M.C. Organic Lasers: Recent Developments on Materials, Device Geometries, and Fabrication Techniques. Chem. Rev. 2016, 116, 12823–12864. [Google Scholar] [CrossRef] [Green Version]
- Xia, H.; Hu, C.; Chen, T.; Hu, D.; Zhang, M.; Xie, K. Advances in conjugated polymer lasers. Polymers 2019, 11, 443. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Grimsdale, A.C.; Zhao, Y. Analyzing the optical properties of a conjugated polymer by the multimode brownian oscillator model. J. Phys. Chem. A. 2010, 114, 504–508. [Google Scholar] [CrossRef]
- Milad, R.; Shi, J.; Aguirre, A.; Cardone, A.; Milián-Medina, B.; Farinola, G.M.; Abderrabba, M.; Gierschner, J. Effective conjugation in conjugated polymers with strongly twisted backbones: A case study on fluorinated MEHPPV. J. Mater. Chem. C 2016, 4, 6900–6906. [Google Scholar] [CrossRef]
- Meier, H.; Stalmach, U.; Kolshorn, H. Effective conjugation length and UV/vis spectra of oligomers. Acta Polym. 1997, 48, 379–384. [Google Scholar] [CrossRef]
- Calzado, E.M.; Boj, P.G.; Díaz-García, M.A. Amplified spontaneous emission properties of semiconducting organic materials. Int. J. Mol. Sci. 2010, 11, 2546–2565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, M.S.; Meresa, A.A.; Kwon, C.M.; Kim, F.S. Selective wet-etching of polymer/fullerene blend films for surface- and nanoscale morphology-controlled organic transistors and sensitivity-enhanced gas sensors. Polymers 2019, 11, 1682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garner, L.E.; Park, J.; Dyar, S.M.; Chworos, A.; Sumner, J.J.; Bazan, G.C. Modification of the optoelectronic properties of membranes via insertion of amphiphilic phenylenevinylene oligoelectrolytes. J. Am. Chem. Soc. 2010, 132, 10042–10052. [Google Scholar] [CrossRef] [PubMed]
- Pisignano, D.; Anni, M.; Gigli, G.; Cingolani, R.; Zavelani-Rossi, M.; Lanzani, G.; Barbarella, G.; Favaretto, L. Amplified spontaneous emission and efficient tunable laser emission from a substituted thiophene-based oligomer. Appl. Phys. Lett. 2002, 81, 3534–3536. [Google Scholar] [CrossRef]
- Ann, M.; Lattante, S.; Cingolani, R.; Gigli, G.; Barbarella, G.; Favaretto, L. Emission properties of organic random lasers. Phys. Status Solidi C Conf. 2004, 1, 450–453. [Google Scholar] [CrossRef]
- Wang, Y.; Tsiminis, G.; Yang, Y.; Ruseckas, A.; Kanibolotsky, A.L.; Perepichka, I.F.; Skabara, P.J.; Turnbull, G.A.; Samuel, I.D.W. Broadly tunable deep blue laser based on a star-shaped oligofluorene truxene. Synth. Met. 2010, 160, 1397–1400. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Y.; Xu, W.; Li, X.; Liu, J.; Guo, X.; Xia, R.; Huang, W. Efficient amplified spontaneous emission from oligofluorene-pyrene starbursts with improved electron affinity property. Opt. Express 2015, 23, A465–A470. [Google Scholar] [CrossRef]
- Choi, E.Y.; Mazur, L.; Mager, L.; Gwon, M.; Pitrat, D.; Mulatier, J.C.; Monnereau, C.; Fort, A.; Attias, A.J.; Dorkenoo, K.; et al. Photophysical, amplified spontaneous emission and charge transport properties of oligofluorene derivatives in thin films. Phys. Chem. Chem. Phys. 2014, 16, 16941–16956. [Google Scholar] [CrossRef]
- Aljaafreh, M.J.; Prasad, S.; AlSalhi, M.S.; Alahmed, Z.A. Ultrafast dynamics of laser from green conjugated-oligomer in solution. Polymer (Guildf). 2019, 169, 106–114. [Google Scholar] [CrossRef]
- Morales-Vidal, M.; Boj, P.G.; Villalvilla, J.M.; Quintana, J.A.; Yan, Q.; Lin, N.T.; Zhu, X.; Ruangsupapichat, N.; Casado, J.; Tsuji, H.; et al. Carbon-bridged oligo(p-phenylenevinylene)s for photostable and broadly tunable, solution-processable thin film organic lasers. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz-García, M.A.; Morales-Vidal, M.; Ramírez, M.G.; Villalvilla, J.M.; Boj, P.G.; Quintana, J.A.; Retolaza, A.; Merino, S. Solution-processable, photo-stable, low-threshold, and broadly tunable thin film organic lasers based on novel high-performing laser dyes. Org. Light Emit. Mater. Devices XIX 2015, 9566, 95660Q. [Google Scholar] [CrossRef] [Green Version]
- Masilamani, V.; Ghaithan, H.M.; Aljaafreh, M.J.; Ahmed, A.; Al Thagafi, R.; Prasad, S.; Alsalhi, M.S. Using a Spectrofluorometer for Resonance Raman Spectra of Organic Molecules. J. Spectrosc. 2017, 2017, 9830. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, L.; Shao, M.; Wu, Y.; Zeng, D.; Cai, X.; Duan, J.; Zhang, X.; Gao, X. Fine-Tuning the Quasi-3D Geometry: Enabling Efficient Nonfullerene Organic Solar Cells Based on Perylene Diimides. ACS Appl. Mater. Interfaces 2018, 10, 762–768. [Google Scholar] [CrossRef]
- Ahmad, W.; He, J.; Liu, Z.; Xu, K.; Chen, Z.; Yang, X.; Li, D.; Xia, Y.; Zhang, J.; Chen, C. Lead Selenide (PbSe) Colloidal Quantum Dot Solar Cells with >10% Efficiency. Adv. Mater. 2019, 31, 1–9. [Google Scholar] [CrossRef]
- Sánchez-Mendoza, A.V.; Ibarra-García, V.G.; Velázquez-Hernández, J.R.; Hernández-Ortíz, O.J.; Carrillo, J.; Palacios-Huerta, L.; Cosme, I.; Alvarez-Hernandez, A.; Alemán-Ayala, K.; Vázquez-García, R.A. Synthesis, chemical, theoretical studies, electrochemical, electrical and optical characterization of novel oligomer 2,2’-((1E,1’E)(2,5-bis(octyloxy)-1,4-phenylenevinylene)bis(6-(E)-2-(vinylquinolin))quinoline for OLED applications. J. Mater. Sci. Mater. Electron. 2019, 30, 19718–19730. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, K.; Li, Y.; Wei, Q.; Bo, Y.; Wang, L.; Qian, Y.; Xia, R.; Zhang, Q.; Huang, W. Low–threshold sky-blue gain medium from a Triazine-capped ladder-type oligomer neat film. Org. Electron. 2020, 76, 5452. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, Y.; Zhang, Q.; Gao, X. Non-fullerene small molecule acceptors based on perylene diimides. J. Mater. Chem. A 2016, 4, 17604–17622. [Google Scholar] [CrossRef]
- Alfahd, S.A.; Rajendra, S.P.; Al-Mujammi, W.; Devaraj, D.; Masilamani, V.; AlSalhi, M.S. An efficient violet Amplified Spontaneous Emission (ASE) from a conjugated polymer (PFO-co-pX) in solution. Materials 2017, 10, 265. [Google Scholar] [CrossRef] [Green Version]
- Lattante, S.; De Giorgi, M.L.; Pasini, M.; Anni, M. Low threshold Amplified Spontaneous Emission properties in deep blue of poly[(9,9-dioctylfluorene-2,7-dyil)-alt-p-phenylene] thin films. Opt. Mater. 2017, 72, 765–768. [Google Scholar] [CrossRef]


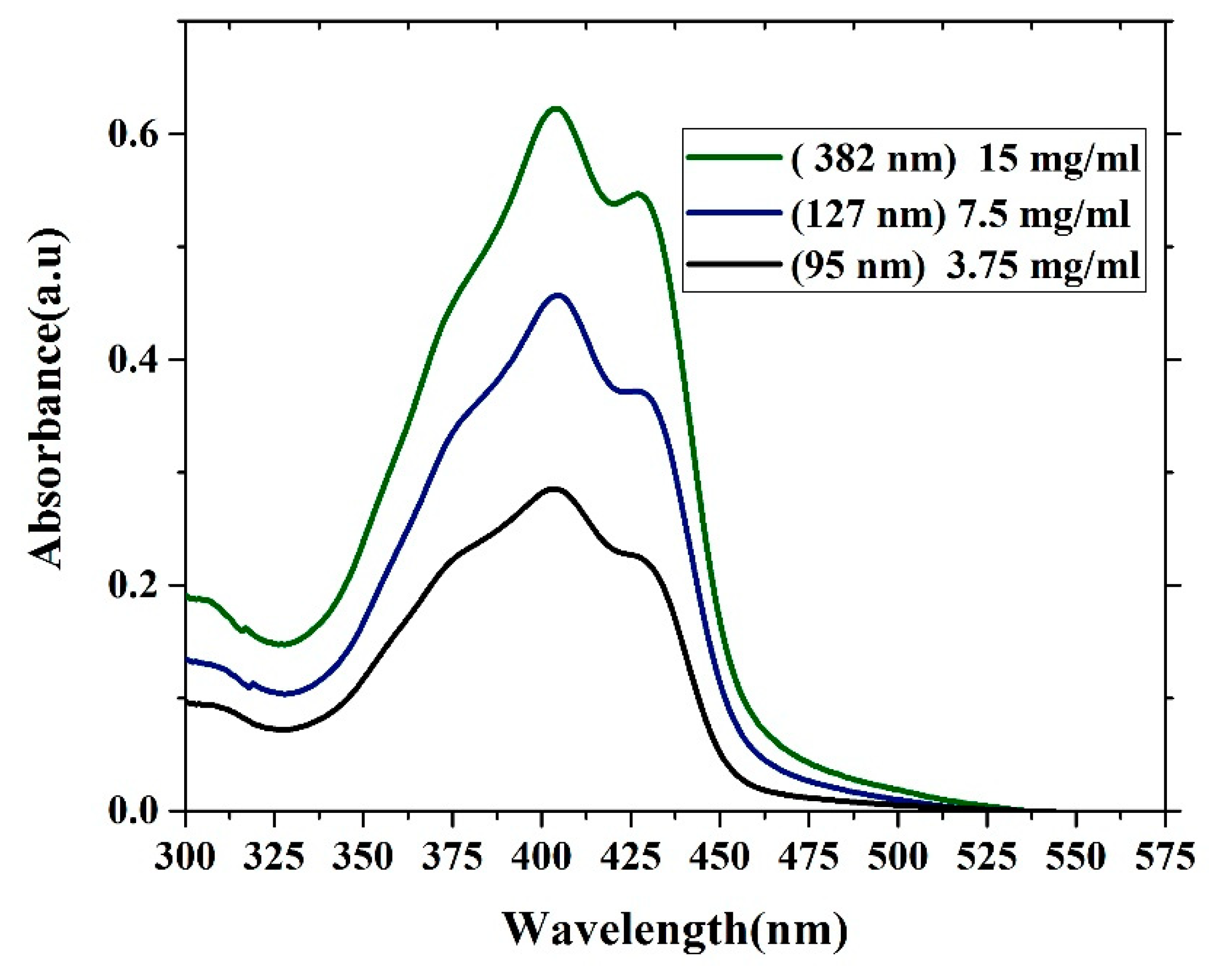

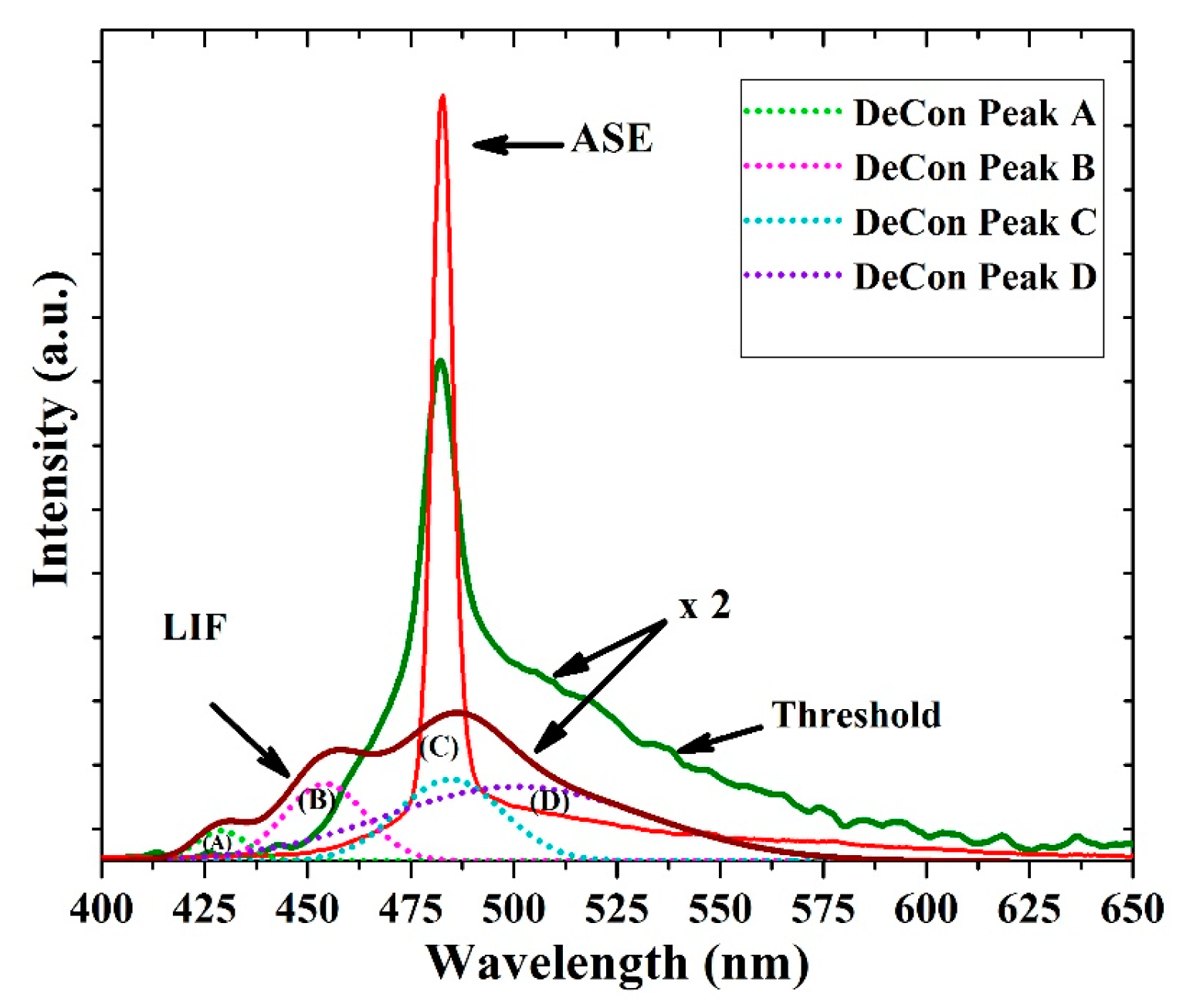
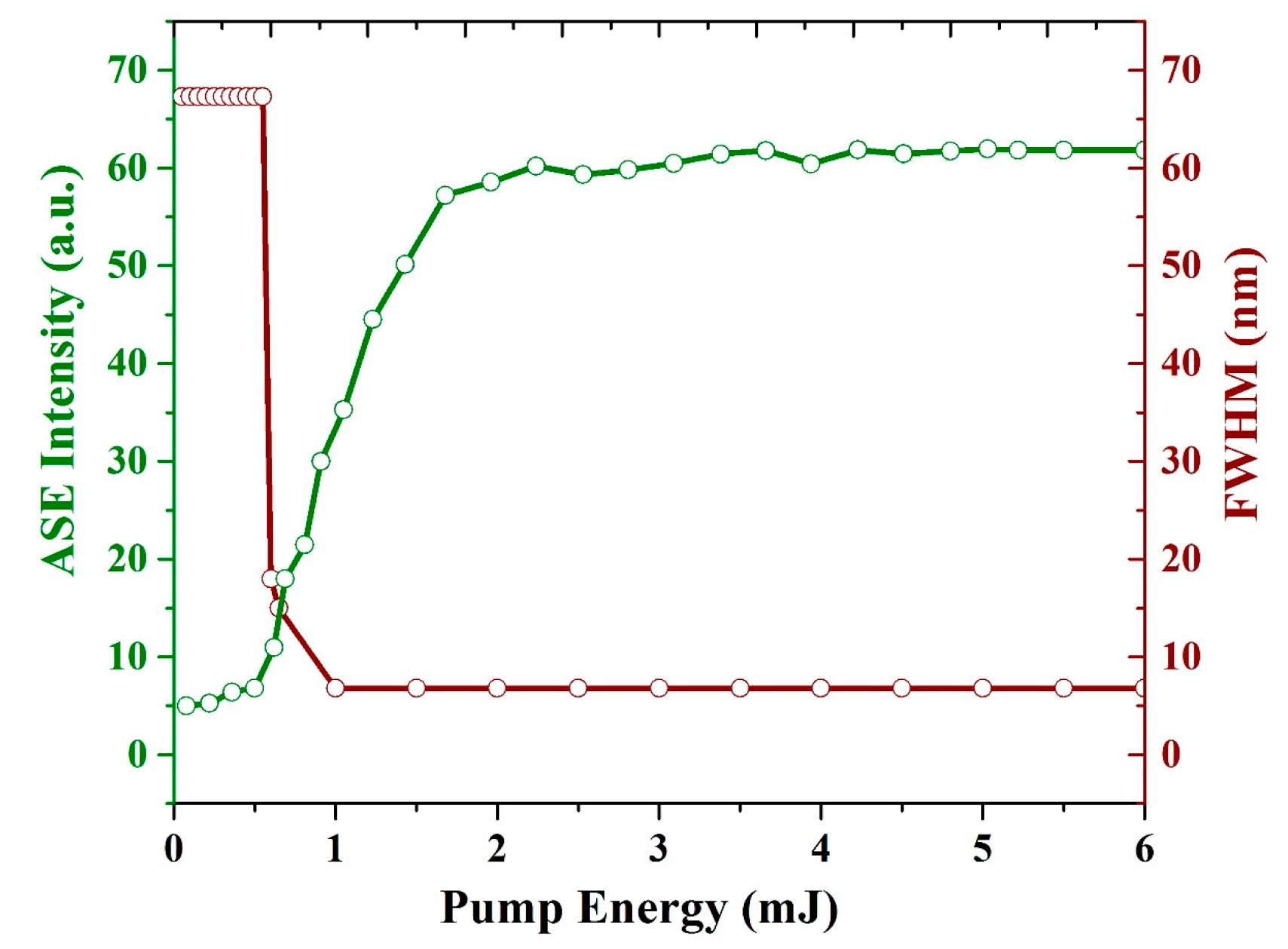
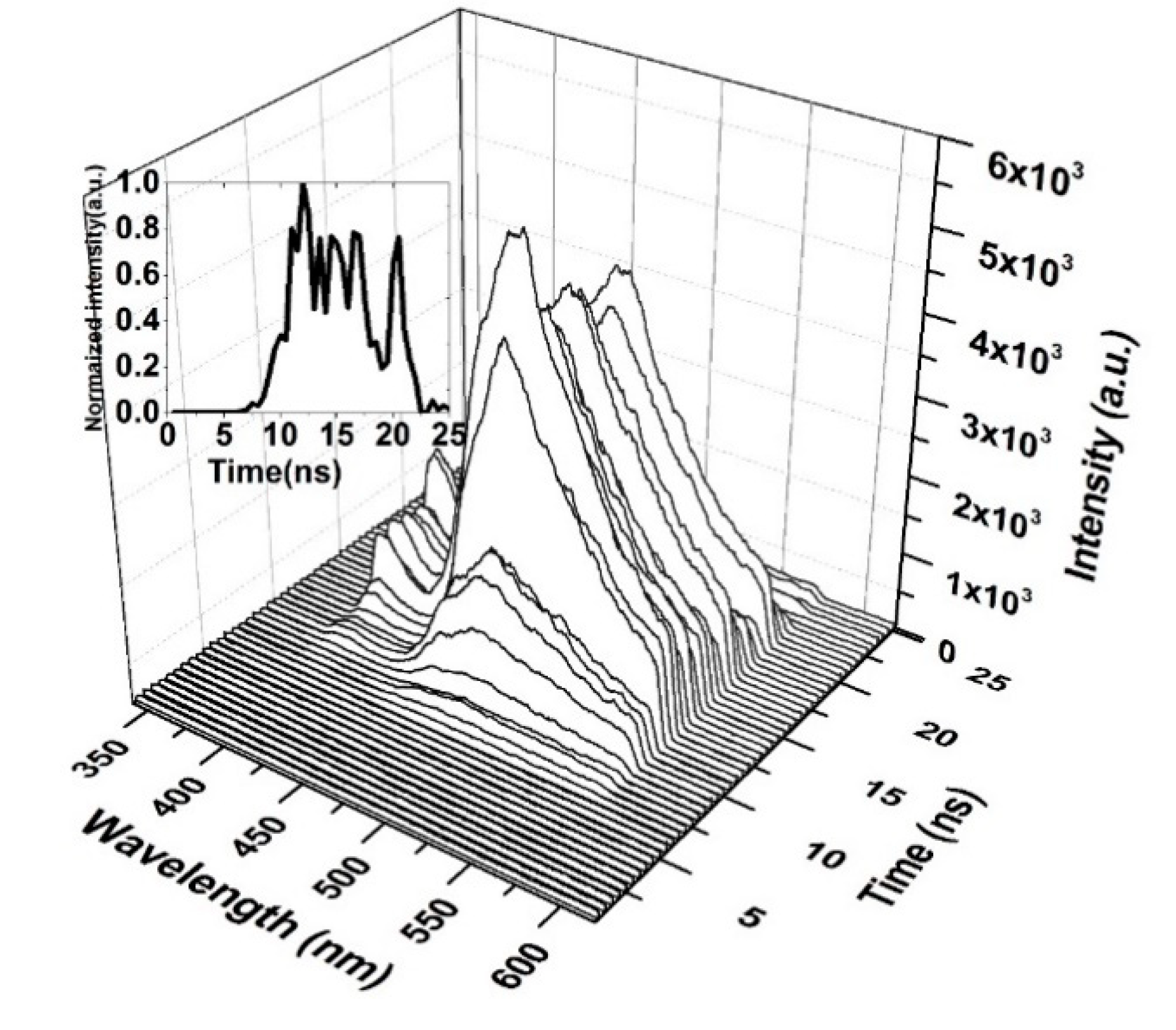

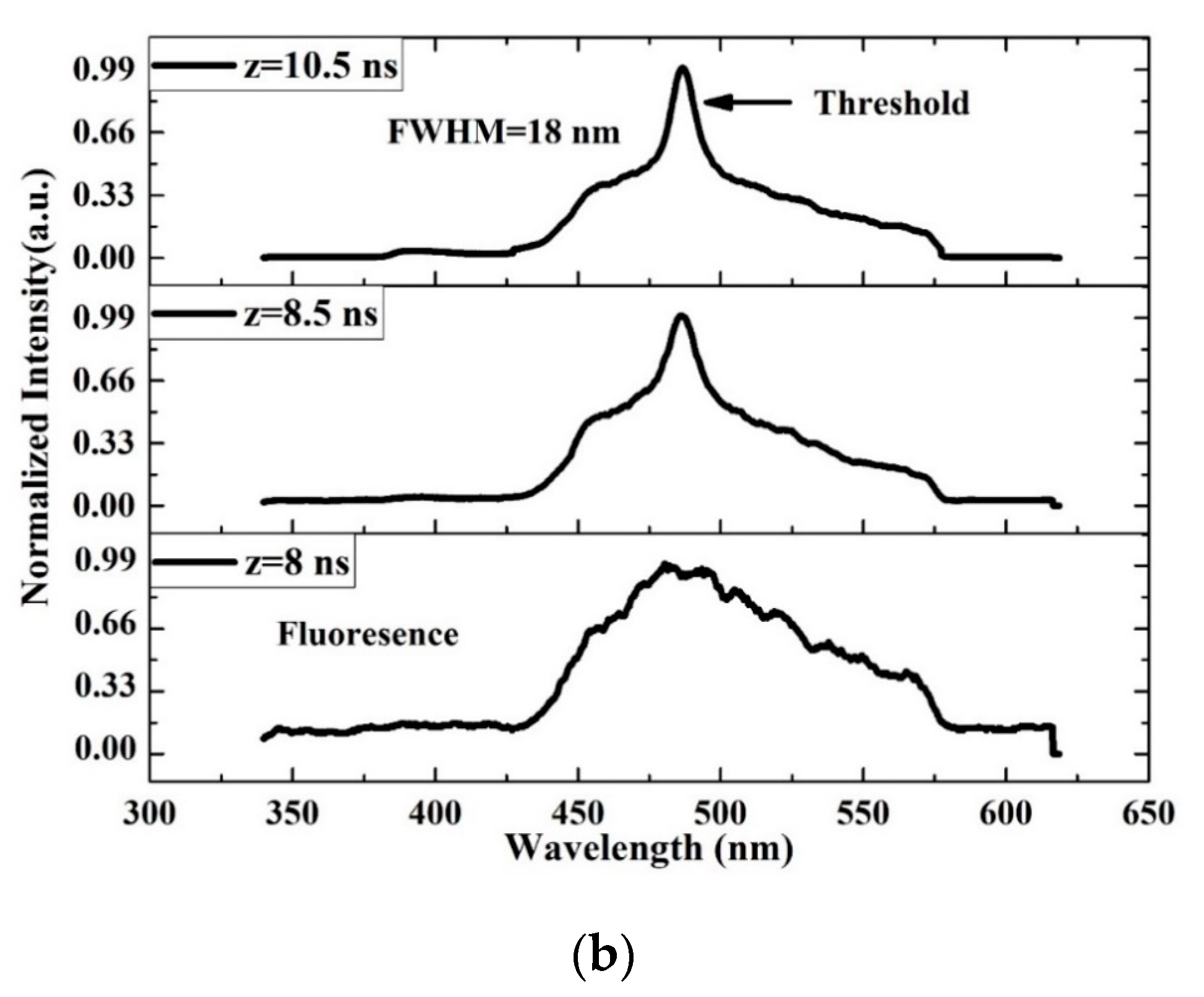



| Absorbance (Units) | Ratio (%) | ||||
|---|---|---|---|---|---|
| Samples (Concentration/Thickness) | V1 375 nm | V2 403 nm | D 428 nm | R (V1/V2) % | R (D/V2)% |
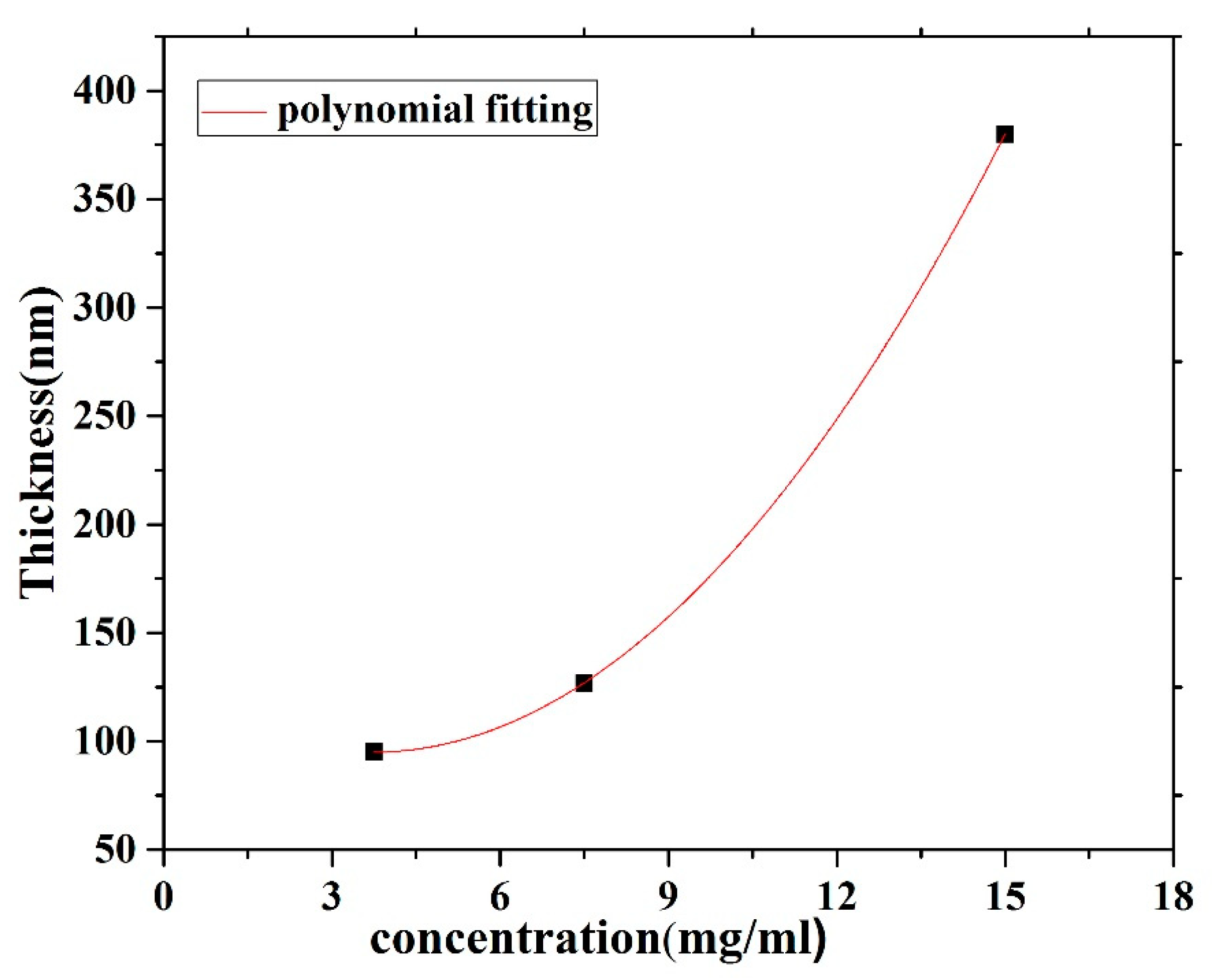
| 3.75 mg/mL 95 nm | 0.227 | 0.285 | 0.244 | 78.59 | 79.64 |
| 7.5 mg/mL 127 nm | 0.335 | 0.455 | 0.370 | 73.62 | 81.31 |
| 15 mg/mL 380 nm | 0.449 | 0.622 | 0.546 | 72.18 | 87.8 |
| Samples (Concentration/Thickness) | PL intensity (a.u) | Ratio (%) | |||||
|---|---|---|---|---|---|---|---|
| V0 at 411 nm | V1 at 455 nm | V2 at 480 nm | Shoulder at 523 nm | (V0/V2) | (V1/V2) | (Sh/V2) | |
| 3.75 mg/mL 95 nm | 452 | 653 | 892 | 629 | 50.07 | 73.2 | 70.5 |
| 7.5 mg/mL 127 nm | 220 | 506 | 671 | 465 | 32.8 | 75.4 | 69.3 |
| 15 mg/mL 380 nm | 127 | 234 | 307 | 200 | 41.4 | 76.2 | 65.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlSalhi, M.S.; Aljaafreh, M.J.; Prasad, S. Narrowband Spontaneous Emission Amplification from a Conjugated Oligomer Thin Film. Polymers 2020, 12, 232. https://doi.org/10.3390/polym12010232
AlSalhi MS, Aljaafreh MJ, Prasad S. Narrowband Spontaneous Emission Amplification from a Conjugated Oligomer Thin Film. Polymers. 2020; 12(1):232. https://doi.org/10.3390/polym12010232
Chicago/Turabian StyleAlSalhi, Mohamad S., Mamduh J. Aljaafreh, and Saradh Prasad. 2020. "Narrowband Spontaneous Emission Amplification from a Conjugated Oligomer Thin Film" Polymers 12, no. 1: 232. https://doi.org/10.3390/polym12010232