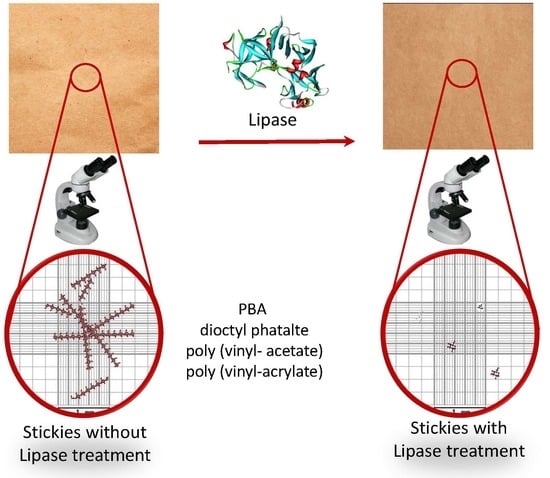
Chemical Characterization and Enzymatic Control of Stickies in Kraft Paper Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Stickies Sampling
- B3—Coil 3;
- B4—Coil 4;
- LS—Dirty felt.
- M—Original sticky, product sampling;
- E—Extracted with ethanol;
- PE—Precipitated from ethanol extract;
- EA—Extracted with ethyl acetate;
- CH—Extracted with cyclohexane;
- DM—Extracted with dichloromethane;
- D—Sticky remaining in the Soxhlet thimble at the end of the entire extraction.
2.2. Fourier-Transform Infrared Spectrometry
2.3. Thermogravimetric Analysis
2.4. Enzyme Preparation and Sample Treatment
2.5. Stickies Quantification to Evaluate Enzymatic Treatment
3. Results
3.1. Stickies Extraction, TGA, and FTIR
3.2. Determining the Purity and Activity of Lipase on Naturally Occurring Stickies
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Licursi, D.; Antonetti, C.; Martinelli, M.; Ribechini, E.; Zanaboni, M.; Galletti, A.M.R. Monitoring/characterization of stickies contaminants coming from a papermaking plant–Toward an innovative exploitation of the screen rejects to levulinic acid. Waste Manag. 2016, 49, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.; Balea, A.; Blanca, E.; Carrillo, I.; Blanco, A. Identification of Recalcitrant Stickies and Their Sources in Newsprint Production. Ind. Eng. Chem. Res. 2008, 47, 6239–6250. [Google Scholar] [CrossRef] [Green Version]
- Blanco, A.; Miranda, R.; Monte, M. Extending the limits of paper recycling-improvements along the paper value chain. For. Syst. 2013, 22, 471–483. [Google Scholar] [CrossRef] [Green Version]
- Bajpai, P. Biotechnology for Pulp and Paper Processing; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Yaguchi, T. Quantitative Estimation of Macro Stickies in Recycled Pulps for Linerboard. Jpn. Tappi J. 2007, 61, 54–58. [Google Scholar] [CrossRef]
- Doshi, M.R.; Moore, W.J.; Venditti, R.; Copeland, K.; Chang, H.-M.; Putz, H.-J.; Delagoutte, T.; Houtman, C.; Tan, F.; Davie, L. Comparison of macrostickies measurement methods. Prog. Pap. Recycl. 2003, 12, 34–43. [Google Scholar]
- Monte, M.; Sánchez, M.; Blanco, A.; Negro, C.; Tijero, J. Improving deposition tester to study adherent deposits in papermaking. Chem. Eng. Res. Des. 2012, 90, 1491–1499. [Google Scholar] [CrossRef] [Green Version]
- Doshi, M.; Dyer, J.; Aziz, S.; Jackson, K.; Abubakr, S.M. Quantification of micro stickies. In Paper Recycling Challenge; Doshi & Associates Inc.: Appleton, WI, USA, 1997; pp. 119–122. [Google Scholar]
- Klungness, J.H.; Doshi, M.R. Adhesive Contaminants (Stickies) and Methods for Removal; MRS Online Proceedings Library Archive; Materials Research Society: San Francisco, CA, USA, 1992; Volume 266, pp. 257–267. [Google Scholar]
- Monte, M.C.; Blanco, A.; Negro, C.; Tijero, J. Development of a methodology to predict sticky deposits due to the destabilisation of dissolved and colloidal material in papermaking—Application to different systems. Chem. Eng. J. 2004, 105, 21–29. [Google Scholar] [CrossRef] [Green Version]
- CEPI. Tackling the Challenges in Commodity Markets and on Raw Materials; Annual Recycling Statistics edn.; European Commission: Brussels, Belgium, 2011. [Google Scholar]
- Saint Amand, F.J. Hydrodynamics of deinking flotation. Int. J. Miner. Process. 1999, 56, 277–316. [Google Scholar] [CrossRef]
- Park, S.-B.; Lee, J.-M.; Eom, T.-J. The control of sticky contaminant with enzymes in the recycling of wastepaper. J. Ind. Eng. Chem. 2004, 10, 72–77. [Google Scholar]
- Gao, Y.; Qin, M.; Li, C.; Yu, H.; Zhang, F. Control of sticky contaminants with cationic talc in deinked pulp. BioResources 2011, 6, 1916–1925. [Google Scholar]
- Genest, S.; Petzold, G.; Schwarz, S. Removal of micro-stickies from model wastewaters of the paper industry by amphiphilic starch derivatives. Colloids Surf. A Physicochem. Eng. Asp. 2015, 484, 231–241. [Google Scholar] [CrossRef]
- Jones, D.R. Enzymes: Using Mother Nature’s tools to control man-made stickies. Pulp Pap. Can. 2005, 106, 23–25. [Google Scholar]
- Jones, D.R.; Fitzhenry, J.W. Esterase-type enzymes offer recycled mills an alternative approach to stickies control. Pulp Pap. 2003, 77, 28. [Google Scholar]
- Zhang, Z.; Lan, D.; Zhou, P.; Li, J.; Yang, B.; Wang, Y. Control of sticky deposits in wastepaper recycling with thermophilic esterase. Cellulose 2017, 24, 311–321. [Google Scholar] [CrossRef]
- Pei, J.C.; Dun, Q.X.; Wang, H.Y.; Zhang, F.D. Clean method for stickies control with StickAway enzyme in ONP pulps. Adv. Mater. Res. 2013, 690, 1426–1430. [Google Scholar] [CrossRef]
- Liu, T.Z.; Wang, D.F.; Zhao, H.Y.; Zheng, K.Y. Stickies Treatments of Recycled Fiber Pulping Wastewater by Lipases. Adv. Mater. Res. 2012, 534, 225–229. [Google Scholar] [CrossRef]
- Sykes, M.S.; Klungness, J.H.; Tan, F.; Abubakr, S.M. Enzymatic Removal of Stickle Contaminants. In Proceedings of the Tappi Pulping Conference, Forest Products Laboratory, Madison, WI, USA, 19–23 October 1997; pp. 687–692. [Google Scholar]
- Gutiérrez, A.; José, C.; Martínez, A.T. Microbial and enzymatic control of pitch in the pulp and paper industry. Appl. Microbiol. Biotechnol. 2009, 82, 1005–1018. [Google Scholar]
- Blanco, A.; Negro, C.; Borch, K.; Minning, S.; Hannuksela, T.; Holmbom, B. Pitch control in thermomechanical pulping and papermaking by enzymatic treatments. Appita J. 2005, 58, 358. [Google Scholar]
- Gutiérrez, A.; del Río, J.C.; Martínez, M.J.; Martínez, A.T. The biotechnological control of pitch in paper pulp manufacturing. Trends Biotechnol. 2001, 19, 340–348. [Google Scholar] [CrossRef]
- Hata, K.; Matsukura, M.; Taneda, H.; Fujita, Y. Mill-Scale Application of Enzymatic Pitch Control During Paper Production; American Chemical Society: Washington, DC, USA, 1996. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680. [Google Scholar] [CrossRef]
- Derakhshanian, V.; Banerjee, S. Cyclodextrin inhibits calcium carbonate crystallization and scaling. Ind. Eng. Chem. Res. 2012, 51, 4463–4465. [Google Scholar] [CrossRef]
- Blanco, A.; Miranda, R.; Negro, C.; Garcia-Suarez, C.; Garcia-Prol, M.; Sanchez, A. Full characterization of stickies in a newsprint mill: The need for a complementary approach. Tappi J. 2007, 6, 19. [Google Scholar]
- Cao, B.; Heise, O. Analyzing contaminants in OCC wax or not wax. Pulp Pap. Can. 2005, 106, 41–46. [Google Scholar]
- Lee, H.L.; Kim, J.M. Quantification of macro and micro stickies and their control by flotation in OCC recycling process. Appita J. J. Tech. Assoc. Aust. N. Z. Pulp Pap. Ind. 2006, 59, 31. [Google Scholar]
- Castro, C.; Dorris, G. Measuring microstickies deposition by monitoring pressure drop through a collector. Prog. Pap. Recycl. 2004, 13, 23–33. [Google Scholar]
- Johansson, H.; Wikman, B.; Lindstrom, E.; Osterberg, F. Detection and evaluation of micro-stickies. Prog. Pap. Recycl. 2003, 12, 4–12. [Google Scholar]
- Janiroz, N. Determination of vinyl acetate (VA) content of ethylene-vinyl acetate (EVA) copolymers in thick films by infrared spectroscopy. J. Chem. Soc. Pak. 2003, 25, 84–87. [Google Scholar]
- Higgins, F.R.A. Quantitative Analysis of Copolymers Using the Cary 630 FTIR Spectrometer, Application Note, Materials Testing, and Research; Agilent Technologies: Danbury, CT, USA, 2011. [Google Scholar]
- Wang, G. Synthesis of poly (n-butyl acrylate) homopolymers by activators generated by electron transfer (AGET) ATRP using FeCl3·6H2O/succinic acid catalyst. Iran Polym. J. 2011, 20, 931–938. [Google Scholar]
- Sjarfrom, J.; Holmboh, B.; Wiklunde, L. Chemical characteristics of paper machine deposits from impurities inydeinked pulp. Nord. Pulp Pap. Res. J. 1987, 2, 123–126. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Martínez, M.J.; Del Río, J.C.; Romero, J.; Canaval, J.; Lenon, G.; Martínez, Á.T. Fungal pretreatment of eucalyptus wood can strongly decrease the amount of lipophilic extractives during chlorine free kraft pulping. Environ. Sci. Technol. 2000, 34, 3705–3709. [Google Scholar] [CrossRef]
- Qin, M.; Xu, Q.; Shao, Z.; Gao, Y.; Fu, Y.; Lu, X.; Gao, P.; Holmbom, B. Effect of bio-treatment on the lipophilic and hydrophilic extractives of wheat straw. Bioresour. Technol. 2009, 100, 3082–3087. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.J.; Ge, W.; Buswell, J.A. Secretion, purification and characterisation of a recombinant Volvariella volvacea endoglucanase expressed in the yeast Pichia pastoris. Enzym. Microb. Technol. 2002, 31, 621–626. [Google Scholar] [CrossRef]
- Deng, T.; Lu, L.; Gao, S.; Lin, Y.; Han, S. Endoglucanase enzymatic modification of kraft pulp during recycling. Biotechnol. Lett. 2016, 38, 1139–1145. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Ding, S.; Zhou, R.; Li, Z. Comparative characterization of a recombinant Volvariella volvacea endoglucanase I (EG1) with its truncated catalytic core (EG1-CM), and their impact on the bio-treatment of cellulose-based fabrics. J. Biotechnol. 2007, 130, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xie, W.; Lin, Y.; Lin, X.; Zheng, S.; Han, S. Combined strategies for improving the heterologous expression of an alkaline lipase from Acinetobacter radioresistens CMC-1 in Pichia pastoris. Process Biochem. 2013, 48, 1317–1323. [Google Scholar] [CrossRef]
- Verma, P.K.; Bhardwaj, N.K.; Singh, S.P. Improving the material efficiency of recycled furnish for papermaking through enzyme modifications. Can. J. Chem. Eng. 2016, 94, 430–438. [Google Scholar] [CrossRef]
- Barba Cedillo, V.; Prieto, A.; Martínez, M.J. Potential of Ophiostoma piceae sterol esterase for biotechnologically relevant hydrolysis reactions. Bioengineered 2013, 4, 249–253. [Google Scholar] [CrossRef] [Green Version]






| Solvent | n | er | m | g(er)-f(n) | dH |
|---|---|---|---|---|---|
| Cyclohexane | 1.426 | 2.024 | 0 | 0 | 16.8 |
| Ethyl Acetate | 1.372 | 6.03 | 1.78 | 0.41 | 18.4 |
| Dichloromethane | 1.424 | 9.02 | 1.6 | 0.46 | 20.3 |
| Ethanol | 1.359 | 24.55 | 1.66 | 0.62 | 26.2 |
| Sample | Total Extracted Fraction | Fraction with Ethanol | Fraction with Ethyl Acetate | Fraction with Cyclohexane | Fraction with Dichloromethane |
|---|---|---|---|---|---|
| B3 | 0.219 | 0.133 | 0.044 | 0.016 | 0.026 |
| B4 | 0.532 | 0.361 | 0.054 | 0.117 | ND |
| Sample | Comp A | Comp B | Comp C | Carbon | CO2 % | CaCO3 % | CaO % | % Solvent | % Organic | % Inorganic Fraction | % Inorganic without CaO |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 7B3M | 29.23 | 20.90 | 24.21 | 0.67 | 3.81 | 8.66 | 4.84 | 3.05 | 75.01 | 18.13 | 13.29 |
| 7B3E | 80.18 | 0 | 0 | 15.63 | 0 | 0 | 0 | 2.51 | 95.81 | 1.68 | 1.68 |
| 7B3PE | 58.98 | 29.50 | 0 | 10.33 | 0 | 0 | 0 | 0.38 | 98.81 | 0.80 | 0.81 |
| 7B3AE | 38.65 | 43.46 | 0 | 10.90 | 0 | 0 | 0 | 6.42 | 93.01 | 0.57 | 0.57 |
| 7B3CH | 54.19 | 14.30 | 18.49 | 5.98 | 0 | 0 | 0 | 3.62 | 93.05 | 3.33 | 3.33 |
| 7B3DM | 87.79 | 5.234 | 0 | 1.78 | 0 | 0 | 0 | 5.197 | 94.80 | 0 | 0 |
| 7DB3 | 3.63 | 49.30 | 0 | 4.50 | 8.69 | 19.78 | 11.06 | 3.22 | 57.42 | 30.64 | 19.58 |
| Stickies (Million Particles/mL) | Control | Lipase 30 G Concentration (g/L) | |||
| 0.11 | 0.22 | 0.33 | 0.44 | ||
| Media | 156.44 | 130.64 | 121.94 | 112.44 | 100.76 |
| Standard Deviation | 14 | 9 | 9 | 14 | 8 |
| % Removal | - | 16.49 | 22.05 | 28.12 | 35.59 |
| Stickies | Control | SebOil DG Concentration (g/L) | |||
| 0.11 | 0.22 | 0.33 | 0.44 | ||
| Media | 145.03 | 124.12 | 126.02 | 113.80 | 118.42 |
| Standard Deviation | 10 | 2 | 4 | 9 | 4 |
| % Removal | - | 14.41 | 13.10 | 21.5 | 18.34 |
| SebOil DG | Lipase 30 G | |
|---|---|---|
| % Stickies without Treatment (Control) | 1.43 | 2.55 |
| % Stickies with Enzyme | 1.06 | 0.63 |
| % Stickies Removal | 25.87 | 75.29 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ballinas-Casarrubias, L.; González-Sánchez, G.; Eguiarte-Franco, S.; Siqueiros-Cendón, T.; Flores-Gallardo, S.; Duarte Villa, E.; de Dios Hernandez, M.; Rocha-Gutiérrez, B.; Rascón-Cruz, Q. Chemical Characterization and Enzymatic Control of Stickies in Kraft Paper Production. Polymers 2020, 12, 245. https://doi.org/10.3390/polym12010245
Ballinas-Casarrubias L, González-Sánchez G, Eguiarte-Franco S, Siqueiros-Cendón T, Flores-Gallardo S, Duarte Villa E, de Dios Hernandez M, Rocha-Gutiérrez B, Rascón-Cruz Q. Chemical Characterization and Enzymatic Control of Stickies in Kraft Paper Production. Polymers. 2020; 12(1):245. https://doi.org/10.3390/polym12010245
Chicago/Turabian StyleBallinas-Casarrubias, Lourdes, Guillermo González-Sánchez, Salvador Eguiarte-Franco, Tania Siqueiros-Cendón, Sergio Flores-Gallardo, Eduardo Duarte Villa, Miguel de Dios Hernandez, Beatriz Rocha-Gutiérrez, and Quintín Rascón-Cruz. 2020. "Chemical Characterization and Enzymatic Control of Stickies in Kraft Paper Production" Polymers 12, no. 1: 245. https://doi.org/10.3390/polym12010245
APA StyleBallinas-Casarrubias, L., González-Sánchez, G., Eguiarte-Franco, S., Siqueiros-Cendón, T., Flores-Gallardo, S., Duarte Villa, E., de Dios Hernandez, M., Rocha-Gutiérrez, B., & Rascón-Cruz, Q. (2020). Chemical Characterization and Enzymatic Control of Stickies in Kraft Paper Production. Polymers, 12(1), 245. https://doi.org/10.3390/polym12010245